Abstract
Objective
To systematically review the diagnostic accuracy of clinical features associated with colorectal cancer (CRC) presenting in primary care.
Data sources
MEDLINE and EMBASE were searched for studies in primary care that provided information on clinical features predictive of CRC. Positive predictive values were used to guide the determination of clinical features associated with increased risk of CRC.
Study selection
Systematic reviews or primary studies that provided possible clinical features predictive of CRC were included.
Synthesis
Clinical features of patients presenting in primary care that are associated with increased risk of CRC, listed in descending order of association, included palpable rectal or abdominal mass; rectal bleeding combined with weight loss; iron deficiency anemia; rectal bleeding mixed with stool; rectal bleeding in the absence of perianal symptoms; rectal bleeding combined with change in bowel habits; dark rectal bleeding; rectal bleeding and diarrhea; and change in bowel habits. Being male and increasing age were also, in general, associated with increased risk of CRC.
Conclusion
Recognition of clinical features associated with increased risk of CRC by FPs might help with earlier identification and referral among patients presenting in primary care. This review might help inform providers and CRC diagnostic assessment programs about indications for assessment and further investigation.
Résumé
Objectif
Faire une revue systématique de la valeur diagnostique des caractéristiques cliniques associées au cancer colorectal (CCR) observé en première ligne.
Sources des données
On a consulté MEDLINE et EMBASE à la recherche d’études effectuées en première ligne qui contenaient des informations sur les caractéristiques cliniques prédictives de CCR. La présence d’une valeur prédictive positive pour ces caractéristiques a permis d’établir celles qui sont associées à un risque augmenté de CCR.
Choix des études
On a retenu les revues systématiques ou les études primaires qui portaient sur des caractéristiques cliniques pouvant être prédictives de CCR.
Synthèse
Chez les patients du milieu des soins primaires, les caractéristiques cliniques associées à un risque accru de CCR sont, par ordre décroissant de probabilité, une masse rectale ou abdominale palpable; un saignement rectal avec amaigrissement; une anémie ferriprive; la présence de sang mélangé aux selles; un saignement rectal sans symptômes péri-anaux; un saignement rectal avec changement des habitudes intestinales; un saignement rectal foncé; un saignement rectal avec diarrhée; et un changement des habitudes intestinales. Le fait d’être un homme et d’avoir un certain âge était aussi associé à un risque accru de CCR.
Conclusion
Le MF de première ligne qui connaît les caractéristiques cliniques associées à un risque accru de CCR devrait être en mesure d’identifier plus tôt les patients à risque et de les diriger en spécialité. Cette revue pourrait servir à informer les soignants ainsi que les programmes d’évaluation du diagnostic du CCR sur les cas où une évaluation et une investigation additionnelle sont indiquées.
Colorectal cancer (CRC) is one of the most common types of cancer in Canada.1 There is strong evidence that screening using fecal occult blood testing (FOBT) reduces mortality from CRC and it should be strongly encouraged.2 Although many jurisdictions across Canada have introduced population-based screening programs, screening rates are still low.3 Patients who have either not been screened or who have interval signs or symptoms of CRC despite regular screening will rely on their FPs or other primary care providers to detect CRC. Cancer Care Ontario’s Provincial Primary Care and Cancer Network has collaborated with the Program in Evidence-based Care to develop this updated systematic review and address the following question: What signs, symptoms, and other clinical features of patients who present in primary care are predictive of CRC?
DATA SOURCES
As a foundation, the authors chose a priori to update the literature review used to support the New Zealand Guidelines Group (NZGG) 2009 guidelines and the National Institute for Health and Care Excellence (NICE) 2005 guidelines.4,5 These guidelines were considered to be of high quality based on assessment using the AGREE II (Appraisal of Guidelines Research and Evaluation) tool,6,7 as well as being comprehensive, recent, and relevant to this topic.
The search strategies from the NZGG 2009 and NICE 2005 guidelines were kindly provided.4,5 MEDLINE and EMBASE were searched for additional English-language papers published from January 2005 to August 2011. Reference lists of papers and review articles were scanned for further citations. Details about the search strategy are available upon request.
Evidence was selected by a methodologist (E.T.V.) and reviewed by the other authors. Systematic reviews and meta-analyses were assessed for quality using the AMSTAR (Assessment of Multiple Systematic Reviews) tool.8 The quality of primary studies was assessed using a modified QUADAS (Quality Assessment of Diagnostic Accuracy Studies) tool, which is based on the Cochrane Collaboration method for assessing the methodologic quality of diagnostic studies.9 Data were not pooled owing to considerable heterogeneity among studies.
Study selection
Systematic reviews or primary studies that provided possible clinical features predictive of CRC were included. An attempt was made to include only studies conducted in primary care. Studies conducted in secondary care settings were included if they provided predictive information about signs or symptoms for suspected CRC. Studies that did not have CRC as the main outcome, case reports, and CRC screening studies were excluded.
A post hoc decision was made to focus on positive predictive values (PPVs). A PPV is the probability that the disease is truly present when the test result is positive. Because PPVs are affected by the prevalence in the population, PPVs from studies conducted in secondary care settings were excluded. Studies that did not provide PPVs or for which the PPVs could not be calculated were excluded. Median PPVs were calculated only if PPVs for a specific clinical feature were reported in at least 4 studies.
Because many jurisdictions consider a positive guaiac FOBT screening result to be an indicator of increased risk of CRC, PPVs relative to a screen-positive FOBT result were used to stratify risk for individual or combinations of signs and symptoms of CRC. The median PPV for the combined guaiac FOBTs evaluated in a review was 5.7%.10 Furthermore, a recent report evaluating the FOBT used in the ColonCancerCheck program in Ontario showed a PPV of 5.4% for single (1-time) testing in asymptomatic patients.11 Clinical features with pooled PPVs from our systematic review and from published meta-analyses that were equal to or greater than the PPV for a positive asymptomatic screening FOBT result (5.4%) were considered high risk for CRC. A PPV greater than 5.4% is above the prevalence of CRC in Canada, which is 0.3% for all age groups and both sexes combined, and also above the prevalence in the demographic groups that have the highest prevalence of CRC (men aged 70 to 79 years and women aged 80 and older, each with a prevalence of 2%).1
SYNTHESIS
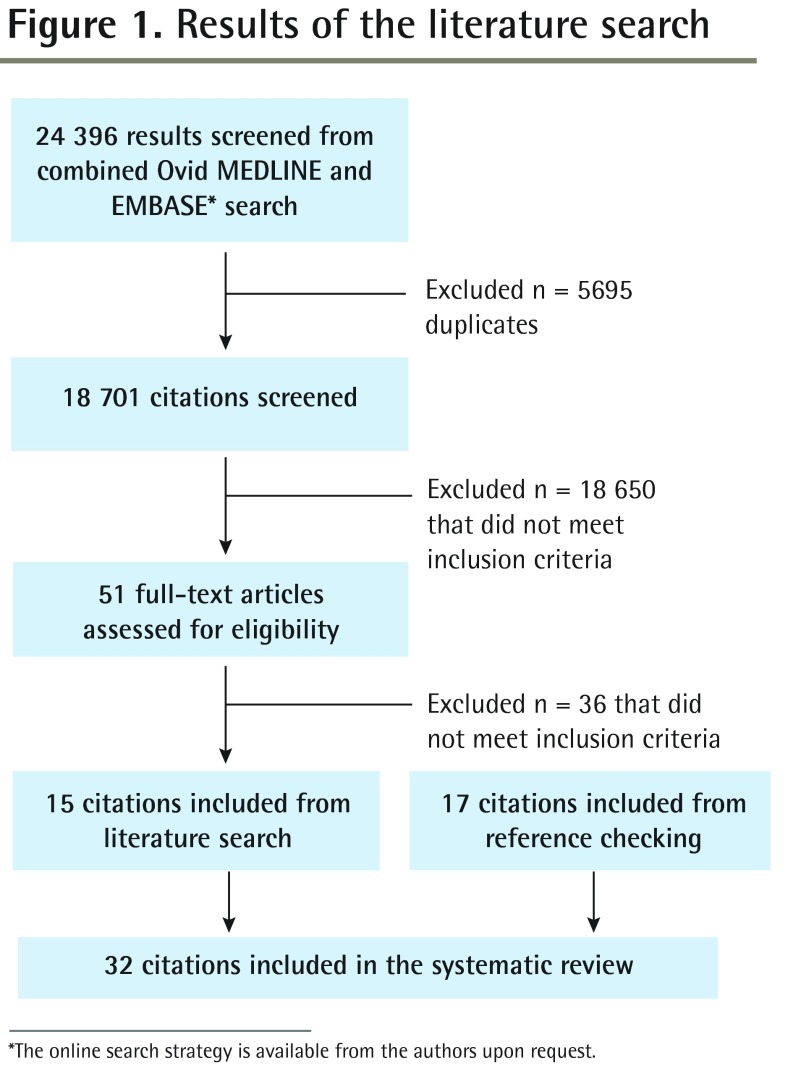
Literature search results
Of 12 013 articles on CRC published since the NICE and NZGG guidelines searches, 51 were deemed relevant (Figure 1).4,5 After full article reviews, 6 systematic reviews and 9 primary studies met the revised inclusion criteria and were retained.12–26 In addition, 17 primary study articles were found in reference lists.27–43
Figure 1.
Results of the literature search
*The online search strategy is available from the authors upon request.
Study design and quality
Reviews
Six systematic reviews, 5 with meta-analyses, investigated the diagnostic accuracy of symptoms or signs of CRC.12,15,19,20,23,25 Table 1 shows how the systematic reviews scored on each of the 11 AMSTAR items.12,15,19,20,23,25 The 5 reviews with meta-analyses had high overall scores. The meta-analyses by Ford et al15 and Jellema et al19 focused on primary care referral and therefore were included in this current review despite their inclusion of secondary care studies. Jellema et al included 2 secondary care studies that were classified as primary care studies by Ford et al.15,19 Jellema et al included secondary care studies only if the prevalence of CRC was less than 15%, which was the highest prevalence reported in primary care studies.19 The meta-analysis by Olde Bekkink et al included studies only from primary care; however, all the studies selected for patients with rectal bleeding.23 Owing to low AMSTAR scores, the meta-analysis by John et al from 2011 was used only to search the reference list for additional studies.20
Table 1.
Evaluation of systematic reviews and meta-analyses using AMSTAR
| ASTIN ET AL,12 2011 | FORD ET AL,15 2008 | JELLEMA ET AL,19 2010 | JOHN ET AL,20 2011 | OLDE BEKKINK ET AL,23 2010 | SHAPLEY ET AL,25 2010 | |
|---|---|---|---|---|---|---|
| No. of studies included | 23 | 15 | 47 | 85 | 8 | 25 |
| No. of patients included | 81464 | 19443 | NR | NR | 2323 | NR |
| AMSTAR items | ||||||
| • Was an a priori design provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| • Were there duplicate study selection and data extraction? | Yes | Yes | Yes | Yes | Yes | Yes |
| • Was a comprehensive literature search performed? | Yes | Yes | Yes | Yes | Yes | Yes |
| • Was the status of publication (eg, gray literature) used as an inclusion criterion? | Yes | Yes | Yes | Yes | Yes | Yes |
| • Was a list of studies (included and excluded) provided? | No | Yes | Yes | Yes | Yes | No |
| • Were the characteristics of the included studies provided? | Yes | Yes | Yes | No | Yes | Yes |
| • Was the scientific quality of the included studies assessed and documented? | Yes | Yes | Yes | No | Yes | Yes |
| • Was the scientific quality of the included studies used appropriately in formulating conclusions? | Yes | Yes | Yes | No | Yes | Yes |
| • Were the methods used to combine the findings of the studies appropriate? | Yes | Yes | Yes | NA | Yes | Yes |
| • Was the likelihood of publication bias assessed? | No | No | Yes | No | No | No |
| • Was the conflict of interest stated? | No | No | No | No | No | No |
| Total AMSTAR points* | 8 | 9 | 10 | 5 | 8 | 8 |
AMSTAR—Assessment of Multiple Systematic Reviews, NA—not applicable, NR—not reported.
Out of a possible total of 11.
Primary studies
Relevant primary studies from the NZGG and NICE reviews and those identified in the current literature search and reference lists included 18 prospective studies14,18,22,24,27,29–37,40–43; 5 retrospective studies13,21,26,28,39; and 3 case-control studies.16,17,38 The details and factors that might have affected the quality of each of these studies are highlighted in Table 2.13,14,16–18,21,22,24,26–43 Because studies conducted in the UK 2-week referral clinics occurred at the interface between primary and secondary care, they were retained for this current review.13,28,31
Table 2.
Characteristics of studies exploring signs or symptoms predictive of CRC: Prospective, retrospective, and case-control studies conducted in primary care.
| STUDY | STUDY TYPE, LENGTH OF STUDY, AND COUNTRY | NO. OF PATIENTS | SPECIFIC INCLUSION AND EXCLUSION CRITERIA | NO. OF PATIENTS WITH CRC (%) | INVESTIGATION USED TO CONFIRM CRC (LENGTH OF FU) | CONSECUTIVE PATIENTS | BLINDING TO INDEX | MISSING DATA EXPLAINED | WITHDRAWALS EXPLAINED |
|---|---|---|---|---|---|---|---|---|---|
| Barwick et al,13 2004 | Retrospective, 8 mo, UK | 144 | 2WW | 14 (9.7) | Various, including BE, SIG, US, CS | NR | No | NR | Yes |
| Bat et al,27 1992 | Prospective, NR, Israel | 101 | ≥ 80 y with rectal bleeding | 29 (29.7) | CS | NR | No | No | No |
| Chohan et al,28 2005 | Retrospective, 18 mo, UK | 462 | 2WW | 64 (13.9) | Histopathology | Yes | No | Yes | Yes |
| Du Toit et al,29 2006 | Prospective, > 10 y, UK | 265 | Age ≥ 45 y with rectal bleeding | 15 (5.7) | Mostly SIG with BE, SIG, or CS (> 10 y) | Yes | No | No | Yes |
| Ellis and Thompson,14 2005 | Prospective, 18 mo, UK | 319 | Rectal bleeding | 11 (3.4) | SIG and BE or CS (18 mo) | Yes | NR | Yes | Yes |
| Fijten et al,30 1995 | Prospective, 20 mo, Netherlands | 269 | Rectal bleeding | 9 (3.3) | CS, radiography, SIG, proctoscopy, US (> 12 mo, mean 20 mo) | Yes | Yes | NR | Yes |
| Flashman, et al,31 2004 | Prospective, 1 y, UK | 695 | 2WW | 65 (9.4) | NR | Yes | No | Yes | Yes |
| Hamilton et al,16 2008 | Case control, NR, UK | 3183 cases, 10 514 controls | Hb measured in year before diagnosis | 3183 (100.0) | Electronic records | No | NR | Yes | NR |
| Hamilton et al,17 2005 | Case control, NR, UK | 349 cases, 1744 controls | NA | 349 (100.0) | Cancer registry | No | Yes | Yes | Yes |
| Heintze et al,18 2005 | Prospective, 1 y, Germany | 422 | Rectal bleeding | 17 (4.0) | CS, SIG, rectoscopy | No | Yes | Yes | Yes |
| Helfand et al,32 1997 | Prospective, NR, United States | 201 | Rectal bleeding | 13 (6.5) | SIG plus BE (6–12 mo) | No | No | Yes | Yes |
| Jones et al,21 2007 | Retrospective, 7 y, UK | 7523 men, 7766 women | Rectal bleeding | 184 (2.4) men, 154 (2.0) women | NR from research database | No | No | Yes | Yes |
| Lawrenson et al,22 2006 | Prospective, NR, UK | 2 793 468 | Age 40–89 y | 9143 (0.3) | Medical database (1 y) | Yes | No | No | Yes |
| Mant et al,33 1989 | Prospective, > 11 mo, Australia | 145 | Age > 40 y with rectal bleeding | 16 (11.0) | Most CS, some SIG and BE, histopathology | Yes | No | NR | Yes |
| Metcalf et al,34 1996 | Prospective, NR, UK | 99 | Age > 40 y with rectal bleeding | 8 (8.1) | CS and histopathology | Yes | No | Yes | Yes |
| Muris et al,35 1993 | Prospective, > 15 mo, Netherlands | 578 | Abdominal pain | 3 (0.5) | X-ray scan, US (15 mo) | Yes | NR | No | Yes |
| Nørrelund and Nørrelund,36 1996 | Prospective; study 1: 3 y, study 2: 2 y; Denmark |
Study 1: 208, study 2: 209 | New rectal bleeding | Study 1: 32 (15.4), study 2: 22 (10.5) | BE and CS, histopathology, (study 1: 32–57 mo, study 2: 22–36 mo) | Yes | NR | No | Yes |
| Panzuto et al,37 2003 | Prospective, 2 mo, Italy | 280 | Exclude previous CRC, recent large bowel examination | 41 (14.6) | CS or BE | Yes | No | Yes | Yes |
| Park et al,38 2009 | Nested case control, NR, UK | 159 cases, 771 controls | NA | 159 (100.0) | National cancer registry (average 12 y) | No | NR | NR | Yes |
| Parker et al,39 2007 | Retrospective, 5 y, UK | 29 007 | Age ≥ 25 y with rectal bleeding | 645 (2.2) | PC medical database (2 y) | Yes | No | Yes | Yes |
| Robertson et al,24 2006 | Prospective, 4 y, UK | 604 | Rectal bleeding | 22 (3.6) | SIG and hospital records (> 4 y) | No | No | Yes | Yes |
| Sánchez et al,40 2005 | Prospective, > 3 mo, Spain | 126 | Rectal bleeding | 6 (4.8) | CS | Yes | No | Yes | Yes |
| Steine et al,41 1994 | Prospective, 9 mo, Norway | 1852 | NA | 55 (3.0) | BE | Yes | Yes | Yes | Yes |
| Stellon and Kenwright,42 1997 | Prospective, > 5 y, UK | 26 | > 50 y with IDA | 2 (7.7) | SIG with or without BE | Yes | No | Yes | Yes |
| Wauters et al,43 2000 | Prospective, 2 y, Belgium | 386 | Rectal bleeding | 27 (7.0) | Histopathology (18–30 mo) | Yes | NR | Yes | NR |
| Yates et al,26 2004 | Retrospective, 4 y, UK | 431 | New IDA | 37 (8.6) | Various (≥ 12 mo) | Yes | No | Yes | Yes |
2WW—2-wk wait program, BE—barium enema, CRC—colorectal cancer, CS—colonoscopy, FU—follow-up, Hb—hemoglobin, IDA—iron deficiency anemia, NA—not applicable, NR—not reported, PC—primary care, SIG—sigmoidoscopy, US—ultrasound, UK—United Kingdom.
What signs, symptoms, and other clinical features of patients who present in primary care are predictive of CRC?
Table 3 provides individual and median PPVs for CRC for each clinical feature from primary studies, as well as pooled PPVs, pooled positive likelihood ratios (PLRs), and diagnostic performance measures including sensitivities and specificities from meta-analyses.12–15,17–19,21,23–26,28–37,39–43 Table 4 lists the clinical features of patients presenting in primary care that are associated with increased risk of CRC, listed in descending order of association.
Table 3.
Clinical features of CRC: Summary of findings. Empty cells indicate that the study did not examine the sign or symptom.
| STUDY | IDA | RB | RB AND MALE | RB AND FEMALE | RB AND ≥50 OR ≥55 Y | RB AND ≥60 OR ≥65 Y | RB AND ≥70 OR ≥ 75 Y | DARK RB | RB MIXED WITH STOOL | RB AND NO PERIANAL SYMPTOMS | RB AND ABD PAIN | RB AND WT LOSS | RB AND CBH | CBH OR DIARRHEA* | WT LOSS | ABD PAIN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary studies | ||||||||||||||||
| PPV, % (95% CI) | ||||||||||||||||
| • Barwick et al,13 2004† | 11 | 16 (9–28) | 5 (2–12) | 6.9 (14–24) | 5 | 7 | ||||||||||
| • Chohan et al,28 2005† | 34 (21–50) | 18 | 14 (9.5–19) | |||||||||||||
| • Du Toit et al,29 2006 | 5.7 (3.4–9.2) | 6.1 (3.6–10) | 8.6 (5–15) | 7.9 (3.6–16) | ||||||||||||
| • Ellis and Thompson,14 2005 | 3.4 (3.6–16) | 5.2 (2.6–10) | 9.7 (3.2–26) | 3.0 (0.4–19) | 11 (5.4–22) | 9.2 (4.7–15.9) | ||||||||||
| • Fitjen et al,30 1995 | 3.3 (1.7–6.3) | 5.9 (2.9–12) | 1.0 (0.3–5.1) | 20 (10–35) | 14 (3.6–43) | 2.2 (0.5–6.4) | 9.5 (2.7–22.6) | |||||||||
| • Flashman et al,31 2004† | 10.9 (5–22) | 10.6 (6.7–16) | ||||||||||||||
| • Hamilton et al,17 2005† | 2.4 (1.9–3.2) | 3.1 (1.9–5.3) | 4.7 | 0.9* (0.7–1.1) | 1.2 (0.9–1.6) | 1.1 (0.9–1.3) | ||||||||||
| • Heintze et al,18 2005 | 4.0 (2.5–6.4) | 5.6 (3.4–9.1) | ||||||||||||||
| • Helfand et al,32 1997 | 6.5 (3.8–11) | |||||||||||||||
| • Jones et al,21 2007 | 2.2 (2.0–2.5) | 2.4 (2.1–2.8) | 2.0 (1.7–2.3) | |||||||||||||
| • Mant et al,33 1989 | 10 (6.3–16) | 9.1 (4.4–18) | 13 (7–24) | 17 (6.7–38) | 21 (11–36) | 9.3 (2.6–22.1) | 14.3 (1.8–42.8) | 10.7 (4.0–21.9) | ||||||||
| • Metcalf et al,34 1996 | 8.1 (4.1–15) | 9.7 (3.2–26) | 11 (4.6–24) | 7.1 (1.5–19.5) | 13.3 (1.7–40.5) | 10.3 (2.9–24.2) | ||||||||||
| • Muris et al,35 1993 | 0.5 (0.1–1.5) | |||||||||||||||
| • Nørrelund and Nørrelund,36 1996 | 14 (11–19) | 17 (12–24) | 13 (9–18) | 31 | 23 (16–33) | 22.7 (7.8–45.4) | 27 (19–36) | |||||||||
| • Panzuto et al,37 2003† | 40.6 (28.9–53.1) | 16 (10–24) | 12 (6.4–21) | 35.7 (9.3–18.6) | 13.5 (9.3–18.7) | |||||||||||
| • Parker et al,39 2007 | 2.2 (2.1–2.4) | 4.0 (3.7–4.3) | 4.6 (4.2–5.1) | 4.9 (4.3–5.6) | ||||||||||||
| • Robertson et al,24 2006 | 3.6 (2.4–5.5) | 4.8 (2.8–8) | 2.7 (1.4–5.1) | 5.7 (3.7–8.7) | 7.5 (3.8–14) | 7.4 (3.9–14) | 5.4 (3.4–8.5) | 1.7 (0.5–4.4) | 4.8 (1.0–13.5) | |||||||
| • Sánchez et al,40 2005 | 4.8 | 9.5 | ||||||||||||||
| • Steine et al,41 1994 | 5.9 (3.7–9.3) | 3.0 (2.1–4.5) | 4.8 (3–7.6) | 2.1 (1.4–3.0) | ||||||||||||
| • Stellon and Kenwright,42 1997 | 7.7 | |||||||||||||||
| • Wauters et al,43 2000 | 7.0 (4.6–10) | 11 (7–15) | 13 (9–19) | 15 (9–22) | 16 (4.5–36) | |||||||||||
| • Yates et al,26 2004† | 8.6 (6.1–11.6) | |||||||||||||||
|
| ||||||||||||||||
| Median PPV (95% CI) across primary studies | 11 | 5.3 | 7.5 | 3.9 | 5.9 | 8.6 | 7.9 | 9.7 | 11 | 10.8 | 5.1 | 13 | 10.5 | 7.5 | 4.9 | 2.1 |
|
| ||||||||||||||||
| Meta-analyses | ||||||||||||||||
| Pooled PPV, % (95% CI) | ||||||||||||||||
| • Astin et al,12 2011 | 9.7 (3.5–26.8) | 8.1 (6.0–10.8) | 7.6 (3.0–19.2) | 13.4 (8.2–21.9) | 11.8 (6.8–20.4) | 3.3 (0.7–15.7) | ||||||||||
| • Jellema et al,19 2010‡ | 13‡ (11–34) | 7 (5–12) | 10 (7–13) | 9 (8–10) | 13 (8–31) | 14 (9–21) | 6 (4–10) | 5‡ (0–23) | 9‡ (5–23) | 9‡ (5–50) | ||||||
| • Shapley et al,25 2010 | 4.6 (3.7–5.5) | 5.5 (3.9–7.2) | ||||||||||||||
| Pooled PLR (95% CI) | ||||||||||||||||
| • Astin et al,12 2011 | 5.3 (1.7–17.1) | 1.0 (0.6–1.7) | 1.9 (1.3–2.8) | 1.8 (1.3–2.5) | 2.4 (1.6–3.8) | 3.5 (2.1–5.8) | 2.5 (1.1–5.6) | |||||||||
| • Ford et al,15 2008 | 1.4 (0.8–2.7) | 1.3 (1.2–1.5) | 3.8 (2.6–5.6) | 1.3 (1.1–1.6) | 2.0 (1.3–3.1) | |||||||||||
| • Olde Bekkink et al,23 2010† | 3.7 (1.3–10.4) | 1.2 (1.0–1.5) | 2.8 (2.0–3.9) | 1.4 (0.6–3.3) | 1.9 (0.8–5.5) | 0.9 (0.2–1.6) | 1.9 (1.0–3.1) | 1.9 (0.5–3.6) | ||||||||
| Pooled sensitivity (95% CI) | ||||||||||||||||
| • Astin et al,12 2011 | 17 (16–18) | 33 (24–43) | 19 (12–28) | 58 (49–67.3) | 11 (11–12) | 31 (30–32) | ||||||||||
| • Ford et al,15 2008 | 17 (5.5–33) | 64 (55–73) | 15 (3–34) | 41 (23–60) | 22 (14–31) | |||||||||||
| • Jellema et al,19 2010§ | 13 (11–34) | 62 (44–78) | 91 (86–96) | 83 (73–93) | 50 (36–63) | 35 (25–41) | 51 (9–77) | 35 (0–40) | 20 (13–44) | 52 (10–100) | ||||||
| • Olde Bekkink et al,23 2010† | 17 (5–35) | 58 (48–67) | 66 (45–83) | 22 (13–34) | 40 (4–93) | 25 (4–62) | 17 (6–37) | 62 (18–94) | ||||||||
| Pooled specificity (95% CI) | ||||||||||||||||
| • Astin et al,12 2011 | 98 (98–99) | 63 (60–65) | 89 (87–90) | 63 (60–65) | 96 (96–96) | 91 (91–92) | ||||||||||
| • Ford et al,15 2008 | 90 (87–92) | 52 (42–63) | 96 (93–98) | 69 (58–78) | 89 (81–95) | |||||||||||
| • Jellema et al,19 2010 | 92 (92–94) | 55 (46–57) | 36 (39–46) | 55 (52–88) | 79 (72–83) | 85 (69–87) | 71 (49–95) | 59 (49–91) | 89 (85–94) | 61 (55–93) | ||||||
| • Olde Bekkink et al,23 2010† | 95 (93–96) | 52 (48–56) | 76 (68–83) | 84 (69–93) | 81 (23–98) | 73 (52–89) | 91 (83–96) | 68 (53–80) | ||||||||
ABD—abdominal, CBH—change in bowel habits, CRC—colorectal cancer, Hb—hemoglobin, IDA—iron deficiency anemia, PLR—positive likelihood ratio, PPV—positive predictive value, RB—rectal bleeding, WT—weight.
Values for diarrhea were used when data on CBH were not available.
Definitions of anemia: Barwick et al13 and Chohan et al28 used IDA and Hb < 100 g/L; Flashman et al31 used IDA and Hb < 110 g/L for men and Hb < 100 g/L for women; Hamilton et al17 used Hb < 99 g/L; Panzuto et al37 used IDA and Hb < 140 g/L for men and Hb < 120 g/L for women; Yates et al26 used IDA and Hb < 120 g/L for men and < 110 g/L for women; Olde Bekkink et al23 used Hb < 133 g/L for men and Hb < 120 g/L for women.
Where data are not homogeneous, median and range are provided.
Median and range are provided for all.
Table 4.
Clinical features that indicate increased risk of CRC
| CLINICAL FEATURE | MEDIAN PPV, % (RANGE) |
|---|---|
| Palpable rectal or abdominal mass | NA* |
| Rectal bleeding combined with weight loss | 13.0 (4.7–23) |
| Iron deficiency anemia | 11.0 (7.7–41) |
| Rectal bleeding mixed with stool | 11.0 (3.0–21) |
| Rectal bleeding in the absence of perianal symptoms | 10.8 (6.9–18) |
| Rectal bleeding combined with change in bowel habits | 10.5 (9.2–27) |
| Dark rectal bleeding | 9.7 (7.4–17) |
| Rectal bleeding and diarrhea | 9.0 (3.4–19) |
| Rectal bleeding and age ≥ 60 or ≥ 65 y | 8.6 (4.6–20) |
| Rectal bleeding and age ≥ 70 or ≥ 75 y | 7.9 (4.9–31) |
| Change in bowel habit or diarrhea | 7.5 (0.94–14) |
| Rectal bleeding and male | 7.5 (2.4–17) |
| Rectal bleeding and age ≥ 50 or ≥ 55 y | 5.9 (4–11) |
| Rectal bleeding (undefined) | 5.3 (2.2–16) |
| Rectal bleeding and abdominal pain | 5.1 (1.7–23) |
| Rectal bleeding, first episode | 5.0 (2.2–14) |
CRC—colorectal cancer, NA—not available, PPV—positive predictive value.
Median not available; individual studies reported PPVs > 15%.
Rectal or abdominal mass: Three studies conducted in the UK 2-week referral clinics found rectal and abdominal masses to be significant predictors of CRC.13,28,31 The PPV for combined rectal or abdominal masses was 16.7% in one study.13 Two studies found the PPVs were 80%28 and 22.6%31 for rectal masses and 41%28 and 16.3%31 for abdominal masses, respectively.
Anemia or iron deficiency anemia (IDA): For anemia defined as a hemoglobin level of less than 100 g/L, 2 different case-control studies by Hamilton et al found PPVs of 2.3% and 2.0% for all ages and both sexes combined.16,17 When Hamilton et al (2008) and Lawrenson et al (2006) stratified patients by sex and age group, PPVs generally increased with age and were higher in men compared with women within each age group.16,22 The highest PPVs in the Lawrenson et al study were found among men with anemia aged 70 to 79 (PPV = 3.38%) and among women with anemia aged 80 to 89 (PPV = 2.01%).22 In the Hamilton et al study, PPVs were higher than 5% in both men or women older than 60 years of age with hemoglobin levels less than 90 g/L and men aged 60 to 69 or older than 79 years with hemoglobin levels of 90 to 99 g/L.16 In most cases, these PPVs increased to greater than 10% if the patients were also iron deficient.
Six studies13,26,28,31,37,42 that examined IDA found PPVs ranging from 7.7% to 40.6%, with a median of 11% (Table 3).12–15,17–19,21,23–26,28–37,39–43 Regression analyses in both of the case-control studies by Hamilton et al found lower hemoglobin levels were significantly associated with increased CRC risk (P < .001).16,17 Furthermore, microcytosis (mean corpuscular volume < 80.0 fL) and low ferritin (< 20 ng/mL) were both strongly associated with CRC.16 Panzuto et al (2003) also found IDA (hemoglobin < 140 g/L for men and < 120 g/L for women, with ferritin < 30 µg/L and mean corpuscular volume of < 80 fL) to be a significant predictor of CRC (P < .001).37
Rectal bleeding: Twenty-one primary studies provided PPVs on rectal bleeding as a single presenting symptom.13,14,17,18,21,22,24,27–34,36,37,39–41,43 In more than half of the studies of rectal bleeding broadly classified,29,32–34,36,37,40,41,43 PPVs were greater than or equal to 5% (Table 3).12–15,17–19,21,23–26,28–37,39–43
Five of 6 studies13,21,24,30,33,36 found higher PPVs for men (median PPV = 7.5%, range 2.4% to 17%) compared with women (median PPV = 3.9%, range 1.0% to 13%), collapsed across all ages (Table 3).12–15,17–19,21,23–26,28–37,39–43 Patients 50, 60, and 70 years of age or older with rectal bleeding had median PPVs for CRC of 5.9%, 8.6%, and 7.9%, respectively.14,18,24,29,30,36,39,40,43 Jellema et al found a pooled PPV of 13% in patients older than 70 years of age.19 In patients presenting with rectal bleeding, both Lawrenson and colleagues (2006)22 and Jones and colleagues (2007)21 observed increasing risk of CRC with increasing age; however, men had higher PPVs compared with women within each age group. Similarly, in a multivariate analysis of 29 007 patients with rectal bleeding, Parker et al (2007) found that the risk of CRC was strongly associated with age and was higher in men than in women.39 Olde Bekkink et al found that being aged 60 years or older among patients with rectal bleeding almost tripled the posttest probability of CRC (pooled PLR = 2.8, 95% CI 2.0 to 3.9).23
Four studies13,14,28,31 examined rectal bleeding without anal symptoms such as hemorrhoids and perianal eczema (median PPV = 10.8%, range 6.9% to 18%) (Table 3).12–15,17–19,21,23–26,28–37,39–43 One study found that rectal bleeding with and without perianal symptoms had PPVs of 2% and 11%, respectively.14
In patients with rectal bleeding, the PPV for CRC of dark blood was higher than that for bright red blood within each of the 3 studies that compared shade of blood. The PPVs for dark rectal bleeding were 17.0%, 9.7%, and 9.7% compared with those for bright-red rectal bleeding of 9.9%, 4.0%, and 8.6%, in the 3 studies respectively.14,33,34 Jellema et al found a pooled PPV of 14% (95% CI 9% to 21%) in patients with dark rectal bleeding.19 In regression analyses, Ford et al found that the presence of dark rectal blood nearly quadrupled the posttest probability of CRC (PLR = 3.8, 95% CI 2.6 to 5.6).15
In 3 of 5 studies that explored location of blood in relation to stool, the PPVs for CRC when blood was mixed with stool (5.4%, 21%, and 14%, respectively) were higher compared with the PPVs for blood separate from stool (1.9%, 6.6%, and 7%, respectively).24,30,33 Jellema et al found a pooled PPV of 6% in patients with rectal bleeding mixed with stool.19 In contrast, Ellis and Thompson found a PPV of 3.0% for rectal blood mixed with stool compared with 4.3% for rectal bleeding not mixed with stool.14 In regression analyses, blood mixed with stool was a significant predictor of CRC.24,30
Robertson et al (2006) found higher PPVs when rectal blood was both mixed with stool and dark (10%), compared with when it was neither dark nor mixed with stool (1.9%).24
Change in bowel habits: Two studies investigated undefined or undifferentiated change in bowel habits,37,41 3 studies investigated diarrhea,17,28,37 and 2 studies examined constipation17,37 as predictors of CRC (Table 3).12–15,17–19,21,23–26,28–37,39–43 In one study, the PPVs of change in bowel habits appeared to increase with age; however, for men PPVs were greater than 5% beginning at age 60 years, whereas for women the PPV never exceeded 4.09% even in the oldest age group.22
In regression analysis, change in bowel habits, including constipation or diarrhea, was found to be a significant predictor of CRC in 3 studies.17,30,36 A study examining the association between the characteristics of changes in bowel habits and risk of CRC found that loose stools significantly increased the risk of CRC compared with soft stools, after adjusting for age, sex, and lifestyle variables.38 Frequency of bowel movement, stool quantity, feelings of discomfort, and laxative use were not significantly associated with risk of CRC.
Weight loss: The median PPV among the 4 studies13,17,37,41 that looked at weight loss as a predictor of CRC was 4.9% (range 1.2% to 35.7%) (Table 3). 12–15,17–19,21,23–26,28–37,39–43 In regression analysis, loss of weight was found to be a significant predictor of CRC in 2 of these studies.17,41 Significantly high PLRs for weight loss were found by Astin et al (PLR = 3.5, 95% CI 2.1 to 5.8) and by Ford et al (PLR = 2.0, 95% CI 1.3 to 3.1).12,15
Abdominal pain: In 5 studies13,17,35,37,41 that explored abdominal pain or bloating as a predictor of CRC, the PPVs ranged from 0.5% to 13.5%, with a median of 2.1% (Table 3).12–15,17–19,21,23–26,28–37,39–43 In regression analysis, abdominal pain and tenderness were each reported as significant predictors by Hamilton et al (P < .001).17
Rectal bleeding in combination with other symptoms and signs: In studies that examined the combination of rectal bleeding and change in bowel habits or diarrhea, or rectal bleeding and weight loss,14,17,24,28,30,31,33,34,36,43 higher PPVs for the combination of symptoms compared with rectal bleeding alone were reported (median PPV for rectal bleeding = 5.3%; median PPV for rectal bleeding and weight loss = 13%; median PPV for rectal bleeding and change in bowel habits = 10.5%) (Table 3).12–15,17–19,21,23–26,28–37,39–43 The median PPV of rectal bleeding and diarrhea, derived from 7 studies, was 9% (data not shown).14,17,24,28,30,31,34 In 3 studies, higher PPVs were seen for the combination of rectal bleeding and diarrhea compared with rectal bleeding and constipation.14,17,34 Astin et al found that the probability of CRC almost doubled when there was a change in bowel habits (PLR = 1.8, 95% CI 1.3 to 2.5) or weight loss (PLR = 1.9, 95% CI 1.3 to 2.8) in patients with rectal bleeding.12
In only 2 of 6 studies, the combination of rectal bleeding and abdominal pain as presenting symptoms had a higher PPV compared with rectal bleeding alone.17,24,30,33,34,36 Both Astin et al12 and Olde Bekkink et al23 also found low PLRs for abdominal pain in patients with rectal bleeding (Table 3).12–15,17–19,21,23–26,28–37,39–43
The PPVs in 2 studies that specifically reported on rectal bleeding and hemorrhoids were 3.1% and 3.3%.24,33 Fijten et al reported a PPV of 18% in patients who presented with rectal bleeding and perianal eczema.30
In a meta-analysis by Olde Bekkink et al, IDA in patients with rectal bleeding had one of the highest PLRs (PLR = 3.7, 95% CI 1.3 to 10.4) compared with all other signs or symptoms examined.23
DISCUSSION
Clinical features of patients presenting in primary care that might raise a suspicion of CRC were evaluated. Signs and symptoms presenting in primary care that were considered to be associated with increased risk of CRC (listed in descending order of associated risk) included a palpable rectal or abdominal mass; rectal bleeding combined with weight loss; IDA; rectal bleeding mixed with stool; rectal bleeding in the absence of perianal symptoms; rectal bleeding combined with change in bowel habits; dark rectal bleeding; rectal bleeding and diarrhea; and change in bowel habits. A combination of clinical features, increasing age, and male sex generally increased the risk of CRC.
Limitations
The current literature search was an update of the searches completed for the NICE and NZGG guidelines.4,5 We trusted that the original searches were equally extensive and that relevant articles were not missed. This review is limited to only those studies published in English. The consistency of results seen among primary studies, meta-analyses, and systematic reviews provides some reassurance that irretrievable literature would be unlikely to show contradicting evidence.
We were limited by the number of rigorous prospective studies that assessed signs or symptoms of CRC. Some studies did not recruit consecutive patients or were not blinded to the patients’ diagnoses. There were also studies that did not adequately explain missing data, data that could not be interpreted, or the reasons for patient withdrawals. In addition, the reference standard of colonoscopy for detecting CRC was not always used. It was difficult to pool data owing to this considerable heterogeneity among studies. Furthermore, some studies selected patients with specific signs or symptoms such as rectal bleeding and, therefore, might not be representative of a primary care population.
As electronic medical records in primary care practices become more widely used, opportunities for rigorous large-sample prospective studies exploring the presentation of clinical features of cancers presenting in primary care will likely become increasingly more feasible.
Conclusion
This systematic review and objective approach to stratifying clinical features into low and increased levels of CRC risk helped assist in the development of recommendations for symptomatic patients who should be referred at least as quickly as asymptomatic patients with positive FOBT screening results.44 This information might also be of value for informing indications for expedited assessment and investigation in CRC diagnostic assessment programs. Knowledge dissemination of clinical features that should raise suspicion of CRC could help with earlier identification and referral by primary care physicians, in addition to those patients identified through routine CRC screening.
EDITOR’S KEY POINTS
Colorectal cancer (CRC) is a leading cause of cancer death in Canada. Early detection and management can lead to considerable reductions in mortality. For FPs and other primary care providers it is often difficult to distinguish the early presentation of CRC from other benign conditions.
This systematic review and objective approach to stratifying clinical features into low and increased levels of CRC risk helped in the development of recommendations for symptomatic patients who should be referred at least as quickly as asymptomatic patients with positive fecal occult blood test screening results.
POINTS DE REPÈRE DU RÉDACTEUR
Au Canada, le cancer colorectal (CCR) est une des principales causes de mortalité due au cancer. Un diagnostic et un traitement précoces peuvent entraîner une diminution importante de la mortalité. Il est souvent difficile pour les MF et pour les autres soignants de première ligne de distinguer un CCR à un stade précoce de certaines affections bénignes.
Cette revue systématique, complétée par une classification objective des caractéristiques cliniques selon qu’elles représentent un risque de CCR faible ou plus grand, a permis d’élaborer des recommandations pour identifier les patients qui devraient être orientés en spécialité au moins aussi rapidement que les patients asymptomatiques qui ont eu une recherche positive du sang occulte dans les selles.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors contributed to the literature review and interpretation, and to preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Canadian Cancer Society [website]. Cancer statistics at a glance. Toronto, ON: Canadian Cancer Society; 2014. Available from: www.cancer.ca/en/cancer-information/cancer-101/cancer-statistics-at-a-glance/?region=on. Accessed 2014 Jul 2. [Google Scholar]
- 2.Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, hemoccult. Cochrane Database Syst Rev. 2007;1:CD001216. doi: 10.1002/14651858.CD001216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Care Ontario [website]. More Ontarians need to be screened for colorectal cancer (Sept. 2012). Toronto, ON: Cancer Care Ontario; 2014. Available from: www.cancercare.on.ca/cms/one.aspx?portalId=1377&pageId=157009. Accessed 2011 Jan 26. [Google Scholar]
- 4.National Institute for Health and Care Excellence [website]. Referral guidelines for suspected cancer. NICE guidelines CG27. London, UK: National Institute for Health and Care Excellence; 2005. Available from: www.nice.org.uk/CG027. Accessed 2014 Jul 2. [Google Scholar]
- 5.New Zealand Guidelines Group. Suspected cancer in primary care: guidelines for investigation, referral and reducing ethnic disparities. Wellington, NZ: New Zealand Guidelines Group; 2009. Available from: www.midlandcancernetwork.org.nz/file/fileid/17510. Accessed 2014 Jul 2. [Google Scholar]
- 6.AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancerview [website]. Standards and guidelines evidence (SAGE) directory of evidence guidelines. Toronto, ON: Canadian Partnership Against Cancer; Available from: www.cancerview.ca/cv/portal/Home/TreatmentAndSupport/TSProfessionals/ClinicalGuidelines/GRCMain/GRCSAGE?_afrLoop=899435266845824&lang=en&_afrWindowMode=0&_adf.ctrl-state=fyx7b0hj8_85. Accessed 2014 Jul 2. [Google Scholar]
- 8.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reitsma JB, Rutjes AWS, Whiting P, Vlassov VV, Leeflang MM, Deeks JJ. Chapter 9: assessing methodological quality. In: Deeks JJ, Bossuyt PM, Gatsonis C, editors. Cochrane handbook for systematic reviews of diagnostic test accuracy version 1.0.0. London, UK: The Cochrane Collaboration; 2009. Available from: http://srdta.cochrane.org/sites/srdta.cochrane.org/files/uploads/ch09_Oct09.pdf. Accessed 2014 Jul 2. [Google Scholar]
- 10.Rabeneck L, Zwaal C, Goodman JH, Mai V, Zamkanei M. Cancer Care Ontario guaiac fecal occult blood test (FOBT) laboratory standards: evidentiary base and recommendations. Clin Biochem. 2008;41(16–17):1289–305. doi: 10.1016/j.clinbiochem.2008.08.069. Epub 2008 Aug 29. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Care Ontario. ColonCancerCheck. 2010 Program report. Toronto, ON: Cancer Care Ontario; 2012. Available from: www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=156747. Accessed 2014 Jul 2. [Google Scholar]
- 12.Astin M, Griffin T, Neal RD, Rose P, Hamilton W. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract. 2011;61(586):e231–43. doi: 10.3399/bjgp11X572427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barwick TW, Scott SB, Ambrose NS. The two week referral for colorectal cancer: a retrospective analysis. Colorectal Dis. 2004;6(2):85–91. doi: 10.1111/j.1463-1318.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- 14.Ellis BG, Thompson MR. Factors identifying higher risk rectal bleeding in general practice. Br J Gen Pract. 2005;55(521):949–55. [PMC free article] [PubMed] [Google Scholar]
- 15.Ford AC, Veldhuyzen van Zanten SJ, Rodgers CC, Talley NJ, Vakil NB, Moayyedi P. Diagnostic utility of alarm features for colorectal cancer: systematic review and meta-analysis. Gut. 2008;57(11):1545–53. doi: 10.1136/gut.2008.159723. Epub 2008 Aug 1. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton W, Lancashire R, Sharp D, Peters TJ, Cheng KK, Marshall T. The importance of anaemia in diagnosing colorectal cancer: a case-control study using electronic primary care records. Br J Cancer. 2008;98(2):323–7. doi: 10.1038/sj.bjc.6604165. Epub 2008 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer. 2005;93(4):399–405. doi: 10.1038/sj.bjc.6602714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heintze C, Matysiak-Klose D, Kröhn T, Wolf U, Brand A, Meisner C, et al. Diagnostic work-up of rectal bleeding in general practice. Br J Gen Pract. 2005;55(510):14–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Jellema P, van der Windt DA, Bruinvels DJ, Mallen DJ, van Weyenberg SJ, Mulder CJ, et al. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ. 2010;340:c1269. doi: 10.1136/bmj.c1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John SK, George S, Primrose JN, Fozard JB. Symptoms and signs in patients with colorectal cancer. Colorectal Dis. 2011;13(1):17–25. doi: 10.1111/j.1463-1318.2010.02221.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones R, Latinovic R, Charlton J, Gulliford MC. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ. 2007;334(7602):1040. doi: 10.1136/bmj.39171.637106.AE. Epub 2007 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrenson R, Logie J, Marks C. Risk of colorectal cancer in general practice patients presenting with rectal bleeding, change in bowel habit or anaemia. Eur J Cancer Care (Engl) 2006;15(3):267–71. doi: 10.1111/j.1365-2354.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 23.Olde Bekkink M, McCowan C, Falk GA, Teljeur C, Van de Laar FA, Fahey T. Diagnostic accuracy systematic review of rectal bleeding in combination with other symptoms, signs and tests in relation to colorectal cancer. Br J Cancer. 2010;102(1):48–58. doi: 10.1038/sj.bjc.6605426. Epub 2009 Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson R, Campbell C, Weller DP, Elton R, Mant D, Primrose J, et al. Predicting colorectal cancer risk in patients with rectal bleeding. Br J Gen Pract. 2006;56(531):763–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Shapley M, Mansell G, Jordan JL, Jordan KP. Positive predictive values of ≥5% in primary care for cancer: systematic review. Br J Gen Pract. 2010;60(578):e366–77. doi: 10.3399/bjgp10X515412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yates JM, Logan EC, Stewart RM. Iron deficiency anaemia in general practice: clinical outcomes over three years and factors influencing diagnostic investigations. Postgrad Med J. 2004;80(945):405–10. doi: 10.1136/pgmj.2003.015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bat L, Pines A, Shemesh E, Levo Y, Zeeli D, Scapa E, et al. Colonoscopy in patients aged 80 years or older and its contribution to the evaluation of rectal bleeding. Postgrad Med J. 1992;68(799):355–8. doi: 10.1136/pgmj.68.799.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chohan DP, Goodwin K, Wilkinson S, Miller R, Hall NR. How has the ‘two-week wait’ rule affected the presentation of colorectal cancer? Colorectal Dis. 2005;7(5):450–3. doi: 10.1111/j.1463-1318.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 29.Du Toit J, Hamilton W, Barraclough K. Risk in primary care of colorectal cancer from new onset rectal bleeding: 10 year prospective study. BMJ. 2006;333(7558):69–70. doi: 10.1136/bmj.38846.684850.2F. Epub 2006 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fijten GH, Starmans R, Muris JW, Schouten HJ, Blijham GH, Knottnerus JA. Predictive value of signs and symptoms for colorectal cancer in patients with rectal bleeding in general practice. Fam Pract. 1995;12(3):279–86. doi: 10.1093/fampra/12.3.279. [DOI] [PubMed] [Google Scholar]
- 31.Flashman K, O’Leary DP, Senapati A, Thompson MR. The department of health’s “two week standard” for bowel cancer: is it working? Gut. 2004;53(3):387–91. doi: 10.1136/gut.2003.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helfand M, Marton KI, Zimmer-Gembeck MJ, Sox HC., Jr History of visible rectal bleeding in a primary care population. Initial assessment and 10-year follow-up. JAMA. 1997;277(1):44–8. [PubMed] [Google Scholar]
- 33.Mant A, Bokey EL, Chapuis PH, Killingback M, Hughes W, Koorey SG, et al. Rectal bleeding: do other symptoms aid in diagnosis? Dis Colon Rectum. 1989;32(3):191–6. doi: 10.1007/BF02554525. [DOI] [PubMed] [Google Scholar]
- 34.Metcalf JV, Smith J, Jones R, Record CO. Incidence and causes of rectal bleeding in general practice as detected by colonoscopy. Br J Gen Pract. 1996;46(404):161–4. [PMC free article] [PubMed] [Google Scholar]
- 35.Muris JW, Starmans R, Fijten GH, Crebolder HF, Krebber TF, Knottnerus JA. Abdominal pain in general practice. Fam Pract. 1993;10(4):387–90. doi: 10.1093/fampra/10.4.387. [DOI] [PubMed] [Google Scholar]
- 36.Nørrelund N, Nørrelund H. Colorectal cancer and polyps in patients aged 40 years and over who consult a GP with rectal bleeding. Fam Pract. 1996;13(2):160–5. doi: 10.1093/fampra/13.2.160. [DOI] [PubMed] [Google Scholar]
- 37.Panzuto F, Chiriatti A, Bevilacqua S, Giovannetti P, Russo G, Impinna S, et al. Symptom-based approach to colorectal cancer: survey of primary care physicians in Italy. Dig Liver Dis. 2003;35(12):869–75. doi: 10.1016/j.dld.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Park JY, Mitrou PN, Luben R, Khaw KT, Bingham SA. Is bowel habit linked to colorectal cancer?—results from the EPIC-Norfolk study. Eur J Cancer. 2009;45(1):139–45. doi: 10.1016/j.ejca.2008.10.002. Epub 2008 Nov 14. [DOI] [PubMed] [Google Scholar]
- 39.Parker C, Hippisley-Cox J, Coupland C, Vinogradova Y. Rectal and post-menopausal bleeding: consultation and referral of patients with and without severe mental health problems. Br J Gen Pract. 2007;57(538):371–6. [PMC free article] [PubMed] [Google Scholar]
- 40.Sánchez A, Muñoz C, Bujanda L, Iriondo C, Gil-Molet A, Cosme A, et al. The value of colonoscopy to assess rectal bleeding in patients referred from primary care units. Rev Esp Enferm Dig. 2005;97(12):870–6. doi: 10.4321/s1130-01082005001200003. [DOI] [PubMed] [Google Scholar]
- 41.Steine S, Stordahl A, Laerum F, Laerum E. Referrals for double-contrast barium examination. Factors influencing the probability of finding polyps or cancer. Scand J Gastroenterol. 1994;29(3):260–4. doi: 10.3109/00365529409090474. [DOI] [PubMed] [Google Scholar]
- 42.Stellon AJ, Kenwright SE. Iron deficiency anaemia in general practice: presentations and investigations. Br J Clin Pract. 1997;51(2):78–80. [PubMed] [Google Scholar]
- 43.Wauters H, Van Casteren V, Buntinx F. Rectal bleeding and colorectal cancer in general practice: diagnostic study. BMJ. 2000;321(7267):998–9. doi: 10.1136/bmj.321.7267.998. Erratum in: BMJ 2001;322(7284):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Giudice ME, Vella ET, Hey A, Simunovic M, Harris W, Levitt C. Guideline for referral of patients with suspected colorectal cancer by family physicians and other primary care providers. Can Fam Physician. 2014;60:717–23. (Eng), e383–90 (Fr). [PMC free article] [PubMed] [Google Scholar]