Abstract
Objective
To determine the effectiveness of presenting individualized colorectal cancer (CRC) risk information for increasing CRC screening rates in primary care patients at above-average risk of CRC.
Design
Randomized controlled trial.
Setting
Georgia Regents University in Augusta—an academic family medicine clinic in the southeastern United States.
Participants
Outpatients (50 to 70 years of age) scheduled for routine visits in the family medicine clinic who were determined to be at above-average risk of CRC.
Interventions
Individualized CRC risk information calculated from the Your Disease Risk tool compared with a standard CRC screening handout.
Main outcome measures
Intention to complete CRC screening. Secondary measures included the proportions of subjects completing fecal occult blood tests, flexible sigmoidoscopy, and colonoscopy.
Results
A total of 1147 consecutive records were reviewed to determine eligibility. Overall, 210 (37.7%) of 557 eligible participants were randomized to receive either individualized CRC risk information (prepared by a research assistant) or a standard CRC screening handout. The intervention group had a mean (SD) age of 55.7 (4.8) years and the control group had a mean (SD) age of 55.6 (4.6) years. Two-thirds of the participants in each group were female. The intervention group and the control group were matched by race (P = .40). There was no significant difference between groups for intention to complete CRC screening (P = .58). Overall, 26.7% of the intervention participants and 27.7% of the control participants completed 1 or more CRC screening tests (P = .66).
Conclusion
Presentation of individualized CRC risk information by a nonphysician assistant as a decision aid did not result in higher CRC screening rates in primary care patients compared with presentation of general CRC screening information. Future research is needed to determine if physician presentation of CRC risk information would result in increased screening rates compared with research assistant presentation.
Résumé
Objectif
Déterminer si une information personnalisée sur les risques de cancer colorectal (CCR) est une mesure efficace pour augmenter le taux de dépistage du CCR chez des patients des soins primaires présentant un risque supérieur à la moyenne de ce type de cancer.
Type d’étude
Essai randomisé.
Contexte
L’Université Georgia Regents à Augusta, une clinique universitaire de médecine familiale du sud-est des États-Unis.
Participants
Des patients externes de 50 à 70 ans qui venaient à la clinique de médecine familiale pour une visite de routine et chez qui on a établi un risque de CCR supérieur à la moyenne.
Interventions
Une information personnalisée sur les risques de CCR calculés à partir de l’outil Your Disease Risk, en comparaison d’un document standard sur ce dépistage.
Principaux paramètres à l’étude
L’intention d’avoir un dépistage. Les paramètres secondaires incluaient la proportion des sujets ayant subi une recherche du sang occulte dans les selles, une sigmoïdoscopie flexible ou une coloscopie.
Résultats
On a étudié 1147 dossiers consécutifs pour en déterminer l’admissibilité. Parmi les 557 participants admissibles, 210 (37,7 %) ont été randomisés pour recevoir soit des informations personnalisées sur les risques de CCR (préparées par un assistant de recherche) ou un document standard sur le dépistage du CCR. L’âge moyen (ÉT) était de 55,7 (4,8) ans dans le groupe d’intervention contre 55,6 (4,6) ans dans le groupe témoin. Les deux tiers des participants des 2 groupes étaient des femmes. Le groupe d’intervention et le groupe témoin étaient appariés pour l’origine raciale (P = ,40). Il n’y avait pas de différence significative entre les groupes pour ce qui est de l’intention de se soumettre à un dépistage du CCR (P = ,58). Dans l’ensemble, 26,7 % des participants du groupe d’intervention ont subi au moins un des examens de dépistage du CCR contre 27,7 % pour ceux du groupe témoin (P = ,66).
Conclusion
Une information personnalisée sur les risques de CCR donnée par un assistant autre qu’un médecin dans le but d’aider le patient à prendre une décision n’a pas résulté en un taux de dépistage plus élevé qu’un document d’information sur les risques de CCR. Des études additionnelles devront déterminer si le fait que ce soit un médecin plutôt qu’un assistant de recherche qui explique les risques de CCR entraînerait un taux de dépistage supérieur.
Colorectal cancer (CRC) is a leading cause of cancer mortality in Canada.1 In 2009, an estimated 22 000 Canadians were diagnosed with CRC, and approximately 9100 died from this disease.1 The Canadian Association of Gastroenterology position statement for CRC screening of average-risk individuals recommends annual fecal immunochemical or high-sensitivity guaiac-based fecal occult blood tests (FOBTs) and flexible sigmoidoscopy testing every 10 years.1 There is evidence that CRC screening with removal of polyps reduces CRC incidence.2–5
The Your Disease Risk tool (YDR) is a component of an Internet-based health appraisal instrument (www.yourdiseaserisk.wustl.edu)6 developed by an interdisciplinary team with the purpose of educating the public.7,8 Three previous studies have evaluated the usefulness of the YDR in helping patients understand their individualized risk of CRC.9–11 However, the effectiveness of the YDR for increasing CRC screening rates has not been tested in a primary care setting. The purpose of the present study was to examine the effectiveness of the YDR for increasing CRC screening rates in primary care patients at above-average risk of CRC.
METHODS
Participants and study procedures
Electronic medical records for 1147 consecutive adult outpatients with appointments during the study period were prescreened to determine eligibility for the study between October 2007 and December 2009. Subjects were eligible if they were between the ages of 50 and 75 years and had no evidence of CRC screening during the past 10 years, as documented in an enterprise-wide electronic medical record. Patients who were eligible based on prescreening were approached and asked to complete an eligibility survey. Patients were determined to be eligible if they reported never having completed CRC screening and if they were at an increased risk of CRC based on 1 or more of the following 4 risk factors identified in the YDR: body mass index (BMI) greater than 27 kg/m2, inflammatory bowel disease for longer than 10 years, lack of folic acid supplementation, and 1 or more first-degree relatives with CRC. A total of 557 (48.6%) patients were deemed eligible to participate in the study. Potential participants were approached as they presented for regularly scheduled outpatient appointments at Georgia Regents University, an academic family medicine centre in Augusta. Participants were each given a $20 gift card for their participation in the study. Informed consent was obtained from all participants, and this study was approved by our institutional review board.
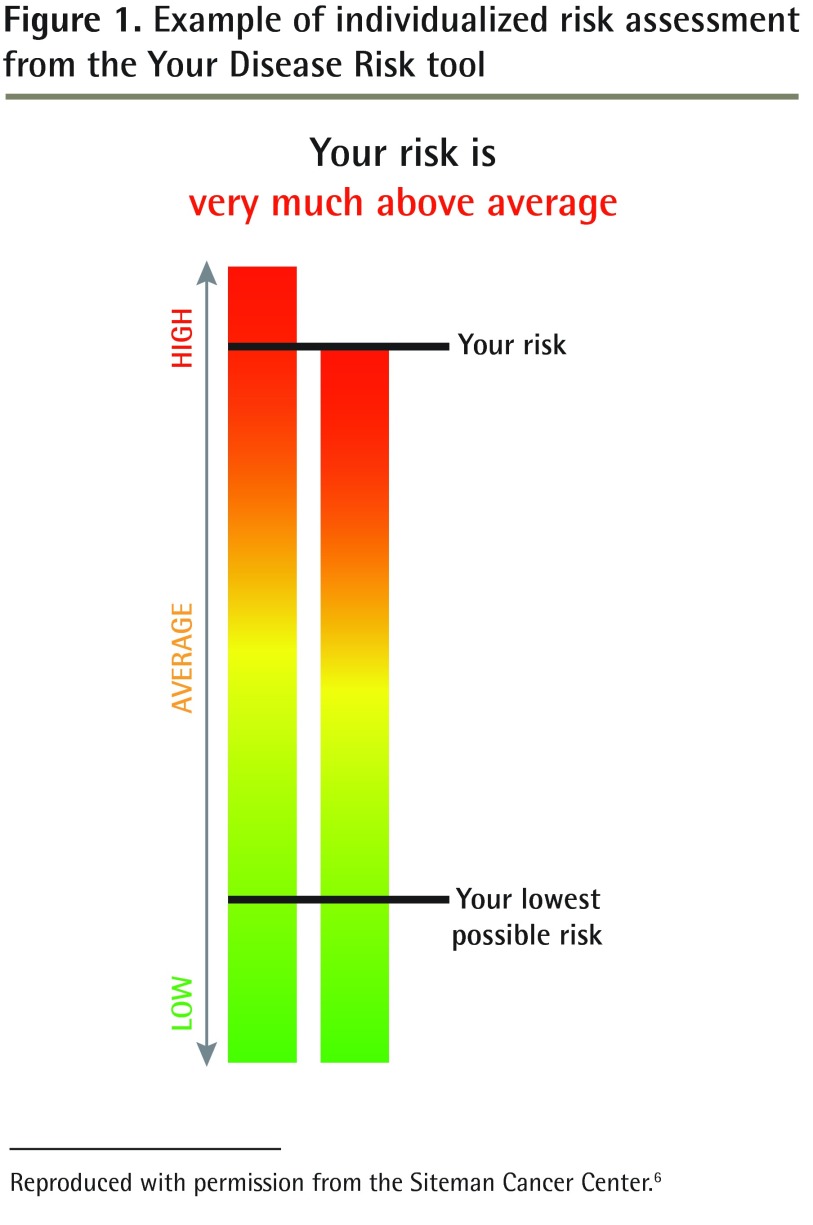
Participants completed a survey before their physician visits to determine their individualized CRC risk and their intention to complete CRC screening. This pre-visit survey assessed demographic factors, education, household income, current health insurance, and BMI. Additionally, numeracy skills (described below), perceived risk of personally developing CRC, and worry about developing CRC were assessed. Investigators assigned participants to the intervention group or the usual care group by opening a numbered opaque envelope that contained a computer-generated, randomized group assignment. The intervention group received from the research assistant individualized CRC risk information calculated from the YDR and presented as a bar graph, plus a handout on CRC screening from the Centers for Disease Control and Prevention. Figure 1 provides an example of the CRC risk information.6 The usual care group received from the research assistant only the handout from the Centers for Disease Control and Prevention on CRC and screening tests. The Internet-based risk appraisal instrument (YDR) for CRC was completed for the patient by the research assistant using the information from the completed surveys. The research assistant provided study material to participants and encouraged participants to read the information.
Figure 1.
Example of individualized risk assessment from the Your Disease Risk tool
Reproduced with permission from the Siteman Cancer Center.6
After reading their study materials, participants continued with their scheduled physician visits and were encouraged to discuss their risk information with their physicians. Following their appointments, participants completed a survey assessing their intention to complete CRC screening. Additionally, risk of and worry about developing CRC were assessed (described below). At the time of completion of the post-visit survey, all participants were given written and verbal invitations to complete 3 FOBTs at home and to make an appointment for flexible sigmoidoscopy. An electronic chart review was conducted 6 months from the index visit for all participants to assess rate of CRC screening (completing 3 FOBTs, flexible sigmoidoscopy, or colonoscopy).
Power analysis indicated that a sample of 99 in each treatment group was necessary to obtain power of 0.80 with α = .05, and that would enable us to observe a small to medium effect (effect size = 0.20). This power analysis used a 1-way ANOVA (analysis of variance) as the model, using intention to complete CRC screening as reported immediately following the intervention (post-visit survey) as the primary outcome variable.
Measures
Intention to be screened is a predictor of future CRC screening.12–15 The intention score for each participant was computed using the average of responses to 2 items: I intend to undergo colorectal screening (completing FOBTs, flexible sigmoidoscopy, or colonoscopy); and I do not intend to go through colorectal screening (FOBTs, flexible sigmoidoscopy, or colonoscopy) (reverse coded). These items were measured using a 4-point Likert scale from strongly disagree (score of 1) to strongly agree (score of 4). We assessed numeracy skills by asking participants to answer 2 questions that have been previously validated. The range of scores on these numeracy skills is from 0 correct (low numeracy skills) to 2 correct (high numeracy skills). Participants were asked about their perceived absolute and comparative (ie, self-compared with others) risk of getting CRC (score 0 to 4). Responses were much below average, below average, average, above average, and much above average. Participants were also asked, “How worried are you about getting colorectal cancer?” The 4 responses included not at all worried, slightly worried, moderately worried, and quite worried. Behavioural measures included responses to an invitation to complete FOBTs and to schedule an appointment for flexible sigmoidoscopy, and a chart review of completed CRC screening tests (eg, FOBTs, flexible sigmoidoscopies, and colonoscopies).
Statistical analysis
To confirm normality of data across categorical demographic variables, χ2 and Fisher exact tests were performed. Mann-Whitney-Wilcoxon analysis was used to compare BMI and age across intervention and control groups to confirm normality of data in these variables. Mann-Whitney-Wilcoxon analyses were performed to compare intention to screen, perceived risk, and worry about CRC, and to compare change in outcomes across the treatment groups. Analysis of variance was used to investigate whether there was a significant difference between the numeracy groups among the participants who discussed CRC with their physicians, while controlling for the baseline score. Data were analyzed in SPSS, version 16.0, and SAS, version 9.2.
RESULTS
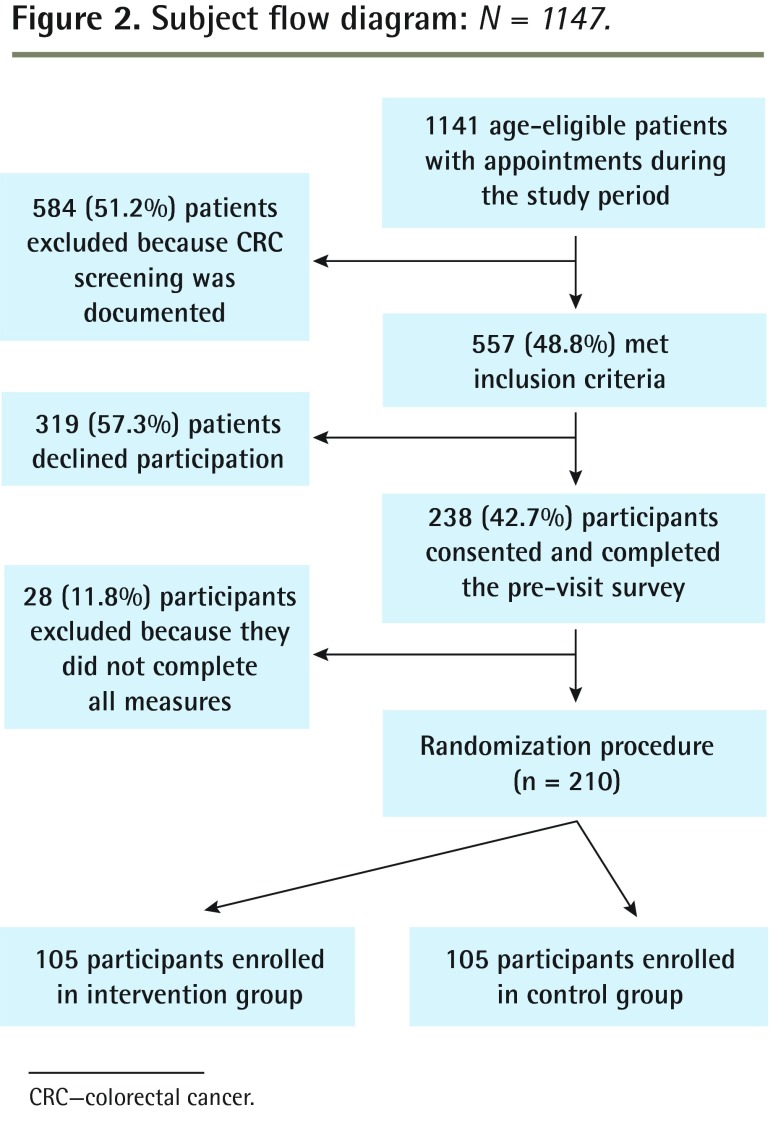
Of the 557 eligible patients, 210 (37.7%) completed the study (ie, completed all measures). Figure 2 provides a study flow diagram. The study population was 66.7% female, 51.4% white, 47.1% African American, 52.9% married, and 43.9% employed. The mean (SD) age was 55.8 (5.1) years, and the mean (SD) BMI was 31.3 (7.6) kg/m2. Most participants had completed high school (76.7%), and nearly half of the sample (43.8%) had a household income of $20 000 (US) or less. Only 24 participants (11.7%) answered the 2 mathematics problems correctly (ie, numeracy score), with the remaining participants roughly split between answering 1 item correctly (44.2%, n = 94) and 0 items correctly (42.7%, n = 88). Participants were randomly assigned in equal numbers to the intervention group and the control group (n = 105 to each group); no significant differences were found between the assigned groups on the above demographic characteristics or numeracy scores. Table 1 provides a detailed summary of demographic characteristics by treatment group.
Figure 2.
Subject flow diagram: N = 1147.
CRC—colorectal cancer.
Table 1.
Treatment groups by demographic characteristics: There were no significant differences between the control and intervention groups.
| CHARACTERISTIC | CONTROL GROUP (N = 105), N (%)* | INTERVENTION GROUP (N = 105), N (%)* |
|---|---|---|
| Female sex | 70 (66.7) | 70 (66.7) |
| Race | ||
| • White | 51 (48.6) | 57 (54.3) |
| • African American | 53 (50.4) | 46 (43.8) |
| • Hispanic, Latino, Asian, or other | 1 (1.0) | 2 (1.9) |
| Current health insurance | 96 (95.0) | 104 (99.0) |
| Marital status | ||
| • Married | 50 (49.5) | 59 (56.2) |
| • Divorced | 21 (20.8) | 25 (23.8) |
| • Other | 30 (29.7) | 21 (20.0) |
| Education | ||
| • Grade 11 or less | 23 (22.8) | 25 (23.8) |
| • Grade 12 or GED | 31 (30.7) | 32 (30.5) |
| • Some college or university | 25 (24.8) | 31 (29.5) |
| • Completed college or university | 22 (21.8) | 17 (16.2) |
| Employment status | ||
| • Full time | 50 (50.5) | 40 (38.5) |
| • Part time | 8 (8.1) | 6 (5.8) |
| • Not employed | 12 (12.1) | 22 (21.2) |
| • Unable to work owing to disability | 29 (29.3) | 36 (34.6) |
| Household income, $ | ||
| • < 20 000 | 43 (43.4) | 45 (44.1) |
| • 20 000–60 000 | 31 (31.3) | 33 (32.4) |
| • 60 000–100 000 | 17 (17.2) | 14 (13.7) |
| • > 100 000 | 8 (8.1) | 10 (9.8) |
GED—general educational development.
Proportions are calculated excluding missing data and might not add to 100% owing to rounding.
No statistically significant differences were found between the control and intervention groups for documented CRC discussions with physicians (P = .75), specific CRC screening tests recommended by their physicians (FOBTs, flexible sigmoidoscopy, or colonoscopy) (P = .31), FOBT cards taken (P = .96), flexible sigmoidoscopy scheduled (P = .96), or any CRC screening test completed (P = .66). Additionally, there were no statistically significant differences between groups completing FOBTs (P = .38) or colonoscopies (P = .95). No barium enemas or flexible sigmoidoscopies were documented as completed by the 6-month chart audit (Table 2). Similarly, there were no significant differences between groups for intention to complete CRC screening (P = .58), perceived risk of CRC (P = .48), perceived risk relative to others (P = .65), or worry about developing CRC (P = .46) (Table 3).
Table 2.
Outcome measures across treatment groups
| VARIABLE | CONTROL GROUP (N = 105), N (%)* | INTERVENTION GROUP (N = 105), N (%)* | P VALUE |
|---|---|---|---|
| Numeracy score | .84† | ||
| • 0 | 45 (44.6) | 43 (41.0) | |
| • 1 | 44 (43.6) | 50 (47.6) | |
| • 2 | 12 (11.9) | 12 (11.4) | |
| FOBT cards taken | 72 (70.3) | 77 (73.3) | .96 |
| CRC screening discussion documented by physician | 52 (51.5) | 57 (54.3) | .75 |
| FOBT recommended by physician | 9 (8.9) | 11 (10.5) | .71 |
| Flexible sigmoidoscopy recommended by physician | 1 (1.0) | 1 (1.0) | .98 |
| Colonoscopy recommended by physician | 49 (48.5) | 56 (53.3) | .49 |
| Barium enema recommended by physician | 7 (6.9) | 4 (3.8) | .32 |
| FOBT completed | 10 (9.9) | 7 (6.7) | .38 |
| Colonoscopy completed | 21 (20.8) | 22 (21.0) | .95 |
| Completed any CRC screening tests | 28 (27.7) | 27 (26.7) | .66 |
CRC—colorectal cancer, FOBT—fecal occult blood test.
Proportions are calculated excluding missing data and might not add to 100% owing to rounding.
Fisher exact test performed in lieu of χ2 test.
Table 3.
Before-and-after changes in outcome measures across treatment groups
| SCORE | CONTROL GROUP (n = 95) | INTERVENTION GROUP (n = 103) | P VALUE* | ||
|---|---|---|---|---|---|
| MEAN (SD) CHANGE | MEDIAN (RANGE) CHANGE | MEAN (SD) CHANGE | MEDIAN (RANGE) CHANGE | ||
| Intention to be screened† | 0.07 (0.6) | 0 (−1 to 2) | 0.13 (0.7) | 0 (−1 to 2) | .58 |
| Risk‡ | 0.08 (0.6) | 0 (−2 to 3) | 0.08 (0.7) | 0 (−4 to 3) | .48 |
| Risk relative to others‡ | 0.04 (0.4) | 0 (−1 to 2) | 0.11 (0.8) | 0 (−4 to 4) | .65 |
| Worry§ | 0.07 (0.4) | 0 (−1 to 3) | 0.05 (0.4) | 0 (−1 to 2) | .46 |
Mann-Whitney-Wilcoxon test was used.
The intention score was the average of responses to 2 items: I intend to undergo colorectal screening (completing fecal occult blood tests, flexible sigmoidoscopy, or colonoscopy); and I do not intend to go through colorectal screening (fecal occult blood tests, flexible sigmoidoscopy, or colonoscopy) (reverse coded). These items were measured using a 4-point Likert scale from strongly disagree (score of 1) to strongly agree (score of 4).
Participants were asked about their perceived absolute and comparative (ie, self-compared with others) risk of getting colorectal cancer (score 0 to 4). Possible responses were much below average, below average, average, above average, and much above average.
Participants were asked, “How worried are you about getting colorectal cancer?” Possible responses were not at all worried (score of 1), slightly worried, moderately worried, and quite worried (score of 4).
Participants who discussed CRC screening with their physicians had a greater increase in their intention to be screened (P = .04) compared with participants who did not discuss CRC screening with their physicians. Among those participants who discussed their CRC risk with their physicians, there was a significant difference between changes in intention score between the numeracy groups (P = .46). Participants (n = 63) with numeracy scores greater than 0 showed almost no change in their mean (SD) intention to screen (0.1 [0.7]), whereas participants (n = 44) with numeracy scores of 0 showed a significant increase in their mean (SD) intention to screen (0.3 [0.6]) (P = .05). Analyses of variance were used to investigate whether there was a significant difference between the treatment groups among the participants who discussed CRC risk with their physicians, while controlling for the baseline score; there were no significant interactions.
No significant association was found between participants who were screened for CRC and various predictor variables (eg, sex, race, education, household income, current health insurance, and numeracy scores). Marital status approached significance, with married participants in the intervention group having higher rates of completed CRC screening than participants in the control group (67.3% vs 46.7%, respectively; P = .05) (Table 4).
Table 4.
Demographic characteristic comparisons of participants by completed screening status
| VARIABLE | ≥ 1 COMPLETED CRC SCREENING TESTS (N = 55), N (%) | NO COMPLETED CRC SCREENING TESTS (N = 135), N (%) | P VALUE |
|---|---|---|---|
| Numeracy | .46 | ||
| • 0 | 22 (40.0) | 62 (45.9) | |
| • ≥ 1 | 33 (60.0) | 73 (54.1) | |
| Female sex | 34 (61.8) | 92 (68.1) | .40 |
| Race | .60* | ||
| • White | 27 (49.1) | 70 (51.9) | |
| • African American | 27 (49.1) | 63 (46.7) | |
| • Hispanic or Latino | 0 (0.0) | 1 (0.7) | |
| • Asian | 1 (1.8) | 0 (0.0) | |
| • Unknown | 0 (0.0) | 1 (0.7) | |
| Marital status | .05* | ||
| • Married | 37 (67.3) | 63 (46.7) | |
| • Divorced | 10 (18.2) | 30 (22.2) | |
| • Widowed | 1 (1.8) | 15 (11.1) | |
| • Separated | 1 (1.8) | 12 (8.9) | |
| • Never married | 5 (9) | 11 (8.1) | |
| • In an unmarried couple | 1 (1.8) | 4 (3.0) | |
| Education | .86 | ||
| • Kindergarten or less | 0 (0.0) | 1 (0.7) | |
| • Grades 1–8 | 7 (13) | 13 (9.6) | |
| • Grades 9–11 | 6 (11) | 17 (12.6) | |
| • Grade 12 or GED | 17 (31) | 40 (29.6) | |
| • Some college or technical school | 17 (31) | 36 (26.7) | |
| • College or university graduate | 8 (15) | 28 (20.7) |
CRC—colorectal cancer, GED—general educational development.
Fisher exact test performed in lieu of χ2 test.
DISCUSSION
We found no statistically significant difference between treatment groups in presenting personalized CRC risk information versus a standard care handout to increase CRC screening rates, and we found no statistically significant difference with respect to intention to be screened for CRC, initiation of screening behaviour (eg, taking FOBT cards or scheduling flexible sigmoidoscopy or colonoscopy after physician visit), or completion of any CRC screening tests. The results of our study are limited by the poor participation rate in the study (37.7%) and the low completion rate of any CRC screening tests (27.7% and 26.7% for control and intervention groups, respectively). In fact, we postulated that our high-risk participants were likely to be the patients most interested in CRC screening, and the failure to show an effect in this select group is a strong indicator of lack of efficacy.
The science of tailored, computer-based risk communication in increasing CRC screening is improving. One study found that substantially more patients given risk communication had accurate perceptions about CRC risk compared with patients not given risk communication.9 Another study involved 353 members of a health maintenance organization who interacted with a computerized program that provided individualized CRC risk information.10 The study found that computerized feedback improved risk perception among participants. A third study involving 119 men and women aged 50 years and older found that providing CRC risk information increased intentions to receive CRC screening and completion of CRC screening tests (screening rate 31%).11
Our study used individualized risk communication as a decision tool to increase CRC screening in a cohort of participants determined to be at high risk of CRC. Another study involving 249 participants used a video-recorded decision aid and found that 36.8% of the intervention group completed CRC screening tests compared with 22.6% of the control group.16 Another study involving 174 participants used a Web-based electronic decision aid and found that 67% of the intervention group completed CRC screening tests compared with 39% of the control group.17 Therefore, our decision aid showed less of an effect on completion of CRC screening (27.7% and 26.7% for control and intervention groups, respectively) compared with a videorecorded decision aid (37%) and a Web-based electronic decision aid (67%).
Our results raise the question of whether the ineffectiveness of the intervention in this study was related to the utility of the CRC risk information or related to who provided the information (ie, nonphysician vs physician). A subsequent, multi-component study is needed in which intervention type (ie, CRC risk vs general CRC screening information) and provider type (ie, nonphysician vs physician) are examined for effect on completion of CRC screening. The engagement of physicians in the intervention process appears to be an essential component for patients to complete CRC screening. Patients in the current study who discussed CRC screening with their physicians after they received the risk information changed their intention to complete screening more often compared with patients who did not have such discussions.
An alternative approach to providing CRC risk information to patients is to provide physicians with this information (eg, determine if providing physicians with their patients’ CRC risk information positively affects physician behaviour and, more important, if it improves patient adherence to CRC screening recommendations). If screening rates are improved, additional studies would be warranted to determine how to systematically provide such information to physicians (eg, automated CRC risk score from the electronic health record with a chart prompt to complete CRC screening) rather than during a brief research study. A study of 270 primary care physicians found that it takes about 4 minutes to properly describe CRC screening.18 This study found that the most important factors for physicians to discuss with patients regarding CRC screening included benefits of screening, making a plan during the visit for screening, and suggesting the best test for patients.18 For CRC screening, physicians most commonly discuss screening colonoscopy (84%) and less commonly FOBTs (49%) and flexible sigmoidoscopy (34%).18
Another potential explanation for the minimal beneficial effect of providing CRC risk information is that provision of information is inadequate without additional consideration of patient readiness for change and personal resources to change. A variety of health behaviour change models, such as motivational interviewing,19,20 conviction and confidence,21 and theory of planned behaviour,22,23 emphasize that physicians and health personnel must identify and address patients beliefs about their health, including norms and patients’ perceived abilities to make changes. From these models, provision of risk information could only be useful within the context of a more comprehensive discussion of patient beliefs, skills, and intent to change.
Our treatment and control groups were well matched; there were no significant differences between groups with regard to any demographic factor or the numeracy score. The average age of participants in this study was 55 years, and most participants were obese (BMI > 30 kg/m2). The mean BMI was 31 kg/m2, and obesity (ie, BMI > 30 kg/m2) was the most common CRC risk factor in our study. Most of our participants were female (66.7%) and either white (51.4%) or African American (47.1%). Most of our participants had a high school education or above, but most participants were at or below the poverty level. However, nearly all participants had health insurance (97.1%).
Limitations
We chose to assess numeracy scores but did not assess health literacy scores in our study. A future study should assess the association of numeracy scores, health literacy, and interpretation of a CRC risk assessment with subsequent completed CRC screening. Another limitation of our study is that nearly all of our participants were either white or African American, and our results might not be generalizable to other races. Another limitation was that nearly all participants had health insurance and our results might not be generalizable to individuals without health insurance.
Conclusion
Colorectal cancer risk information presented by a non-physician assistant as a decision aid to primary care patients at above-average risk did not increase intention to complete CRC screening or actual CRC screening rates compared with a standard handout on CRC screening.
Acknowledgments
This research was funded by a grant from the American Academy of Family Physicians Foundation. This paper was presented at the American College of Gastroenterology Annual Meeting in San Diego, Calif, in October 2009.
EDITOR’S KEY POINTS
Colorectal cancer (CRC) risk information presented by a nonphysician assistant as a decision aid to primary care patients at above-average risk did not increase intention to complete CRC screening or actual CRC screening rates compared with a standard handout on CRC screening.
Results of this study raise the question of whether the ineffectiveness of the intervention was related to the utility of the CRC risk information or related to who provided the information (ie, nonphysician versus physician). A subsequent, multi-component study is needed in which intervention type (ie, CRC risk vs general CRC screening information) and provider type (ie, nonphysician vs physician) are examined for effect on completion of CRC screening.
POINTS DE REPÈRE DU RÉDACTEUR
Des explications personnalisées sur les risques de cancer colorectal (CCR) données par un assistant non médecin à des patients présentant des risques supérieurs à la moyenne afin de les aider à prendre une décision n’a pas augmenté leur intention de se soumettre à un dépistage ni les taux réels de dépistage, par rapport à la distribution d’une documentation standard sur ce dépistage.
Les résultats de cette étude soulèvent la possibilité que l’échec de l’intervention soit en rapport avec l’utilité de l’information sur les risques de CCR ou plutôt avec la personne qui donnait cette information (non médecin vs médecin). Il y a lieu de faire une étude à composantes multiples pour vérifier l’effet sur le taux de dépistage du type d’intervention (information sur les risques de CCR vs information générale sur le dépistage du CCR) ainsi que l’effet de la personne qui fournit l’information (non médecin vs médecin).
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors contributed to the concept and design of the study; data gathering, analysis, and interpretation; and preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Leddin DJ, Enns R, Hilsden R, Plourde V, Rabeneck L, Sadowski DC, et al. Canadian Association of Gastroenterology position statement on screening individuals at average risk for developing colorectal cancer: 2010. Can J Gastroenterol. 2010;24(12):705–14. doi: 10.1155/2010/683171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M, Italian Multicentre Study Group Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48(6):812–5. doi: 10.1136/gut.48.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoff G, Sauar J, Vatn MH, Larsen S, Langmark F, Moen IE, et al. Polypectomy of adenomas in the prevention of colorectal cancer: 10 years’ follow-up of the Telemark Polyp Study I. A prospective, controlled population study. Scand J Gastroenterol. 1996;31(10):1006–10. doi: 10.3109/00365529609003121. [DOI] [PubMed] [Google Scholar]
- 4.Thiis-Evensen E, Hoff GS, Sauar J, Langmark F, Majak BM, Vatn MH. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol. 1999;34(4):414–20. doi: 10.1080/003655299750026443. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 6.Siteman Cancer Center . Your disease risk. The source on prevention. St Louis, MO: Barnes-Jewish Hospital, Washington University School of Medicine; 2013. Available from: www.yourdiseaserisk.wustl.edu. Accessed 2014 Jul 2. [Google Scholar]
- 7.Colditz GA, Atwood KA, Emmons K, Monson RR, Willett WC, Trichopoulos D, et al. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention. Cancer Causes Control. 2000;11(6):477–88. doi: 10.1023/a:1008984432272. [DOI] [PubMed] [Google Scholar]
- 8.Emmons KM, Koch-Weser S, Atwood K, Conboy L, Rudd R, Colditz G. A qualitative evaluation of the Harvard Cancer Risk Index. J Health Commun. 1999;4(3):181–93. doi: 10.1080/108107399126904. [DOI] [PubMed] [Google Scholar]
- 9.Emmons KM, Wong M, Puleo E, Weinstein N, Fletcher R, Colditz G. Tailored computer-based cancer risk communication: correcting colorectal cancer risk perception. J Health Commun. 2004;9(2):127–41. doi: 10.1080/10810730490425295. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein ND, Atwood K, Puleo E, Fletcher R, Colditz G, Emmons K. Colon cancer: risk perceptions and risk communication. J Health Commun. 2004;9(1):53–65. doi: 10.1080/10810730490271647. [DOI] [PubMed] [Google Scholar]
- 11.Lipkus IM, Green LG, Marcus A. Manipulating perceptions of colorectal cancer threat: implications for screening intentions and behaviors. J Health Commun. 2003;8(3):213–28. doi: 10.1080/10810730305684. [DOI] [PubMed] [Google Scholar]
- 12.Myers RE, Ross EA, Wolf TA, Balshem A, Jepson C, Millner L. Behavioral interventions to increase adherence in colorectal cancer screening. Med Care. 1991;29(10):1039–50. doi: 10.1097/00005650-199110000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Myers RE, Vernon SW, Tilley BC, Lu M, Watts BG. Intention to screen for colorectal cancer among white male employees. Prev Med. 1998;27(2):279–87. doi: 10.1006/pmed.1998.0264. [DOI] [PubMed] [Google Scholar]
- 14.Sutton S, Wardle J, Taylor T, McCaffery K, Williamson S, Edwards R, et al. Predictors of attendance in the United Kingdom flexible sigmoidoscopy screening trial. J Med Screen. 2000;7(2):99–104. doi: 10.1136/jms.7.2.99. [DOI] [PubMed] [Google Scholar]
- 15.Watts BG, Vernon SW, Myers RE, Tilley BC. Intention to be screened over time for colorectal cancer in male automotive workers. Cancer Epidemiol Biomarkers Prev. 2003;12(4):339–49. [PubMed] [Google Scholar]
- 16.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening. A randomized, controlled trial. Ann Intern Med. 2000;133(10):761–9. doi: 10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
- 17.Ruffin MT, 4th, Fetters MD, Jimbo M. Preference-based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial. Prev Med. 2007;45(4):267–73. doi: 10.1016/j.ypmed.2007.07.003. Epub 2007 Jul 14. [DOI] [PubMed] [Google Scholar]
- 18.Wolf MS, Baker DW, Makoul G. Physician-patient communication about colorectal cancer screening. J Gen Intern Med. 2007;22(11):1493–9. doi: 10.1007/s11606-007-0289-y. Epub 2007 Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527–37. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rollnick S, Miller WR, Butler CC. Motivational interviewing in health care. Helping patients change behavior. New York, NY: Guilford Press; 2008. [Google Scholar]
- 21.Keller VF, Kemp White M. Choices and changes: a new model for influencing patient health behavior. J Clin Outcomes Management. 1997;4(6):33–6. [Google Scholar]
- 22.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179–211. [Google Scholar]
- 23.Kiviniemi MT, Bennett A, Zaiter M, Marshall JR. Individual-level factors in colorectal cancer screening: a review of the literature on the relation of individual-level health behavior constructs and screening behavior. Psychooncology. 2011;20(10):1023–33. doi: 10.1002/pon.1865. Epub 2010 Oct 27. [DOI] [PMC free article] [PubMed] [Google Scholar]