Abstract
Objective
To systematically review the literature and provide an update and integration of existing peer-reviewed guidelines with recent systematic reviews and with primary studies related to the early recognition and management of lung cancer in primary care.
Data sources
MEDLINE and EMBASE were searched for relevant articles. The quality of the evidence to support existing guideline recommendations, and the consistency of recommendations with updated evidence, were assessed. Applicability in a Canadian primary care setting was also evaluated.
Study selection
All studies that explored signs or symptoms of or risk factors for lung cancer in the primary care setting were included. All diagnostic studies in which symptomatic primary care patients underwent 1 or more investigations were also searched.
Synthesis
Recommendations were consistent among guidelines despite a paucity of supporting evidence. Updated evidence provided further support for the recommendations. Recommendations for identifying signs and symptoms of lung cancer presenting in primary care and for initial management can be adopted and applied within a Canadian primary care setting.
Conclusion
This updated review of recommendations might help promote evidence-based practice and, ultimately, more timely management and improved prognosis for lung cancer patients. It might also assist in the development of lung cancer diagnostic assessment programs.
Résumé
Objectif
Faire une revue systématique de la littérature, et proposer une mise à jour et une intégration des lignes directrices ayant fait l’objet d’une revue par des pairs et qui reposent sur des revues systématiques récentes et sur des études primaires relatives au diagnostic précoce et au traitement du cancer pulmonaire en contexte de soins primaires.
Sources des données
On a consulté MEDLINE et EMBASE à la recherche d’articles pertinents. La qualité des preuves à l’appui des directives existantes et de leur cohérence par rapport aux données plus récentes ont été évaluées. On a également vérifié si ces directives étaient applicables dans le contexte des soins primaires au Canada.
Choix des études
Toutes les études traitant des signes, des symptômes et des facteurs de risque de cancer du poumon dans les contextes de soins primaires ont été retenues. On a également recherché toutes les études portant sur le diagnostic dans lesquelles des patients des soins primaires présentant des symptômes ont été l’objet d’au moins une investigation.
Synthèse
Les recommandations étaient pratiquement les mêmes dans les différentes lignes directrices, malgré le peu de preuves à l’appui. La mise à jour des preuves a confirmé ces recommandations. Les recommandations portant sur l’identification des signes et des symptômes et sur le traitement initial d’un cancer pulmonaire rencontré en première ligne peuvent être adoptées et appliquées au niveau des soins primaires au Canada.
Conclusion
Cette mise à jour des recommandations pourrait servir à promouvoir la pratique d’une médecine fondée sur des données probantes et, ultimement, permettre une prise en charge précoce et un meilleur pronostic pour le cancer pulmonaire. Elle pourrait aussi favoriser le développement de programmes d’évaluation portant sur le diagnostic du cancer pulmonaire.
Lung cancer is the most common cause of cancer death in Canada.1 Tobacco use is the primary cause of lung cancer, accounting for an estimated 86% of cases.2 The chance of surviving lung cancer in Canada is low, with a 5-year survival rate for all types and all stages combined of 13% for men and 19% for women.1 Lung cancers are most frequently diagnosed at a late stage, when prognosis is very poor. Delays in the diagnosis of lung cancer have, in part, been found to be associated with physicians failing to recognize early signs and symptoms.3,4 This could be owing to both physicians and patients attributing the often common, atypical, or nonspecific signs and symptoms of lung cancer to other benign diseases.3–5
A working group was formed to develop an updated comprehensive consensus document for primary care providers that would assist them in the early identification and management of patients with lung cancers. The systematic review presented here formed the basis of a companion guideline, and investigated what signs, symptoms, and other clinical features of patients who present in primary care are predictive of lung cancer.6,7 This updated review of the literature is intended to promote evidence-based practice and, ultimately, more timely management and improved prognosis of lung cancer patients.
DATA SOURCES
This systematic review was initiated by the Cancer Care Ontario Provincial Primary Care and Cancer Network in collaboration with the Program in Evidence-based Care. A working group was assembled consisting of 9 members including 5 FPs (M.E.D., S.Y., M.A., P.B., C.L.), 1 medical oncologist (A.R.), 1 respirologist (R.S.), 1 radiation oncologist (Y.U.), 1 thoracic surgeon (R.Z.), and 1 methodologist (E.T.V.). The specific objectives of this initiative were to update and integrate existing peer-reviewed evidence-based guidelines with recent systematic reviews and primary studies related to signs, symptoms, and other clinical features predictive of lung cancer; risk factors for lung cancer; and the diagnostic accuracy of early investigations for lung cancer. The final guideline was developed using the methods of practice guideline development cycle.8 An environmental scan was initially conducted (March 5 to 8, 2010). The 2009 guidelines from the New Zealand Guidelines Group (NZGG) and the 2005 guidelines from the National Institute for Health and Care Excellence (NICE) were chosen a priori as baseline documents for the development of the current updated systematic review.9,10 These guidelines were considered to be of high quality, comprehensive, recent, and relevant to this topic.9,10 Updated literature searches of the NZGG 2009 or NICE 2005 systematic reviews were completed. Additional relevant guidelines, systematic reviews, and prospective and retrospective studies were selected and evaluated.
Literature search strategy
The search strategies from NZGG 2009 and NICE 2005 were kindly provided to us.9,10 An updated search since the NZGG 2009 publication using MEDLINE (Ovid, August 2007 to week 3 of February 2010) and EMBASE (Ovid, 2007 to week 7 of 2010) was performed using the NZGG 2009 literature search strategy for the diagnostic accuracy of signs, symptoms, and investigations.10 Because it was not completed in the NZGG 2009 systematic review, an updated search of lung cancer risk factors since the NICE 2005 publication of MEDLINE (Ovid, June 2004 to week 3 of February 2010) and EMBASE (Ovid, 2004 to week 8 of 2010) was conducted using the NICE 2005 search strategies.9,10 A second literature search update of all strategies for literature available to June 27, 2011, was performed. The detailed search strategies are available upon request.
Study selection
Guidelines and systematic reviews were included if they addressed at least 1 of our objectives. They were also included if they provided different recommendations than or were not cited in the NZGG 2009 or NICE 2005 guidelines.9,10
All prospective or retrospective cohort or case-control studies that explored signs and symptoms of or risk factors for lung cancer in the primary care setting were included. All diagnostic studies in which symptomatic primary care patients underwent 1 or more investigations including complete blood count, chest x-ray scan, spirometry, sputum cytology, and computed tomography (CT) chest scan were also searched. Studies conducted in secondary care settings were included when limited evidence was available from primary care. Screening studies of asymptomatic patients were excluded.
Publications in a language other than English were not eligible owing to a lack of funding for translation. Non-systematic reviews, abstracts, case reports, letters, editorials, and commentaries were excluded.
There was considerable heterogeneity among studies; therefore, data were not pooled.
Quality appraisal of evidence-based guidelines and systematic review
The AGREE II (Appraisal of Guidelines Research and Evaluation) tool was used by 3 independent methodologists to evaluate the quality of the evidence-based guidelines.11,12 Using the AGREE II tool, only clinical practice guidelines in which the objective of the guideline was specifically described and which included a review of the evidence were evaluated.11,12 The AMSTAR (Assessment of Multiple Systematic Reviews) tool was used to assess the quality of the 1 systematic review.13
SYNTHESIS
Literature search results
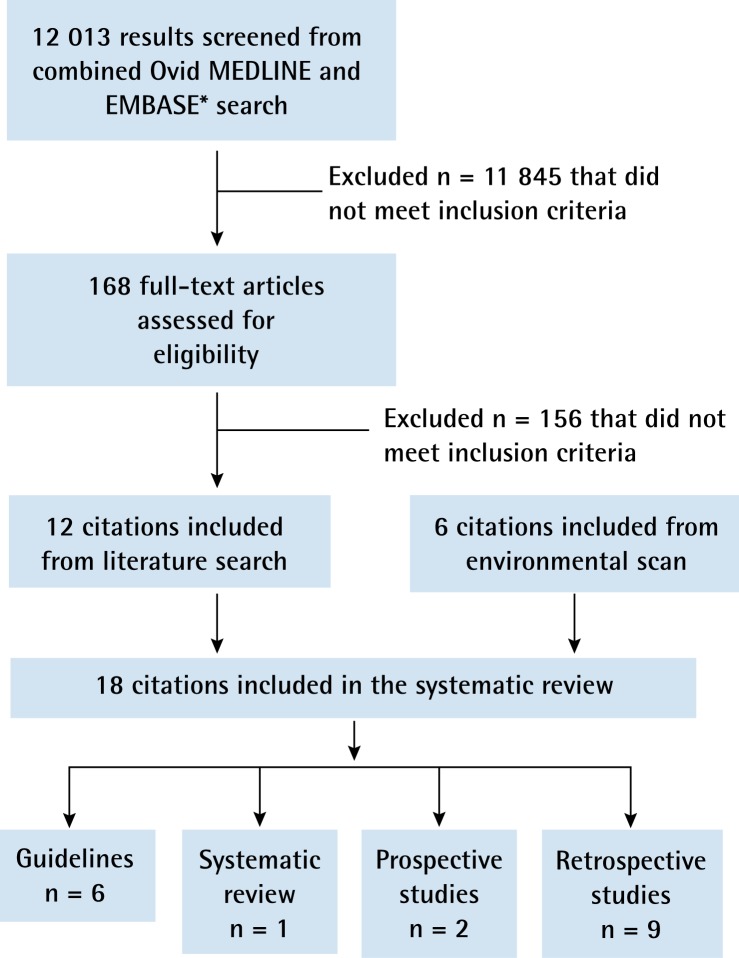
During the initial environmental scan, 6 guidelines in addition to the NICE 2005 and NZGG 2009 guidelines were identified.4,9,10,14–18 Of 12 013 articles identified in the updated literature search, 168 were deemed relevant for a full article review. Of these, 12 articles not included in the NICE 2005 or NZGG 2009 systematic review met the inclusion criteria and were retained (Figure 1).19–30
Figure 1.
Results of the literature search
*The online search strategy is available from the authors upon request.
The quality of the guidelines from NICE 2005, NZGG 2009, the Australian National Health and Medical Research Council, the Scottish Intercollegiate Guidelines Network (SIGN), and the American College of Chest Physicians (ACCP) was assessed using the AGREE II instrument (Table 1).4,9,10,14–18
Table 1.
Results of AGREE II tool quality rating of evidence-based guidelines
| GUIDELINE |
AGREE II DOMAIN SCORES
|
|||||
|---|---|---|---|---|---|---|
| SCOPE AND PURPOSE, % | STAKEHOLDER INVOLVEMENT, % | RIGOUR OF DEVELOPMENT, % | CLARITY AND PRESENTATION, % | APPLICABILITY, % | EDITORIAL INDEPENDENCE, % | |
| NICE,9 2005 | 97.2 | 66.7 | 77.1 | 61.1 | 79.2 | 25.0 |
| NZGG,10 2009 | 74.1 | 74.1 | 66.0 | 75.9 | 51.4 | 75.0 |
| Australian,17 2004 | 80.6 | 94.4 | 74.0 | 86.1 | 27.1 | 58.3 |
| SIGN,18 2005 | 61.1 | 81.5 | 81.9 | 96.3 | 47.2 | 30.6 |
| ACCP (Kvale,15 2006) | 50.0 | 18.5 | 45.1 | 85.2 | 13.9 | 11.1 |
| ACCP (Rivera and Mehta,16 2007) | 72.2 | 57.4 | 61.1 | 92.6 | 36.1 | 50.0 |
| ACCP (Gould et al,14 2007) | 46.3 | 55.6 | 62.5 | 90.7 | 22.2 | 25.0 |
| ACCP (Spiro et al,4 2007) | 66.7 | 64.8 | 70.1 | 79.6 | 30.6 | 66.7 |
ACCP—American College of Chest Physicians, AGREE—Appraisal of Guidelines for Research and Evaluation, NICE—National Institute for Health and Care Excellence, NZGG—New Zealand Guidelines Group, SIGN—Scottish Intercollegiate Guidelines Network.
Summaries of the recommendations, the supporting evidence, and the rating of the evidence to support the recommendations are provided in Table 2.4,9,10,14–18,31,32 Details of the quality of the guidelines that were included in the systematic review are provided below.
Table 2.
Summary of guideline recommendations, literature review, and supporting evidence
| GUIDELINE OR REVIEW | SUMMARY OF RECOMMENDATIONS, LITERATURE REVIEW, OR SUPPORTING EVIDENCE (RATING OR LEVEL OF EVIDENCE*†‡§) |
|---|---|
| Factors that increase the risk of lung cancer | |
| • NICE,9 2005 | Recommendations (C): current or former smokers; smoking-related COPD; previous exposure to asbestos; previous history of cancer (especially head and neck) |
| Summary of literature review (additional risk factors reported): occupational exposure to dust or microscopic particles (eg, wood dust, silica); past medical history of COPD; silicosis or tuberculosis; family history of cancer; exposure to known carcinogens (eg, radon, chromium, nickel) | |
| • NZGG,10 2009 | Cited NICE 2005 recommendations and literature review |
| • SIGN,18 2005 | Recommendations: current or former smokers, especially those older than 40 y; COPD |
| Supporting evidence: cited a study that showed 22% of patients diagnosed with lung cancer had coexistent COPD | |
| • ACCP (Kvale,15 2006) | Summary of literature review: tobacco smoking; passive cigarette smoke exposure; asbestos, radon, and exposure to selected other carcinogens; COPD; family history of lung cancer |
|
| |
| Symptoms and signs that raise suspicion of lung cancer and require further investigation | |
| • NICE,9 2005 | Recommendations:
|
| • NZGG,10 2009 | Recommendations (C):
Summary of literature review (additional factors reported): dysphagia; fever; pneumonia; superior vena cava obstruction; weakness; wheezing |
| • SIGN,18 2005 | Recommendations:
|
| • ACCP (Kvale,15 2006) and ACCP (Spiro et al,4 2007) | Summary of literature review:
|
|
| |
| Recommended initial investigation of suspicious lung cancer symptoms | |
| • NICE,9 2005 | Recommendation: A chest x-ray scan is the principal diagnostic investigation for lung cancer in primary care (D) |
| • NZGG,10 2009 | Recommendation: Urgent chest x-ray scan is recommended in patients presenting with symptoms and signs raising suspicion of lung cancer (C) |
| • SIGN,18 2005 | Recommendation: A chest x-ray scan should be performed on all patients being investigated for the possibility of lung cancer (D) Supporting evidence: Partly based on study of 345 patients that showed normal chest x-ray scan findings were seen in only 2% of lung cancer patients; patients with lung cancer often have obstructive features (37%) and pleural effusions (22%) |
| • ACCP (Spiro et al,4 2007) | Provided laboratory tests that would be useful in evaluating patients for metastatic or paraneoplastic syndromes associated with lung cancer4 |
|
| |
| Recommendations for the use of chest CT scans in the investigation of suspected lung cancer | |
| • SIGN,18 2005 | Recommendations:
Supporting evidence: Review of 4 CT scan studies showing CT scans have good sensitivity (89%–100%) but low specificity (56%–63%) in differentiating malignant from benign solitary pulmonary nodules, which might be improved with serial scans |
| • ACCP (Kvale,15 2006) | Summary of review: Patients with chest x-ray scan results negative for lung cancer might show positive results with bronchoscopy or CT imaging |
|
| |
| Recommendations for further management of symptomatic patients or patients with abnormal results | |
| • NICE,9 2005 | Recommendations:
|
| • NZGG,10 2009 | Recommendations:
|
| • Australian,17 2004 | Recommendation: All individuals with suspected lung cancer should be referred to a specialist with expertise in the management of lung disease for an opinion (IV) |
| • SIGN,18 2005 | Recommendations: Patients should be referred urgently to a chest physician for any of the following (D): persistent hemoptysis in smokers or former smokers older than 40 y of age; chest x-ray scan findings suggestive of or suspicious for lung cancer (including pleural effusion and slowly resolving or recurrent consolidation); signs of superior vena caval obstruction (swelling of the face and or neck with fixed elevation of jugular venous pressure); or stridor (emergency referral) |
| • ACCP (Gould et al,14 2007) | Recommendation: In a patient with a single pulmonary nodule, the clinician should estimate the pretest probability of malignancy either qualitatively by using clinical judgment or quantitatively using a validated model (1C) |
|
| |
| Recommendations for further management of ongoing suspicion of lung cancer despite normal initial investigation findings | |
| • NICE,9 2005 | Recommendation: If chest x-ray scan findings are normal but there is a high suspicion of lung cancer, patients should be offered an urgent referral (D) |
| • NZGG,10 2009 | Recommendation: A person should be referred urgently to a specialist if they have normal chest x-ray scan findings but there is a high suspicion of lung cancer (C) |
| Supporting evidence: Based on a publication that found up to a quarter of lung cancer patients had negative chest x-ray scan results in primary care in the year before diagnosis in people with common symptoms of lung cancer, with the exception of hoarseness,31 suggesting not to over-rely on negative chest x-ray scan findings if there is a suspicion of lung cancer | |
| • SIGN,18 2005 | Recommendation: Even with normal chest x-ray scan findings, patients who have experienced unexplained, nonspecific symptoms (eg, fatigue) potentially attributable to lung cancer, for more than 6 wk should be referred urgently to a respiratory physician (D) |
| • ACCP (Gould et al,14 2007) | Recommendation: In a patient with a single pulmonary nodule that is stable on imaging tests for at least 2 y, no additional diagnostic evaluation should be performed, except for patients with pure ground-glass opacities on CT, for whom a longer duration of annual follow-up should be considered (2C) |
| Supporting evidence: No evidence was found to suggest extending follow-up beyond 2 y that would detect more malignant nodules or improve patient outcomes | |
|
| |
| Recommendations for the use of sputum cytology in the investigation of suspected lung cancer | |
| • NICE,9 2005 | Recommendation: Sputum cytology is not a discriminatory investigation in symptomatic patients (C) |
| • NZGG,10 2009 | Recommendation: Sputum cytology is not recommended for the investigation of lung cancer (√) |
| • Australian,17 2004 | Recommendation: Sputum cytology is recommended to help establish a positive diagnosis of lung cancer in individuals with a central pulmonary mass (III) |
| Supporting evidence: Based on 5 studies. The sensitivity of sputum cytology increases with the number of specimens obtained—from about 50% with a single specimen up to almost 90% with 3 or more specimens. The use of induced ultrasonic nebulized sputum and optimal processing also increases the sensitivity of sputum cytology for the detection of lung cancer. Sensitivity is highest with centrally placed squamous cell carcinomas and lowest with both peripheral tumours and centrally placed small cell carcinomas. In an editorial,32 a specificity of 97.9% was reported | |
| • SIGN,18 2005 | Recommendation: Sputum cytology should only be used in patients with large central lesions, for whom bronchoscopy or other diagnostic tests are deemed unsafe (D) |
| Supporting evidence: Based on 3 studies that showed a wide variation in sensitivity (10% to 97%) in diagnosis of lung cancer that was dependent upon the techniques of sample collection | |
| • ACCP (Rivera and Mehta,16 2007) | Recommendation: In a patient suspected of having lung cancer who presents with a central lesion with or without radiographic evidence of metastatic disease, in whom a semi-invasive procedure such as bronchoscopy or transthoracic needle aspiration might pose a higher risk, sputum cytology is recommended as an acceptable method of establishing the diagnosis. However, the sensitivity of sputum cytology varies by the location of the lung cancer. It is recommended that further testing be performed with a nondiagnostic sputum cytology test if the suspicion of lung cancer remains (1C) |
| Supporting evidence: Based on 17 studies with a pooled sensitivity of 66% and pooled specificity of 99% for sputum cytology. Sensitivity was highly variable across studies with no explanation | |
|
| |
| Recommended wait timelines | |
| • NICE,9 2005 | Recommendation: A report of chest x-ray scan findings should be made back to the referring primary health care professional within 5 d |
| • NZGG,10 2009 | Recommendation: After urgent referral for chest x-ray scan, the scan should be completed and reported within 1 wk (√) |
| • SIGN,18 2005 | Recommendations:
|
ACCP—American College of Chest Physicians, COPD—chronic obstructive pulmonary disease, CT—computed tomography, NICE—National Institute for Health and Care Excellence, NZGG—New Zealand Guidelines Group, SIGN—Scottish Intercollegiate Guidelines Network.
For NICE, a rating of C means the recommendation is based directly on level III evidence or extrapolated from level I or level II evidence; a rating of D means the recommendation is based directly on level IV evidence or extrapolated from level I, level II, or level III evidence. Levels of evidence are defined by NICE as follows: Ia—systematic review or meta-analysis of randomized controlled trials; Ib—at least 1 randomized controlled trial; IIa—at least 1 well designed controlled study without randomization; IIb—at least 1 well designed quasi-experimental study, such as a cohort study; III—well designed non-experimental descriptive studies, case-control studies, and case series; IV—expert committee reports and opinions or clinical experience of respected authorities.
For NZGG, a rating of C means the recommendation is supported by international expert opinion. In the NZGG guideline, where no evidence is available, best practice recommendations are made based on the experience of the Guideline Development Team or on feedback from consultation within New Zealand; these recommendations are identified by √. Levels of evidence for the Australian 2004 guideline are defined as follows: III-1—evidence obtained from well designed pseudo-randomized controlled trials (alternate allocation or some other method); III-2—evidence obtained from comparative studies with concurrent controls and allocation not randomized (cohort studies), case-control studies, or interrupted time series with a control group; III-3—evidence obtained from comparative studies with historical control, 2 or more single-arm studies, or interrupted time series without a parallel control group; IV—evidence obtained from case series, either posttest or pretest and posttest.
For SIGN, a rating of D means the recommendation is based on level 3 or 4 evidence or is extrapolated evidence from studies rated as 2+. Recommended best practice based on the clinical experience of the guideline development group is identified by √. Levels of evidence are defined by SIGN as follows: 2+ ratings are well conducted case-control or cohort studies with a low risk of confounding or bias and a moderate probability that the relationship is causal; 2- ratings are case-control or cohort studies with a high risk of confounding or bias and a substantial risk that the relationship is not causal; 3 ratings are nonanalytic studies (eg, case reports, case series); 4 ratings are expert opinion.
For the ACCP guidelines, evidence graded as low to very low quality is based on observational studies or case series; 1C evidence is low to very low quality.
The systematic review for signs and symptoms of lung cancer conducted by NICE in 2005 included 3 guidelines, only 1 of which provided a table of common signs and symptoms based on evidence from case series.9 These were not described in detail. Nine additional studies were included in the NICE 2005 systematic review. Data from only 2 of the 9 studies were collected from primary care records. The systematic review conducted by NICE in 2005 also included 1 systematic review with meta-analyses comparing the diagnostic accuracy of cytology, bronchoscopy, transthoracic needle aspirate, or biopsy.9 As well, 3 primary studies were included: 2 regarding chest radiography and 1 regarding bloodwork. The link between the evidence and the recommendations was not always clear.9 They noted that the literature lacked evidence to adequately address the research questions, especially within the context of primary care.
The updated literature search since NICE 2005 by NZGG 2009 for signs and symptoms associated with lung cancer included a case-control study by Hamilton et al from 2005 and a case-series study by Jones et al from 2007.33,34 In their updated systematic review for diagnostic investigations, NZGG identified an additional systematic review and 2 primary studies that provided further information on complete blood count, chest x-ray scans, spirometry, and sputum cytology.31,33,35
The ACCP published a series of evidence-based clinical practice guidelines for the management of patients with lung cancer. Four of these guidelines were included because they addressed at least 1 of the research questions.4,14–16 The ACCP guidelines did not provide lists or details of included studies and did not assess their quality. However, each of the recommendations was followed by a grading of the supporting evidence.4,14–16 A 2006 study by Kvale was included in the first edition of the ACCP clinical practice guidelines.15 Kvale focused on the management of cough associated with lung tumours; although MEDLINE was searched, only 2 terms were listed: cough and lung neoplasms.15 Studies by Spiro et al,4 Gould et al,14 and Rivera and Mehta16 were included in the second addition of the ACCP clinical practice guidelines. Spiro et al conducted a systematic review of the initial symptoms and signs of lung cancer, as well as the symptoms, signs, and laboratory tests that could be used in a standardized evaluation for systemic metastases and paraneoplastic syndromes associated with lung cancer.4 The search terms in MEDLINE and the inclusion and exclusion criteria were not outlined in the article.4 Gould et al performed a systematic review on the diagnosis and management of patients with pulmonary nodules, which did not distinguish between screen-detected nodules and nodules that were detected incidentally.14 Rivera and Mehta looked at 17 studies on sputum cytology.16 Rivera and Mehta and Gould et al searched more than 1 database and included their research questions, as well as their inclusion and exclusion criteria.14,16
Two additional guidelines were developed by SIGN in 2005 and the Australian National Health and Medical Research Council in 2004.17,18 The SIGN 2005 guidelines included evidence summaries from their systematic review with each of their recommendations, and also included a grading of the strength of the evidence for each recommendation.18 They provided their search strategies for MEDLINE, but their inclusion and exclusion criteria were not clearly defined. This guideline addressed the management of patients with lung cancer, including 4 studies on CT scans. As a result, studies completed in a primary care setting were not a priority. The Australian guidelines covered a broad spectrum of care for patients with lung cancer, from prevention and diagnosis to management.17 Studies were not selected on the basis of whether they were relevant to the primary care setting. The authors did not include their search strategy or their inclusion or exclusion criteria. They provided the strength of the evidence to support their recommendations, as well as the citations for each recommendation.
A systematic review by Shapley et al from 2010 was included in the updated literature search since the NZGG 2009 search.30 This systematic review scored well, with 8 of the 11 items meeting the AMSTAR criteria. The authors did not provide all excluded studies and did not assess the likelihood of publication bias. Although Shapley et al provided a conflict of interest statement, such statements were not acknowledged for the included studies. Shapley et al included studies that had positive predictive values of 5% or more for any sign or symptom, as well as studies with positive predictive values less than of 5% for the same sign or symptom.30 The 2 articles cited for lung cancer were already referenced in the NZGG 2009 guideline.33,34
Of the 11 primary studies published since the NZGG 2009, 9 had retrospective designs.19–29 Details of these studies are provided in Table 3. None of the studies was performed in a primary care setting. Nine of the studies looked at signs, symptoms, and risk factors.19–21,24–29 Only 2 primary studies provided information on the diagnostic accuracy of investigations for lung cancer.22,23 Both of these studies included patients with and without lung cancer and were blinded to the diagnostic results.
Table 3.
Primary studies and results from updated literature review
| STUDY | STUDY TYPE AND SETTING | STUDY DETAILS | STUDY RESULTS |
|---|---|---|---|
| Signs, symptoms, risk factors, and other clinical features associated with the presentation of lung cancer | |||
| • Ak et al,19 2007, Turkey | Retrospective; secondary care | N = 1340 patients with lung cancer; compared symptom and sign presentation between younger (< 50 y, n = 179) and older (≥ 50 y, n = 1161) patients | Chest pain was more common in younger patients, while cough and dyspnea were more common in older patients. Occupational exposure was a risk factor in the younger group, while smoking was a risk factor in the older group |
| • Beatty et al,20 2009, New Zealand | Retrospective; secondary care | N = 159 patients with lung cancer; n = 66 referred by GPs | Of 66 patients referred by GPs: 47% presented with respiratory symptoms, 38% presented with hemoptysis, and 31% presented with no hemoptysis. Symptom duration varied from < 1 wk (35%, n = 16) to > 2 mo (33%, n = 8) |
| • Chandra et al,21 2009, India | Retrospective; tertiary care | N = 165 patients with lung cancer | Main clinical features at the time of diagnosis of lung cancer included coughing (75.2%), shortness of breath (66.9%), weight loss (63.7%), chest pain (63.1%), hemoptysis (33.1%), hoarseness of voice (29.3%), excessive weakness or fatigue (26.8%), clubbing (22.9%), dysphagia (9.3%), and superior vena cava syndrome (8.0%) |
| • Koumarianou et al,24 2009, Greece | Retrospective; cancer registry, mainly from phase II and III trials | N = 1906 with non–small cell lung cancer; compared symptom characteristics of 417 patients ≥ 70 y (elderly); 1374 patients 45–70 y; and 115 patients ≤ 45 y (young) | Most commonly reported symptoms: hemoptysis, cough, and weight loss. Elderly patients presented with more symptoms such as pain, dyspnea, cough, and fatigue compared with young patients |
| • Lovgren et al,25 2008, Sweden | Retrospective; secondary care | N = 314 with lung cancer | Five of the most commonly reported first symptoms were cough, dyspnea, weight loss, fatigue, and thoracic pain. Four of the most common symptoms triggering health care system appointments included cough, dyspnea, and thoracic pain for men and women, and neurologic symptoms for women and hemoptysis for men |
| • Thammakumpee et al,26 2007, Thailand | Retrospective; secondary care | N = 116 with small cell lung cancer | Symptoms and signs, in order of frequency, included cough, weight loss, dyspnea, chest pain, hemoptysis, hoarseness, superior vena cava syndrome, neurologic syndrome, syndrome of inappropriate antidiuretic hormone, Cushing syndrome, and massive hemoptysis |
| • Thomas et al,27 2008, India | Retrospective; tertiary care | N = 25 with pulmonary carcinoid tumours | Presenting symptoms or signs included hemoptysis, cough, breathlessness, chest pain, fever, and superior vena cava syndrome |
| • Uzun et al,28 2010, Turkey | Prospective; tertiary care | N = 178 with hemoptysis; n = 51 (29%) with lung cancer | Of 51 patients with lung cancer: 32% had mild hemoptysis, 38% had moderate hemoptysis, 24% had severe hemoptysis, and 13% had massive hemoptysis |
| • Yaman et al,29 2009, Turkey | Retrospective; secondary care | N = 109 with lung cancer | First symptoms related to lung cancer grouped into 5 categories were cough (32%), dyspnea (21%), hemoptysis (11%), chest pain (20%), and other first symptoms (16%) |
|
| |||
| Diagnostic accuracy of tests to investigate clinical suspicion of lung cancer | |||
| • Choi et al,22 2008, Korea | Retrospective; secondary care | N = 955; 352 histologically confirmed; n = 127 (36%) with lung cancer | Compared the diagnostic accuracy of sputum samples from a hospital using CP vs TP methods.22 The diagnosis of lung cancer was confirmed histologically. The sensitivity of TP and CP were 50.4% and 30.6%, respectively. The specificity was 99.1% with TP and 100.0% with CP |
| • Kemp et al,23 2007, Canada | Prospective; secondary care* | N = 1123 with medical history or clinical symptoms suspicious for lung cancer; n = 370 (33%) with lung cancer | Smears were assessed by conventional cytology (reference standard) or using an automated technique (LungSign test).23 LungSign showed a sensitivity of 40% and a specificity of 91% |
CP—conventional preparation, TP—ThinPrep.
Sponsored by Perceptronix Medical Inc.
DISCUSSION
Owing to a paucity of evidence in the primary care setting for the diagnostic accuracy of signs, symptoms, and risk factors associated with lung cancer, definitive conclusions could not be derived for the recognition of lung cancer in patients presenting in primary care. However, owing to the consistency among systematic reviews, the working group agreed with the signs and symptoms of lung cancer listed in the NICE 2005 and NZGG 2009 guidelines, which included superior vena cava obstruction, stridor, hemoptysis, finger clubbing, enlarged lymph nodes, persistent or unexplained cough, unexplained weight loss, dyspnea, chest or shoulder pain, hoarseness, dysphagia, and abnormal chest x-ray findings.9,10 Patients might also present with signs and symptoms of metastases or paraneoplastic syndromes.4
Our literature review did not provide evidence for additional risk factors associated with lung cancer beyond those listed in the NICE 2005 or NZGG 2009 guidelines.9,10 In addition, no evidence was found to challenge and remove any of the risk factors listed. The risk factors for lung cancer include current or previous smoking, passive exposure to tobacco smoke, chronic obstructive pulmonary disease, previous exposure to asbestos, and a history of cancer (especially head and neck cancer). Other risk factors might include occupational exposure to dust or microscopic particles (eg, wood dust, silica), silicosis or tuberculosis, family history of cancer, and exposure to known carcinogens (eg, radon, chromium, nickel).
Based on the interpretation of the evidence for diagnostic tests, a chest x-ray scan should be ordered as a preliminary investigation for signs or symptoms of lung cancer. There is little evidence to support CT chest scan as an initial investigation. Sputum cytology should not be used as a primary investigation for lung cancer. Further investigation or referral is warranted if there is a suspicion of lung cancer despite a negative chest x-ray scan finding.
Limitations
The current literature search was an update of the searches completed for the NICE and NZGG guidelines, and we trusted that the original searches were equally as extensive and that relevant articles were not missed. This review is limited to only those studies published in English. The consistency of results seen among the primary studies and systematic reviews provides some reassurance that irretrievable information is unlikely to show contradicting evidence.
We were limited by the number of rigorous prospective studies that assessed signs, symptoms, risk factors, and initial investigations for patients presenting with undiagnosed lung cancer, especially in primary care. Most of the evidence supporting existing guidelines and systematic reviews came from case-control studies, case-series studies, and consensus based on clinical experience. Furthermore, some of the systematic reviews of this evidence did not provide search strategies, study inclusion or exclusion criteria, study details, or evaluation of the quality of the studies included.
As electronic medical records in primary care practices become more widely used, opportunities for rigorous large-sample prospective studies exploring the presentation of clinical features and initial investigations of cancer presenting in primary care should become increasingly more feasible.
Conclusion
Early detection of lung cancer is critical for improving survival. While some presenting symptoms might be vague and imprecise, delays in diagnosis might be avoided if patients presenting with suspicious signs and symptoms, especially in the presence of risk factors, receive timely chest x-ray scans and, where warranted, further investigation or referral. This updated review of recommendations might help promote evidence-based practice and, ultimately, more timely management and improved prognosis of lung cancer patients. It might also assist in the development of lung cancer diagnostic assessment programs.
EDITOR’S KEY POINTS
Lung cancer is the leading cause of cancer death in Canada. It is frequently diagnosed at a late stage, when prognosis is very poor. Delays in diagnosis have been associated, in part, with delays in recognizing symptoms. For FPs and other primary care providers it can be difficult to distinguish early presentation of lung cancer from other benign conditions.
This systematic review found the following signs and symptoms to be predictive of lung cancer: superior vena cava obstruction, stridor, hemoptysis, finger clubbing, enlarged lymph nodes, persistent or unexplained cough, unexplained weight loss, dyspnea, chest or shoulder pain, hoarseness, dysphagia, and abnormal chest x-ray findings. Risk factors for lung cancer include current or previous smoking, passive exposure to tobacco smoke, chronic obstructive pulmonary disease, exposure to asbestos, and history of cancer (especially head and neck cancer).
A chest x-ray scan should be the preliminary investigation for signs or symptoms of lung cancer. There is little evidence to support computed tomography chest scan as an initial investigation, and sputum cytology should not be used as a primary investigation. Further investigation or referral is warranted if there is a suspicion of lung cancer despite a negative chest x-ray scan finding.
POINTS DE REPÈRE DU RÉDACTEUR
Au Canada, le cancer pulmonaire est la principale cause de mortalité due au cancer. Ce cancer est souvent diagnostiqué à un stade avancé, son pronostic étant alors très mauvais. Ce diagnostic trop tardif a souvent été attribué en partie au fait que les symptômes n’étaient pas reconnus assez tôt. Pour les MF comme pour les autres soignants de première ligne, il peut être difficile de distinguer les symptômes précoces d’un cancer pulmonaire de ceux d’une autre affection bénigne.
D’après cette revue systématique, les signes et les symptômes suivants sont prédictifs d’un cancer pulmonaire: obstruction de la veine cave supérieure, stridor, hémoptysie, hippocratisme digital, hypertrophie ganglionnaire, toux persistante ou inexpliquée, amaigrissement inexpliqué, dyspnée, douleur au thorax ou à l’épaule, voix rauque, dysphagie et radiographie pulmonaire anormale. Les facteurs de risque du cancer pulmonaire incluent le tabagisme antérieur ou actuel, l’exposition passive à la fumée du tabac, la maladie pulmonaire obstructive, l’exposition à l’amiante et des antécédents de cancer, plus particulièrement au niveau de la tête et du cou.
Une radiographie du thorax devrait être l’examen préliminaire en présence de signes ou de symptômes de cancer pulmonaire. Il y a peu de données indiquant qu’une tomodensitométrie thoracique devrait être utilisée initialement; la cytologie des expectorations ne devrait pas non plus être utilisée comme premier examen. Il y a indication d’examens additionnels ou d’une consultation si on soupçonne un cancer pulmonaire, même en présence d’une radiographie pulmonaire négative.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors contributed to the literature review and interpretation, and to preparing the manuscript for submission.
Competing interests
None declared
References
- 1.National Cancer Institute of Canada . Canadian cancer statistics 2001. Toronto, ON: National Cancer Institute of Canada; 2001. Available from: http://publications.gc.ca/site/eng/398229/publication.html. Accessed 2014 Jul 2. [Google Scholar]
- 2.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M, Comparative Risk Assessment collaborating group (Cancers) Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–93. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 3.Singh H, Sethi S, Raber M, Petersen LA. Errors in cancer diagnosis: current understanding and future directions. J Clin Oncol. 2007;25(31):5009–18. doi: 10.1200/JCO.2007.13.2142. [DOI] [PubMed] [Google Scholar]
- 4.Spiro SG, Gould MK, Colice GL, American College of Chest Physicians Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):149S–60S. doi: 10.1378/chest.07-1358. [DOI] [PubMed] [Google Scholar]
- 5.Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64(9):749–56. doi: 10.1136/thx.2008.109330. [DOI] [PubMed] [Google Scholar]
- 6.Del Giudice L, Young S, Vella E, Ash M, Bansal P, Robinson A, et al. Referral of suspected lung cancer by family physicians and other primary care providers. Toronto, ON: Cancer Care Ontario; 2011. Evidence-based series 24-2. Available from: www.cancer-care.on.ca/common/pages/UserFile.aspx?fileId=155781. Accessed 2014 Jul 2. [Google Scholar]
- 7.Del Giudice ME, Young SM, Vella ET, Ash M, Bansal P, Robinson A, et al. Guideline for referral of patients with suspected lung cancer by family physicians and other primary care providers. Can Fam Physician. 2014;60:711–6. (Eng), e376–82 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 8.Browman GP, Levine MN, Mohide EA, Hayward RS, Pritchard KI, Gafni A, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13(2):502–12. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- 9.Referral guidelines for suspected cancer. NICE guidelines CG27. London, UK: National Institute for Health and Care Excellence; 2005. National Institute for Health and Care Excellence [website] Available from: www.nice.org.uk/CG027. Accessed 2014 Jul 2. [Google Scholar]
- 10.New Zealand Guidelines Group . Suspected cancer in primary care: guidelines for investigation, referral and reducing ethnic disparities. Wellington, NZ: New Zealand Guidelines Group; 2009. Available from: www.midlandcancernetwork.org.nz/file/fileid/17510. Accessed 2014 Jul 2. [Google Scholar]
- 11.AGREE Collaboration Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Standards and guidelines evidence (SAGE) directory of evidence guidelines. Toronto, ON: Canadian Partnership Against Cancer; Cancerview [website] Available from: www.cancerview.ca/cv/portal/Home/TreatmentAndSupport/TSProfessionals/ClinicalGuidelines/GRCMain/GRCSAGE?_afrLoop=899435266845824&lang=en&_afrWindowMode=0&_adf.ctrl-state=fyx7b0hj8_85. Accessed 2014 Jul 2. [Google Scholar]
- 13.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould MK, Fletcher J, Iannettoni MD, Lynch WR, Midthun DE, Naidich DP, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):108S–30S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 15.Kvale PA. Chronic cough due to lung tumors: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):147S–53S. doi: 10.1378/chest.129.1_suppl.147S. [DOI] [PubMed] [Google Scholar]
- 16.Rivera MP, Mehta AC, American College of Chest Physicians Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):131S–48S. doi: 10.1378/chest.07-1357. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Council Australia . Clinical practice guidelines for the prevention, diagnosis and management of lung cancer. Sydney, Aust: National Health and Medical Research Council; 2004. Available from: www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp97.pdf. Accessed 2014 Jul 2. [Google Scholar]
- 18.Scottish Intercollegiate Guidelines Network . Management of patients with lung cancer: a national clinical guideline. Edinburgh, Scot: Scottish Intercollegiate Guidelines Network; 2005. Available from: www.sign.ac.uk/guidelines/fulltext/80/. Accessed 2014 Jul 2. [Google Scholar]
- 19.Ak G, Metintas M, Metintas S, Yildirim H, Erginel S, Alatas F. Lung cancer in individuals less than 50 years of age. Lung. 2007;185(5):279–86. doi: 10.1007/s00408-007-9021-2. Epub 2007 Aug 20. [DOI] [PubMed] [Google Scholar]
- 20.Beatty S, Stevens W, Stevens G, Kolbe J, Cox B. Lung cancer patients in New Zealand initially present to secondary care through the emergency department rather than by referral to a respiratory specialist. N Z Med J. 2009;122(1294):33–41. [PubMed] [Google Scholar]
- 21.Chandra S, Mohan A, Guleria R, Singh V, Yadav P. Delays during the diagnostic evaluation and treatment of lung cancer. Asian Pac J Cancer Prev. 2009;10(3):453–6. [PubMed] [Google Scholar]
- 22.Choi YD, Han CW, Kim JH, Oh IJ, Lee JS, Nam JH, et al. Effectiveness of sputum cytology using ThinPrep method for evaluation of lung cancer. Diagn Cytopathol. 2008;36(3):167–71. doi: 10.1002/dc.20761. [DOI] [PubMed] [Google Scholar]
- 23.Kemp RA, Reinders DM, Turic B. Detection of lung cancer by automated sputum cytometry. J Thorac Oncol. 2007;2(11):993–1000. doi: 10.1097/JTO.0b013e318158d488. [DOI] [PubMed] [Google Scholar]
- 24.Koumarianou A, Fountzilas G, Kosmidis P, Klouvas G, Samantas E, Kalofonos C, et al. Non small cell lung cancer in the elderly: clinico-pathologic, management and outcome characteristics in comparison to younger patients. J Chemother. 2009;21(5):573–83. doi: 10.1179/joc.2009.21.5.573. [DOI] [PubMed] [Google Scholar]
- 25.Lövgren M, Leveälahti H, Tishelman C, Runesdotter S, Hamberg K. Time spans from first symptom to treatment in patients with lung cancer—the influence of symptoms and demographic characteristics. Acta Oncol. 2008;47(3):397–405. doi: 10.1080/02841860701592392. [DOI] [PubMed] [Google Scholar]
- 26.Thammakumpee K, Juthong S, Viriyachaiyo V, Rittirak W, Tanomkiat W. Clinical manifestation and survival of patients with small-cell lung cancer. J Med Assoc Thai. 2007;90(7):1303–8. [PubMed] [Google Scholar]
- 27.Thomas R, Christopher DJ, Balamugesh T, Shah A. Clinico-pathologic study of pulmonary carcinoid tumours—a retrospective analysis and review of literature. Respir Med. 2008;102(11):1611–4. doi: 10.1016/j.rmed.2008.05.003. Epub 2008 Jul 9. [DOI] [PubMed] [Google Scholar]
- 28.Uzun O, Atasoy Y, Findik S, Atici AG, Erkan L. A prospective evaluation of hemoptysis cases in a tertiary referral hospital. Clin Respir J. 2010;4(3):131–8. doi: 10.1111/j.1752-699X.2009.00158.x. [DOI] [PubMed] [Google Scholar]
- 29.Yaman N, Ozgen A, Celik P, Ozyurt BC, Nese N, Coskun AS, et al. Factors affecting the interval from diagnosis to treatment in patients with lung cancer. Tumori. 2009;95(6):702–5. doi: 10.1177/030089160909500611. [DOI] [PubMed] [Google Scholar]
- 30.Shapley M, Mansell G, Jordan JL, Jordan KP. Positive predictive values of ≥5% in primary care for cancer: systematic review. Br J Gen Pract. 2010;60(578):e366–77. doi: 10.3399/bjgp10X515412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stapley S, Sharp D, Hamilton W. Negative chest x-rays in primary care patients with lung cancer. Br J Gen Pract. 2006;56(529):570–3. [PMC free article] [PubMed] [Google Scholar]
- 32.Tockman MS, Mulshine JL. Sputum screening by quantitative microscopy: a new dawn for detection of lung cancer? Mayo Clin Proc. 1997;72(8):788–90. doi: 10.1016/S0025-6196(11)63601-X. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton W, Peters TJ, Round A, Sharp D. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax. 2005;60(12):1059–65. doi: 10.1136/thx.2005.045880. Epub 2005 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones R, Latinovic R, Charlton J, Gulliford MC. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ. 2007;334(7602):1040. doi: 10.1136/bmj.39171.637106.AE. Epub 2007 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton W, Sharp D. Diagnosis of lung cancer in primary care: a structured review. Fam Pract. 2004;21(6):605–11. doi: 10.1093/fampra/cmh605. Epub 2004 Nov 1. [DOI] [PubMed] [Google Scholar]