Abstract
Objective
The aim of this guideline is to assist FPs and other primary care providers with recognizing features that should raise their suspicions about the presence of lung cancer in their patients.
Composition of the committee
Committee members were selected from among the regional primary care leads from the Cancer Care Ontario Provincial Primary Care and Cancer Network and from among the members of the Cancer Care Ontario Lung Cancer Disease Site Group.
Methods
This guideline was developed through systematic review of the evidence base, synthesis of the evidence, and formal external review involving Canadian stakeholders to validate the relevance of recommendations.
Report
Evidence-based guidelines were developed to improve the management of patients presenting with clinical features of lung cancer within the Canadian context.
Conclusion
Earlier identification and referral of patients with lung cancer might ultimately help improve lung cancer morbidity and mortality. These guidelines might also be of value for informing the development of lung cancer diagnostic programs and for helping policy makers to ensure appropriate resources are in place.
Lung cancer is the most common cause of cancer death in Canada.1 Lung cancers are frequently diagnosed at a late stage, and the prognosis is very poor.1 The chance of surviving lung cancer in Canada is low, with a 5-year survival rate of 13% for men and 19% for women.1 Delays in the diagnosis of lung cancer are well documented.2–11 This might in part be owing to patients and clinicians attributing the often common, atypical, or nonspecific symptoms of lung cancer to other, benign diseases.
In order to provide guidance for the introduction of lung cancer diagnostic assessment programs (DAPs) in Ontario, the Cancer Care Ontario (CCO) Provincial Primary Care and Cancer Network initiated a collaboration in October 2009 with CCO’s Program in Evidence-based Care (PEBC) to form the Lung Cancer Referral Working Group. The working group was tasked with providing recommendations that would help FPs and other primary care providers recognize and initiate the management of undiagnosed patients presenting with signs or symptoms of lung cancer. The following questions were evaluated in completing this overall objective.
What main known risk factors are predictive of lung cancer?
What signs, symptoms, and other clinical features are predictive of lung cancer?
What is the diagnostic accuracy of investigations for lung cancer?
Which patient and provider factors are associated with delayed referral?
Does a delay in the time to consultation affect patient outcomes?
The aim of this guideline is to assist primary care clinicians in recognizing and managing clinical features that should raise their suspicion of lung cancer and ultimately lead to more timely and appropriate referrals. The recommendations are targeted to managing patients presenting in primary care settings. They are also intended to help policy makers ensure that resources such as lung cancer DAPs are in place so that target wait times are achieved.
Composition of committee
The working group consisted of 5 FPs (M.E.D., S.Y., M.A., P.B., C.L.), 1 medical oncologist (A.R.), 1 respirologist (R.S.), 1 radiation oncologist (Y.U.), 1 thoracic surgeon (R.Z.), and 1 methodologist (E.T.V.). Committee members included regional primary care cancer leads selected from the Provincial Primary Care and Cancer Network and members of CCO’s Lung Cancer Disease Site Group. Internal and external reviewers included FPs, thoracic surgeons, and radiologists. The work of the PEBC is supported by the Ontario Ministry of Health and Long-Term Care through CCO, and the PEBC is editorially independent from its funding source.
Methods
The guideline was developed using the methods of the practice guideline development cycle, including an environmental scan of existing guidelines, systematic review of the evidence base, evidence synthesis, and input from internal and external reviewers across Canada.12 Further details of the methods and findings of the systematic review are published elsewhere.13,14
Recommendations from the 2009 New Zealand Guidelines Group (NZGG),6 the 2004 Australian National Health and Medical Research Council,15 the 2005 National Institute for Health and Care Excellence (NICE),2 the American College of Chest Physicians’ evidence-based clinical practice guidelines,10,16,17 and the 2005 Scottish Intercollegiate Guidelines Network18 were considered during the guideline adaptation process. Updated evidence from primary studies was also taken into consideration. Many of the specific recommendations from the NZGG 2009 or NICE 2005 guidelines were endorsed or adapted.2,6 The following recommendations reflect the integration of the NZGG 2009 and NICE 2005 recommendations with evidence from level I systematic reviews, level II case-control and cohort studies, and level III expert opinion of the PEBC Lung Cancer Referral Working Group as described below.2,6
The working group held teleconferences to develop and approve the recommendations through informal consensus. Each recommendation took into consideration evidence from the systematic review. Recommended wait times were based on the expert opinion and consensus of the working group that often reflected feasibility in the Canadian health care system. Some members of the working group were part of a Canadian study (Lung Cancer Time to Treat Study), which showed wait times for specialist consultation among patients presenting with signs or symptoms causing suspicion of lung cancer can be reduced from 20 days to 6 days with the implementation of a DAP.5
Report
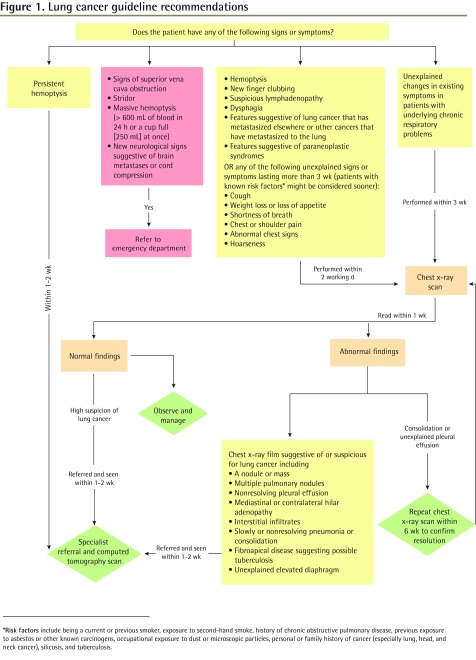
The lung cancer guideline recommendations are presented in Figure 1 and are outlined below.
Figure 1.
Lung cancer guideline recommendations
*Risk factors include being a current or previous smoker, exposure to second-hand smoke, history of chronic obstructive pulmonary disease, previous exposure to asbestos or other known carcinogens, occupational exposure to dust or microscopic particles, personal or family history of cancer (especially lung, head, and neck cancer), silicosis, and tuberculosis.
Factors that increase the risk of lung cancer
The risk factors for lung cancer provided in Box 1 were based on the summary of the NICE 2005 literature review provided by NZGG 2009.2,6 Our updated literature review did not provide evidence for additional risk factors associated with lung cancer beyond those listed in the NICE 2005 or NZGG 2009 guidelines.
Box 1. Factors that increase the risk of lung cancer.
The following factors increase the risk of lung cancer:
|
Indications and recommended initial investigation for suspicious lung cancer
An urgent chest x-ray scan within 48 hours is recommended for all signs and symptoms causing suspicion of lung cancer. The working group adapted the NZGG 2009 guideline indications for urgent chest x-ray scan (Box 2).6,10 Modifications of the NZGG 2009 recommendations included removal of the 3-week time frame for new finger clubbing, suspicious lymphadenopathy, or presentation with clinical features suggestive of cancer metastasis to or from the lung. Dysphagia was added as an indication for chest x-ray scan because it was reported in the NICE 2005 review as a symptom of lung cancer and was found to be a main clinical symptom among lung cancer patients in a tertiary care setting.2,3 Furthermore, paraneoplastic syndromes were also included based on findings of a 2007 review by Spiro et al, who reportedly observed paraneoplastic syndromes in approximately 10% of patients presenting with lung cancer.10
Box 2. Indications for investigation of suspicious lung cancer.
|
Indications for chest x-ray scan A person should have a chest x-ray scan within 2 working days if he or she presents with any of the following:
Or if any of the following unexplained signs or symptoms last more than 3 weeks (patients with known risk factors [Box 1] might be considered sooner):
Also consider the following:
Indications for chest CT scan A person should have a chest CT scan within 2 weeks if he or she has either of the following:
The ordering physician will depend on locally available resources and processes for expedited CT scans |
CT—computed tomography.
For patients with underlying chronic respiratory problems, the working group chose to endorse the recommendation from NICE 2005, which recommended a chest x-ray scan for unexplained changes in existing symptoms lasting more than 3 weeks.2
The recommendation for a 6-week follow-up of a consolidation found on a chest x-ray scan was adapted by the working group with modifications from the NZGG 2009 referral guideline.6 This recommendation was modified to include unexplained pleural effusion and to include all patients regardless of lung cancer risk factors (Box 2).6,10
There was considerable variability among guidelines regarding the indication for sputum cytology when lung cancer is suspected.6,15,17,18 The NICE 2005 and NZGG 2009 guidelines did not recommend sputum cytology.2,6 Because our updated literature search continued to find high specificity but variable sensitivity of sputum cytology in detecting lung cancer,15,17–20 the working group endorsed the NZGG 2009 recommendation against sputum cytology.6
The NICE 2005 and NZGG 2009 guidelines did not provide reviews of or recommendations on the use of thoracic computed tomography (CT) scans in patients with suspected lung cancer.2,6 The updated literature search revealed a paucity of further studies. The working group developed indications for CT thoracic scan based on recommendations provided by the Scottish Intercollegiate Guidelines Network in 2005 and on the expert opinion of the working group (Box 2).6,10,18 These indications included abnormal chest x-ray film findings or normal chest x-ray film findings but a high clinical suspicion of lung cancer.
A recommendation for follow-up of solitary pulmonary nodules on imaging tests was adapted initially from the American College of Chest Physicians’ clinical practice guidelines for pulmonary nodules.16 However, after internal review, the working group chose to remove this recommendation because a patient with a solitary pulmonary nodule, independent of size, should be referred to a specialist.
Indications and recommended referral for suspected lung cancer
Indications for immediate referral to the emergency department (Box 3) were adapted from the NICE 2005 guidelines.2 Massive hemoptysis (defined as more than 600 mL of blood over 24 hours or more than 250 mL at once) and new neurologic signs suggestive of brain metastases or cord compression were added based on the expert opinion and consensus of the working group.5
Box 3. Indications for referral of suspicious lung cancer.
|
Indications for referral to the ED Patients should be referred to the ED if they have any of the following:
Indications for referral to a specialist (respirologist or thoracic surgeon) or DAP Patients should be referred to and expect a consultation with a specialist or, where locally available, to a DAP within 1 to 2 weeks if they have any of the following:
If promptly accessible, a chest CT scan can be simultaneously ordered with the referral while awaiting the specialist’s consultation. This will depend on locally available resources. If the CT scan findings are entirely negative, then further referral to a specialist can be cancelled |
CT—computed tomography, DAP—diagnostic assessment program, ED—emergency department.
The indications for referral to a specialist were adapted by the working group from the NZGG 2009 and NICE 2005 referral guidelines.2,6 An unexplained elevated diaphragm was included, as per the suggestion of an internal reviewer. Additional suspicious abnormal chest x-ray scan findings (Box 3) and practice tips (Box 4) for specialist referral were based on the expert opinion of the working group members, especially from their experience with the Time to Treat program.5
Box 4. Practice tips for investigations and referral of patients with suspected lung cancer.
The requisition for a chest x-ray scan should include the presenting history, including signs and symptoms causing suspicion of lung cancer and whether risk factors (Box 1) exist
To expedite the diagnosis and avoid duplication of investigations, at a minimum provide the specialist consultant with the following information:
|
CT—computed tomography.
Owing to high false-negative results with chest x-ray scans, NICE 2005 and NZGG 2009 guidelines recommended referral to a specialist if there was a high suspicion of lung cancer despite normal chest x-ray scan findings.2,6 Two systematic reviews in our updated search also reported high false-negative results with chest x-ray scans.18,21 Thus, the recommendation urging clinicians to refer to a specialist if there is still a high suspicion of lung cancer was endorsed.
Recommendations to reduce diagnostic delay
The recommendations to reduce diagnostic delay were taken from evidence found in the NZGG 2009 and NICE 2005 guidelines, as well as from the updated literature search (Box 5).2,6,7,9,10 The patient- or FP-related factors that might delay referral or the diagnosis of lung cancer included fear of a diagnosis of cancer, not recognizing the signs and symptoms suggestive of lung cancer, comorbidities, multiple consecutive investigations in primary care, overreliance on chest x-ray scan results to diagnose lung cancer, failure to follow up on imaging results, and initial referral to a non–respiratory physician.2,6,7,9,10
Box 5. Recommendations to reduce diagnostic delay.
The following could contribute to reducing diagnostic delay:
|
Conclusion
The signs and symptoms of lung cancer that warrant further investigation include superior vena cava obstruction, stridor, hemoptysis, finger clubbing, enlarged lymph nodes, persistent or unexplained cough, unexplained weight loss, dyspnea, chest or shoulder pain, hoarseness, dysphagia, and abnormal chest x-ray film findings.2,6 Patients might also present with signs and symptoms of paraneoplastic syndromes or lung metastases.2,3,10 Risk factors for lung cancer include current or previous smoking, chronic obstructive pulmonary disease, previous exposure to asbestos, history of cancer (especially head and neck cancer), occupational exposure to dust or microscopic particles (eg, wood dust, silica), silicosis or tuberculosis, family history of cancer, exposure to known carcinogens (eg, radon, chromium, nickel), and passive exposure to tobacco smoke.2,6
Chest x-ray scans should be ordered as a preliminary investigation for patients presenting with signs or symptoms causing suspicion of lung cancer. Sputum cytology is not recommended. For a high suspicion of lung cancer based on chest x-ray film findings or clinical judgment (despite negative chest x-ray film findings), and depending on locally available resources, a chest CT scan and referral to a respirologist or thoracic surgeon are recommended.
While there is no published evidence on the effects of wait times on patient outcomes, it is generally believed that earlier diagnosis of lung cancer would likely lead to improved prognosis. Delays in diagnosis might be avoided if clinicians have a low threshold for ordering chest x-ray scans for patients presenting with even vague symptoms of lung cancer. If warranted, an expedited referral to a specialist or a DAP for further investigation should also be made. Attempts to address delays in diagnosis should be made at the patient, provider, and policy levels.
This guideline could help reduce delays in lung cancer diagnosis by assisting FPs and other primary care providers in recognizing clinical features that should raise their suspicion about the presence of lung cancer and lead them to more timely and appropriate referrals. It might also guide program development of DAPs for patients with suspected lung cancer and help policy makers to ensure that resources are in place so that target wait times can be achieved.
EDITOR’S KEY POINTS
The signs and symptoms of lung cancer that warrant further investigation include superior vena cava obstruction, stridor, hemoptysis, finger clubbing, enlarged lymph nodes, persistent or unexplained cough, unexplained weight loss, dyspnea, chest or shoulder pain, hoarseness, dysphagia, and abnormal chest x-ray film findings.
Chest x-ray scans should be ordered as a preliminary investigation for patients presenting with signs or symptoms causing suspicion of lung cancer. Sputum cytology is not recommended. For a high suspicion of lung cancer based on chest x-ray film findings or clinical judgment (despite negative chest x-ray film findings), and depending on locally available resources, a chest computed tomography scan and referral to a respirologist or thoracic surgeon are recommended.
Footnotes
This article is eligible for Mainpro-M1 credits. To earn credits, go to www.cfp.ca and click on the Mainpro link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro d’août 2014 à la page e376.
Contributors
All authors contributed to the literature review and interpretation, and to preparing the report for submission.
Competing interests
None declared
References
- 1.National Cancer Institute of Canada. Canadian cancer statistics 2001. Toronto, ON: National Cancer Institute of Canada; 2001. Available from: http://publications.gc.ca/site/eng/398229/publication.html. Accessed 2014 Jun 27. [Google Scholar]
- 2.National Institute for Health and Care Excellence [website]. Referral guidelines for suspected cancer. NICE guidelines CG27. London, UK: National Institute for Health and Care Excellence; 2005. Available from: www.nice.org.uk/CG027. Accessed 2014 Jul 2. [Google Scholar]
- 3.Chandra S, Mohan A, Guleria R, Singh V, Yadav P. Delays during the diagnostic evaluation and treatment of lung cancer. Asian Pac J Cancer Prev. 2009;10(3):453–6. [PubMed] [Google Scholar]
- 4.Liedekerken BM, Hoogendam A, Buntinx F, van der Weyden T, de Vet HC. Prolonged cough and lung cancer: the need for more general practice research to inform clinical decision-making. Br J Gen Pract. 1997;47(421):505. [PMC free article] [PubMed] [Google Scholar]
- 5.Lo DS, Zeldin RA, Skrastins R, Fraser IM, Newman H, Monavvari A, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol. 2007;2(11):1001–6. doi: 10.1097/JTO.0b013e318158d4b6. [DOI] [PubMed] [Google Scholar]
- 6.New Zealand Guidelines Group. Suspected cancer in primary care: guidelines for investigation, referral and reducing ethnic disparities. Wellington, NZ: New Zealand Guidelines Group; 2009. Available from: www.midlandcancernetwork.org.nz/file/fileid/17510. Accessed 2014 Jun 27. [Google Scholar]
- 7.Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64(9):749–56. doi: 10.1136/thx.2008.109330. [DOI] [PubMed] [Google Scholar]
- 8.Rolke HB, Bakke PS, Gallefoss F. Delays in the diagnostic pathways for primary pulmonary carcinoma in Southern Norway. Respir Med. 2007;101(6):1251–7. doi: 10.1016/j.rmed.2006.10.021. Epub 2006 Dec 5. [DOI] [PubMed] [Google Scholar]
- 9.Singh H, Sethi S, Raber M, Petersen LA. Errors in cancer diagnosis: current understanding and future directions. J Clin Oncol. 2007;25(31):5009–18. doi: 10.1200/JCO.2007.13.2142. [DOI] [PubMed] [Google Scholar]
- 10.Spiro SG, Gould MK, Colice GL, American College of Chest Physicians. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):149S–60S. doi: 10.1378/chest.07-1358. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz A, Damadoglu E, Salturk C, Okur E, Tuncer LY, Halezeroglu S. Delays in the diagnosis and treatment of primary lung cancer: are longer delays associated with advanced pathological stage? Ups J Med Sci. 2008;113(3):287–96. doi: 10.3109/2000-1967-236. [DOI] [PubMed] [Google Scholar]
- 12.Browman GP, Levine MN, Mohide EA, Hayward RS, Pritchard KI, Gafni A, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13(2):502–12. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- 13.Del Giudice L, Young S, Vella E, Ash M, Bansal P, Robinson A, et al. Referral of suspected lung cancer by family physicians and other primary care providers. Evidence-based series 24-2. Toronto, ON: Cancer Care Ontario; 2011. Available from: www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=155781. Accessed 2014 Jun 27. [Google Scholar]
- 14.Del Giudice ME, Young SM, Vella ET, Ash M, Bansal P, Robinson A, et al. Systematic review of guidelines for the management of suspected lung cancer in primary care. Can Fam Physician. 2014;60:e395–404. [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Council Australia. Clinical practice guidelines for the prevention, diagnosis and management of lung cancer. Sydney, Aust: National Health and Medical Research Council; 2004. Available from: www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp97.pdf. Accessed 2014 Jun 27. [Google Scholar]
- 16.Gould MK, Fletcher J, Iannettoni MD, Lynch WR, Midthun DE, Naidich DP, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):108S–30S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 17.Rivera MP, Mehta AC, American College of Chest Physicians. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):131S–48S. doi: 10.1378/chest.07-1357. [DOI] [PubMed] [Google Scholar]
- 18.Scottish Intercollegiate Guidelines Network. Management of patients with lung cancer: a national clinical guideline. Edinburgh, Scot: Scottish Intercollegiate Guidelines Network; 2005. Available from: www.sign.ac.uk/guidelines/fulltext/80/. Accessed 2014 Jun 27. [Google Scholar]
- 19.Choi YD, Han CW, Kim JH, Oh IJ, Nam JH, Juhng SW, et al. Effectiveness of sputum cytology using ThinPrep method for evaluation of lung cancer. Diagn Cytopathol. 2008;36(3):167–71. doi: 10.1002/dc.20761. [DOI] [PubMed] [Google Scholar]
- 20.Kemp RA, Reinders DM, Turic B. Detection of lung cancer by automated sputum cytometry. J Thorac Oncol. 2007;2(11):993–1000. doi: 10.1097/JTO.0b013e318158d488. [DOI] [PubMed] [Google Scholar]
- 21.Kvale PA. Chronic cough due to lung tumors: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):147S–53S. doi: 10.1378/chest.129.1_suppl.147S. [DOI] [PubMed] [Google Scholar]