Abstract
Leptin, a pleiotropic adipokine, crosses the blood-brain barrier (BBB) and blood–spinal cord barrier (BSCB) from the periphery and facilitates experimental autoimmune encephalomyelitis (EAE). EAE induces dynamic changes of leptin receptors in enriched brain and spinal cord microvessels, leading to further questions about the potential roles of endothelial leptin signaling in EAE progression. In endothelial leptin receptor specific knockout (ELKO) mice, there were lower EAE behavioral scores in the early phase of the disorder, better preserved BSCB function shown by reduced uptake of sodium fluorescein and leukocyte infiltration into the spinal cord. Flow cytometry showed that the ELKO mutation decreased the number of CD3 and CD45 cells in the spinal cord, although immune cell profiles in peripheral organs were unchanged. Not only were CD4+ and CD8+ T lymphocytes reduced, there were also lower numbers of CD11b+Gr1+ granulocytes in the spinal cord of ELKO mice. In enriched microvessels from the spinal cord of the ELKO mice, the decreased expression of mRNAs for a few tight junction proteins was less pronounced in ELKO than WT mice, as was the elevation of mRNA for CCL5, CXCL9, IFN-γ, and TNF-α. Altogether, ELKO mice show reduced inflammation at the level of the BSCB, less leukocyte infiltration, and better preserved tight junction protein expression and BBB function than WT mice after EAE. Although leptin concentrations were high in ELKO mice and microvascular leptin receptors show an initial elevation before inhibition during the course of EAE, removal of leptin signaling helped to reduce disease burden. We conclude that endothelial leptin signaling exacerbates BBB dysfunction to worsen EAE.
Keywords: EAE, Endothelia, Leptin receptor, Autoimmunity, Blood–spinal cord barrier
1. Introduction
Leptin, a 16 kD soluble protein produced mainly by adipocytes, can reach the CNS by specific transport systems at the blood–brain barrier (BBB) and blood–spinal cord barrier (BCSB) (Banks et al., 1996; Pan and Kastin, 2001; Tu et al., 2008). Leptin is proinflammatory and facilitates autoimmunity. Studies involving leptin treatment, antagonism, and knockout mice have collectively shown that leptin worsens experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS) (Matarese et al., 2001a, 2001c; Sanna et al., 2003). In particular, a serum leptin surge precedes the onset of EAE, and leptin-deficient (ob/ob) mice and leptin receptor ObRb-deficient (db/db) mice are resistant to the induction and progression of EAE. Leptin treatment by peripheral delivery increases susceptibility of mice to EAE. In active EAE brain lesions, leptin is also produced by pathogenic T helper (Th1) cells and macrophages (Sanna et al., 2003). Studies from ob/ob and db/db mice that have defective T cell responses show that leptin stimulates Th1 response but inhibits Th2 response (Lord et al., 1998). However, astrocyte-specific leptin receptor knockout (ALKO) mice show worsened EAE and greater leukocyte infiltration in spinal cord and brain (Mishra et al., 2013), indicating a cell-specific leptin action in the CNS.
The BBB and BSCB are three-dimensional, multicellular neurovascular interfaces. The comprising microvascular endothelial cells are joined by tight junction (TJ) complexes. The TJ complex consists of three major classes of transmembrane proteins: occludins, claudins, and junctional adhesion molecules, and it is reinforced by TJ-associated adaptor proteins, such as zonula occludin (ZO)-1, ZO-2, and ZO-3 that serve as scaffolding proteins to secure transmembrane TJ protein localization on the membrane (Liebner et al., 2000b; Wolburg and Lippoldt, 2002; Abbott et al., 2010). Altered expression of occludin, junctional adhesion molecules, and ZO-1 has been documented in acute and progressive forms of MS, together with changes in the vascular basement membrane and pericytes (Kirk et al., 2003; Alvarez et al., 2011). Inflammation and BSCB breakdown are also major features in different EAE models (Juhler et al., 1984; Pan et al., 1996). In EAE, the reduction of occludin, claudin-1, claudin-3, claudin-5, and ZO-1 coincides with the onset of inflammation and slightly precedes signs of disease and increased permeability of the BSCB (Wolburg et al., 2003; Bennett et al., 2010).
The BBB endothelia express leptin receptors (Hileman et al., 2002; Pan et al., 2008b). Leptin transport involves its specific receptors at the BBB and BCSB (Banks et al., 1996; Pan and Kastin, 2001; Tu et al., 2008), and megalin at the blood-cerebrospinal fluid barrier (Kurrimbux et al., 2004; Dietrich et al., 2008). In mice with EAE, the transport of leptin does not diminish, but rather shows a selective upregulation (Hsuchou et al., 2013b). This is similar to the change of transport of tumor necrosis factor α (TNF) (Pan et al., 1996) but opposite from that of interleukin (IL)-15 (Hsuchou et al., 2009b). Regulated transport may underlie differential effects of leptin in autoimmunity in the periphery and CNS (Mishra et al., 2013), a phenomenon seen for many peptides (Pan and Kastin, 2009). During the course of transcytosis, leptin also modifies cellular signaling with generation of secondary mediators by the BBB (Pan et al., 2011). However, the role of endothelial leptin signaling in the incidence and progression of EAE is not clear. Here, we used endothelial specific leptin receptor mutant (ELKO) mice to determine whether endothelial leptin signaling promotes EAE.
2. Materials and methods
2.1. EAE induction
All experimental procedures followed a protocol approved by the Institutional Animal Care and Use Committee. The generation and characterization of the ELKO mice are described in detail elsewhere (Hsuchou et al., 2011; Pan et al., 2012). The ELKO mice were studied along with their littermate controls on a C57BL/6 strain background (wildtype, WT). All mice were group-housed and fed ad lib in a specific pathogen-free animal facility. Female ELKO and WT mice (8–10 week old) were used to induce EAE after brief anesthesia by inhalation of isofluorane (1 L/min). Mice were immunized by subcutaneous injection of 100 µg of MOG35–55 (Ana-Spec Inc, Fremont, CA) in 100 µl emulsion of 50% complete Freund’s adjuvant (CFA) containing 5 mg/ml of heat-inactivated Mycobacterium tuberculosis (DIFCO Laboratories, Detroit, MI). Three injections (about 33.3 µl each) were delivered at three sites in the lower flank regions. The mice also received 200 ng of pertussis toxin (Sigma, St. Louis, MO) in 200 µl of phosphate-buffered saline (PBS) solution intraperitoneally on the day of immunization (day 0) and again 48 h later (day 2). The adjuvant-only control groups received both CFA and pertussis toxin, but MOG35–55 was omitted. To distinguish them from naïve control mice, this control group is labeled CFA control but pertussis toxin was also present. The mice were monitored daily for clinical signs of EAE and body weight changes. EAE symptoms were scored daily by experienced researchers (Pan et al., 1996; Wu et al., 2010; Mishra et al., 2013). The scores are as follows: 0, no detectable signs of weakness; 0.5, distal tail limpness, mild postural changes, or reduced locomotor activity; 1, completely limp tail; 1.5, limp tail and hind limb weakness (unsteady gait and poor grip with hind limbs); 2, unilateral partial hind limb paralysis; 2.5, bilateral hind limb paralysis; 3, complete bilateral hind limb paralysis; 3.5, complete hind limb paralysis and unilateral forelimb paralysis; 4, total paralysis of hind limbs and forelimbs; 5, moribund or dead.
2.2. qPCR of enriched microvessels from CNS
In the study to determine dynamic changes of ObRs in cerebral and spinal microvessels after EAE induction, five groups of C57 female mice were studied: 0, 6, 13, 17, and 24 d after EAE (n = 3/time point). The 0 time control was naïve mice studied along with the d17 group. The EAE induction occurred on the same day, and tissue collection fell on different days after anesthesia induced by intra-peritoneal injection of a ketamine/xylazine/acepromazine cocktail (80/4/1.6 mg/kg). Microvessels were enriched by use of a capillary depletion procedure as described for RNA analyses in the past (Pan et al., 2009; Ouyang et al., 2014). Cerebral cortex and spinal cord were homogenized separately in capillary buffer (10 mM HE-PES,141 mM NaCl, 4 mM KCl, 2.8 mM CaCl2, 1 mM NaH2PO4, 1 mM MgSO4, and 10 mM glucose) and thoroughly mixed with 26% dextran, to reach a final concentration of dextran slightly above 13.5%. The homogenate was centrifuged at 6400g for 30 min at 4 °C in a swing bucket rotor. The pelleted microvessel enrichment was snap frozen and stored at −80 °C until the time of RNA analysis. Total RNA was extracted and reversely transcribed to cDNA. Real-time PCR was performed by use of the SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) along with the cDNA and gene-specific primers on an ABI7900T device.
To determine the effects of ELKO and EAE on BSCB gene expression and the interactions of the two factors, four groups of mice were studied: WT or ELKO on day 17 of EAE induction, and their littermates studied on the same day (n = 3/group). Anesthesia, tissue collection, enrichment of microvessels, and qPCR analysis were the same as described above. Primer sequences are listed in Table 1. The mRNA expression of ObR subtypes, cytokines, chemokines, and tight junction molecules was quantified by normalization of the cycle threshold (CT) values with those of the reference gene GAP-DH by use of the ΔΔCT method.
Table 1.
List of qPCR primers for SYBR Green qPCR.
| Gene | Forward primer (5′ → 3′) | Reverse primer (5′ → 3′) |
|---|---|---|
| ObRa | GAAGTCTCTCATGACCACTACAGATGA | TTGTTTCCCTCCATCAAAATGTAA |
| ObRb | GCATGCAGAATCAGTGATATTTGG | CAAGCTGTATCGACACTGATTTCTTC |
| ObRc | AGGGCTTTATGTTGTTGTGTTC | TTTCTCTGATCAAATCCCAAAC |
| ObRe | TAATGAAGATGATGGAATGAAG | ATTGCCAGTCTACAGTGTCA |
| GADPH | TGTGTCCGTCGTGGATCTGA | CCTGCTTCACCACCTTCTTGA |
| TNF | CACCGTCAGCCGATTTGC | TTGACGGCAGAGAGGAGGTT |
| IFNγ | TTGGCTTTGCAGCTCTTCCT | TGACTGTGCCGTGGCAGTA |
| CCL5 (RANTES) | TCCAATCTTGCAGTCGTGTTTG | TCTGGGTTGGCACACACTTG |
| CCL12 (MCP5) | CAGTCACGTGCTGTTATAATGTTGTT | TGCTTGTGATTCTCCTGTAGCTCTT |
| CXCL9 (MIG) | ACGGAGATCAAACCTGCCTAGA | CCATTCTTTCATCAGCTTCTTCAC |
| CXCL10 (IP10) | CGCTGCAACTGCATCCATAT | AGCTTCCCTATGGCCCTCAT |
| Claudin-1 | ACTCCTTGCTGAATCTGAACAGT | GGACACAAAGATTGCGATCAG |
| Claudin-2 | CTCCCTGGCCTGCATTATCTC | CAGTGGTGAGTAGAAGTCCCG |
| Claudin-3 | TGGGAGGGCCTGTGGAT | CGTACATTTTGCACTGCATCTGA |
| Claudin-5 | CTGCCTTCCTGGACCACAA | TCCACAGCCCCTTCCAAGT |
| Occludin | TCAAACCGAATCATTATGCACCA | AGATGGCAATGCACATCACAA |
| ZO-1 | GAG CTA CGC TTG CCA CAC TGT | TCG GAT CTC CAG GAA GAC ACT T |
2.3. Measurement of leptin and sObR by enzyme-linked immunosorbent assay (ELISA)
We have shown that naïve ELKO mice have higher levels of leptin and sObR in blood (Hsuchou et al., 2013a). To determine whether this pattern remained the same after EAE, two groups of mice were studied: WT and ELKO mice on day 17 (n = 2–3/group). Mice were anesthetized by intraperitoneal injection of the ketamine/xylazine/acepromazine cocktail (80/4/1.6 mg/kg). Blood was collected by dissection of the common carotid artery immediately before decapitation of the mouse between 11 am and 2 pm. Leptin and sObR concentrations in serum were measured respectively by use of a murine leptin ELISA kit and a mouse Leptin R DuoSet ELISA assay (R &D Systems, Minneapolis, MN) as described previously (Pan et al., 2012; Hsuchou et al., 2013a). The linear range for leptin assay was 0–4 µg/ml. The linear range for sObR was 0–10 ng/ml.
2.4. Morphological analyses
To determine the effects of the ELKO mutation and EAE induction on leukocyte infiltration, four groups of mice were studied (n = 3/group): ELKO and WT mice with adjuvant only (control), or on day 17 of EAE. The mice were anesthetized with ketamine/xylazine/acepromazine as described above, and perfused intracardially with PBS followed by 4% paraformaldehyde (PFA). Spinal cord was post-fixed in 4% PFA, cryoprotected with 15% and then 30% sucrose, and embedded in HistoPrep. The tissue was stored at −80°F until cryosectioning. Spinal cord cross-sections (20 µm) were subjected to permeabilization, blocking, and immunofluorescence staining (Pan et al., 2008a; Hsuchou et al., 2009a; Wu et al., 2013). Separate sections were incubated with CD3 and CD45 antibodies (BioLegend, San Diego, CA, 10 µg/ml final concentration) overnight at 4 °C, washed with PBS thoroughly, and incubated with respective secondary antibodies. The CD45 antibody identifies leukocytes and recognizes a human leukocyte antigen, and the CD3 antibody identifies T lymphocytes and recognizes the epsilon chain of the CD3 antigen/T-cell antigen receptor (TCR) (van Dongen et al., 1988). The negative controls omitted primary antibodies and did not show the presence of fluorescence. A DAPI-containing medium was used to coverslip the sections after immunostaining to demarcate nucleated cells.
2.5. Measurement of BBB permeability
To determine the effects of ELKO and EAE on paracellular permeability of the BBB and BSCB, four groups of mice were studied a month after induction (n = 3/group): ELKO and WT mice receiving adjuvant only (control) or MOG35–55 (EAE). Mice were anesthetized and injected intravenously with 100 µl of sodium fluorescein (10%, i.e. 100 mg/ml, Sigma). Thirty min later, blood was collected by dissecting the right carotid artery to obtain serum, and the mouse was decapitated immediately. Brain and spinal cord were dissected, weighed, and homogenized separately in PBS. The homogenate was centrifuged at 10,000g for 15 min at 4 °C. The fluorescent intensity of sodium fluorescein was measured in 100 µl of supernatant and 50 µl of serum, along with blank controls with PBS only and standards with serial dilutions of sodium fluorescein. The excitation wave length was 493 nm, the emission wave length was 538 nm, and an auto cutoff was applied on the fluorescent microplate reader (Molecular Devices, Sunnyvale, CA). The concentrations were within the linear range of the standard curve. The tissue/serum ratio of fluorescence was determined.
2.6. Flow cytometry of cells in peripheral immune organs and infiltrated into the spinal cord
To determine the effects of ELKO and EAE on immune profiles at the peak of the disease, four groups of mice were studied: WT and ELKO mice receiving CFA (or naïve mice where indicated in the Results section), and those receiving MOG35–55 (n = 3/group). The mice were anesthetized by ketamine/xylaxine/acepromazine. After intracardial perfusion with 25 ml of PBS to clear blood and loosely adherent cells on vessel walls, mice were decapitated. Spinal cord was dissected. Thymus, spleen, and inguinal, axillary, and mesenchymal lymph nodes were also collected into Hanks Balanced Salt Solution. Spinal cord was isolated and homogenized. Infiltrated cells were recovered following an established Percoll gradient protocol (Pino and Cardona, 2011). The supernatant was loaded onto a gradient of 30% and 70% isotonic Percoll. After centrifugation, cells residing at the 30% and 70% interface were collected and washed with PBS. Cells from peripheral immune organs were dissociated by pressing the tissue between two glass slides, and red blood cells from the spleen were removed by RBC lysis buffer containing NH4Cl. Cells were washed and filtered through 100 lm nylon strainers, and collected into RPMI1640 culture medium containing 10% fetal bovine serum. The number of cells was estimated by use of a hemocytometer. The cells were processed immediately for cell surface staining and flow cytometry.
For cell surface immunostaining, the cells were blocked with PBS containing 2% bovine serum albumin for 10 min on ice and then incubated with fluorophore-conjugated antibodies. The antibodies include: FITC-CD4 (2.5 ng/µl final concentration), PE-CD8 (2 ng/µl), Alexa488-CD11b (2.5 ng/µl), APC-CD3 (2 ng/µl), APC-CD45 (2 ng/µl), or APC-Gr1 (2 ng/µl). All were purchased from Biolegend (San Diego, CA). After incubation for 30 min on ice, stained cells were thoroughly washed with ice-cold PBS, then either analyzed immediately or fixed with 1% PFA overnight and stored in PBS for less than a week. Flow cytometry analysis was performed on a FACSCalibur (BD Pharmingen, San Diego, CA) in the Cellular Imaging Core Facility. Negative controls were cells without incubation with the antibodies, or with a single antibody only for triple staining. Data were analyzed by post-acquisition compensation with FlowJo software (Tree Star, Ashland, OR).
2.7. Statistical analysis
Means are presented with their standard errors. The difference of EAE scores between WT and ELKO groups over time was determined by repeated measures two-way ANOVA followed by the Bonferroni’s post hoc test. The difference of leukocyte composition over the course of EAE was determined by randomized block analysis. Effects of strain (WT vs ELKO) and disease (naïve vs EAE) on mRNA expression were determined by two-way ANOVA and single factors were analyzed post hoc.
3. Results
3.1. Dynamic changes of ObR mRNA expression in enriched brain and spinal cord microvessels during the course of EAE
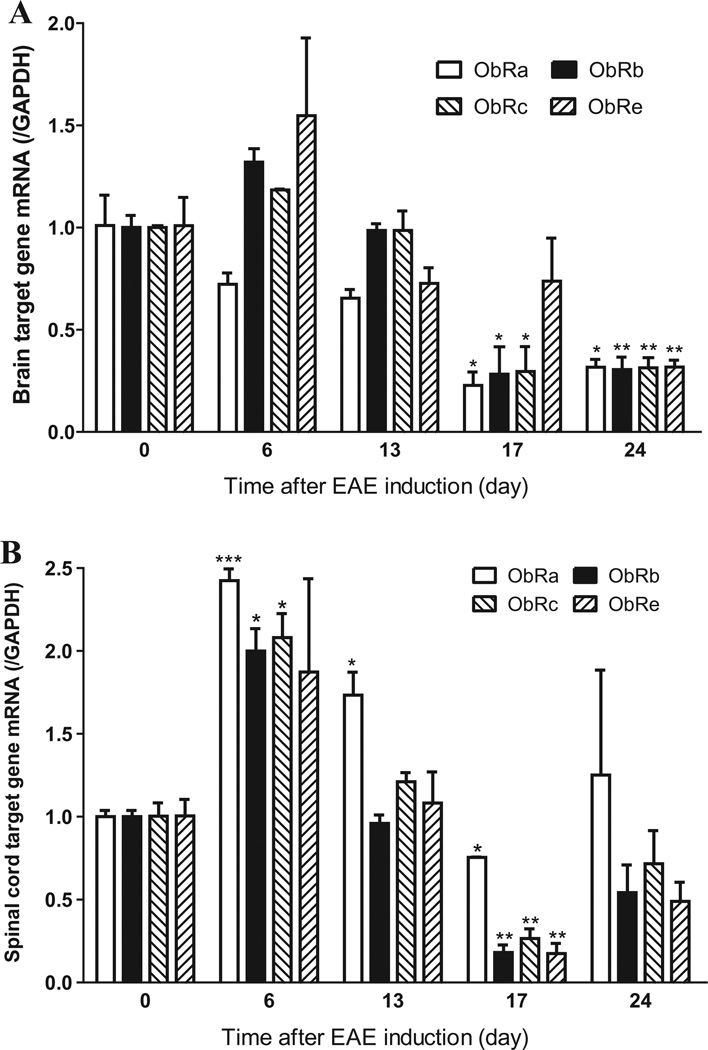
The effect of EAE evolution on mRNA expression of ObR isoforms at the BBB and BSCB was estimated by randomized block analysis, similar to repeated measures ANOVA (n = 3/time point). Although direct comparison among ObR isoforms suffered from differences of amplification efficiency of these splicing variants, a significant effect of time after EAE induction was evident. In enriched cerebral microvessels, there was an initial trend of increase of ObRb, ObRc, and ObRe on day 6 and a later decrease of all ObR isoforms [F(4,33) = 31.04, p < 0.005]. In comparison with the respective time 0 naïve control, post hoc analysis showed that ObRa, ObRb, and ObRc were reduced on day 17, and that all isoforms were reduced on day 24 (Fig. 1A). In the enriched spinal cord microvessels, there was also a significant effect of time [F(4,32) = 53.9, p < 0.005]. There was an initial increase on day 6–13, and a later reduction on day 17. Specifically, ObRa mRNA was increased on day 6 and 13 and reduced on day 17 in comparison with the 0 time control. ObRb and ObRc mRNA were both increased on day 6 and decreased on day 17. ObRe mRNA was decreased on day 17 (Fig. 1B). The differences among ObR isoforms were not uniform and the results were under powered. Overall, there was an initial upregulation of ObR isoforms before the onset of EAE symptoms, and later inhibition of gene expression that persisted on day 24 of the spinal cord samples. The temporal and regional differences in BBB and BSCB expression of ObRs provided a strong basis to determine the effects of ELKO mutation on EAE progression.
Fig. 1.
The mRNA level of ObR isoforms showed a dynamic change during the course of EAE. (A) In enriched microvessels from cerebral cortex, there was an initial non-significant increase on day 6, followed by a significant reduction on day 17 and day 24 for all three membrane-bound leptin receptors. The soluble receptor ObRe also was reduced on day 24. (B) In enriched spinal cord microvessels, there was an initial increase of membrane-bound ObRs on day 6, and subsequent reduction of all isoforms on day 17. *p < 0.05; **p < 0.01; ***p < 0.005 in comparison with 0 time.
3.2. ELKO mice show reduced severity of EAE
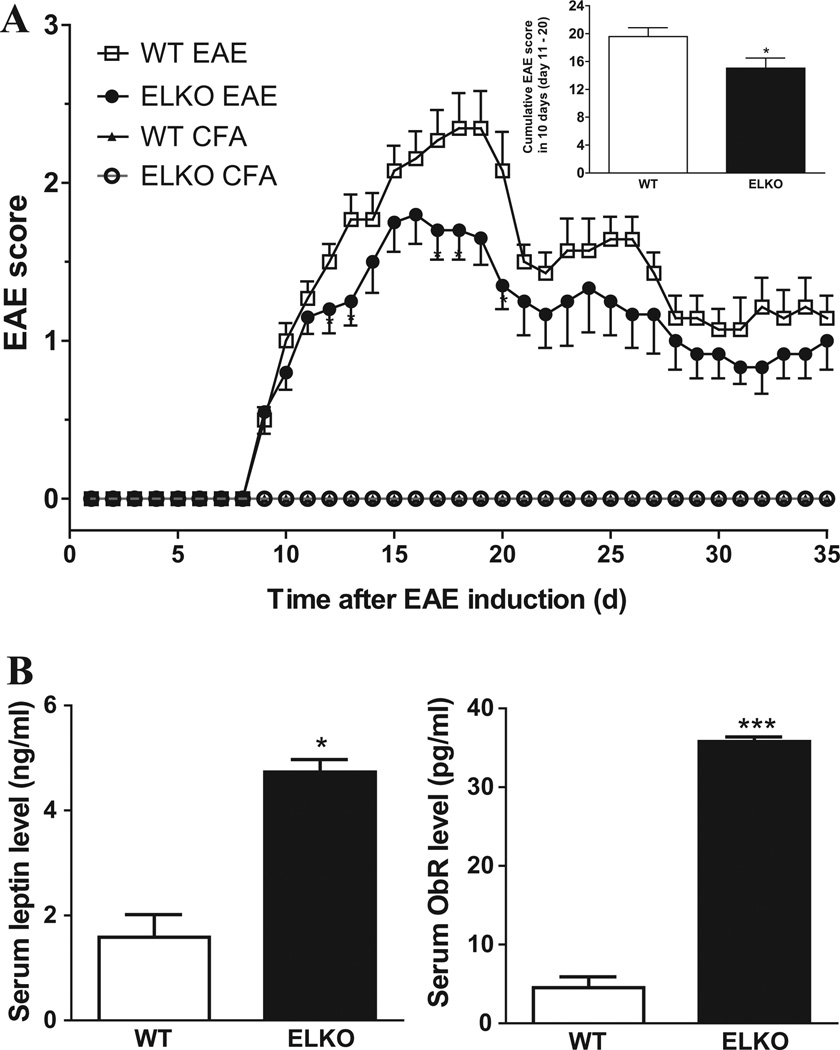
The effects of ELKO and EAE induction on behavioral scores were determined by repeated measures ANOVA on daily EAE scores from four groups of mice till day 35 after induction (n = 6–13/group). The uneven sample size is caused by sample collection in the course of the study. Since several studies used naïve controls instead of CFA control, this resulted in unequal loss of data points among the groups. Nonetheless, EAE score comparison was replicated in four different batches of experiments that were consistent with each other, and results were not pooled from the batches. The adjuvant-treated controls of WT and ELKO mice did not have elevation of EAE scores. In mice injected with MOG35–55, the disease incidence was 100%. Both WT and ELKO mice had onset of symptoms on day 7. The WT mice reached a maximal EAE score on day 17, averaging 2.0 ± 0.28. The maximal EAE score of ELKO mice, also peaking around day 17, was 1.58 ± 0.49. Repeated measures ANOVA on day 1–20 showed a significant effect of time [F(19,589) = 57.01, p < 0.005] and group [F(3,589) = 67.71, p < 0.005]. Their interaction was also significant. Post-hoc analysis showed that ELKO mice had lower scores on day 12–20 (p < 0.05; Fig. 2A). The cumulative scores during the 10 days of peak disease (day 11–20) also had a reduction in ELKO group (p < 0.05), indicating a reduction of an overall disease burden (Fig. 2A, inset). The results were consistent in two subsequent repeats.
Fig. 2.
Effects of ELKO on EAE scores and blood leptin concentrations. (A) The EAE symptoms in ELKO mice were less severe than those of WT mice, supported by the significantly lower EAE scores in the ELKO group on days 12,13,17, 18 and 20 after EAE induction (n = 7/group). The cumulative 10-day score (day 11–20) was also lower in the ELKO mice (inset). (B) On day 17 of EAE, both serum leptin and sObR concentrations were higher in the ELKO mice than in the WT mice (n = 2–3/group). *p < 0.05; **p < 0.01; ***p < 0.005.
We have reported that naïve ELKO mice had higher serum leptin concentration than WT mice (Pan et al., 2012; Hsuchou et al., 2013a). To determine whether this pattern persists after EAE, WT mice on day 17 after EAE induction were compared with their ELKO counterparts (n = 3/group). Serum leptin averaged 4.7 ± 0.2 ng/ml in the ELKO mice with EAE, and 1.6 ± 0.4 ng/ml in WT mice with EAE (Fig. 2B, left panel). Both were lower than the values in naïve mice shown in previous studies. The elevation of serum leptin in ELKO EAE group over WT EAE group was significant (df = 3, 2-tailed t value = 5.4, p = 0.013). In parallel, ELKO mice on day 17 of EAE had serum sObR of 35.8 ± 0.6 pg/ml whereas WT mice at day 17 of EAE had serum ObR of 7.4 ± 3.0 pg/ml. The difference between the two EAE groups was significant (df = 3, 2-tailed t value = 24.8, p = 0.0001; Fig. 2B right panel). Both were lower than those in healthy naïve mice, which have sObR levels of 3.6 ng/ml (ELKO) and 0.1 ng/ml (WT), respectively.
3.3. ELKO mice show better preserved BSCB permeability and less leukocyte infiltration
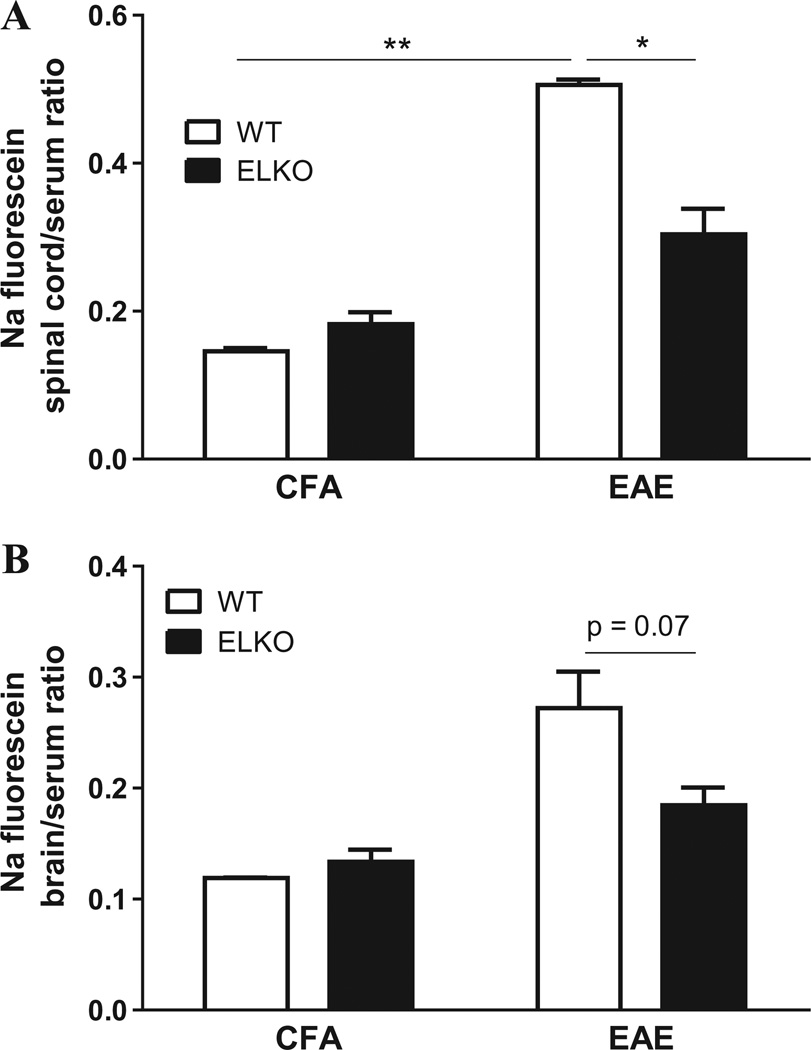
The permeability of BSCB was then quantified by sodium fluorescein uptake on day 30 in 4 groups of mice (n = 3/group): WT or ELKO mice receiving CFA (control groups), and those with EAE. Two-way ANOVA of spinal cord samples showed an effect of the ELKO mutation [F(1,5) = 9.55, p < 0.05] and of EAE [F(1,5) = 80.5, p < 0.005], and the interaction was also significant. Post-hoc analysis showed that the spinal cord/serum ratio of sodium fluorescein was higher in WT mice with EAE than in ELKO mice with EAE (Fig. 3A). In the brain, there was an effect of EAE (p = 0.005) and a trend of significance of ELKO mutation (p = 0.08), without interaction of the two factors (Fig. 3B). Post-hoc analysis showed that the increase of brain/serum ratio of sodium fluorescein in the WT EAE mice tended to be higher than that in the ELKO EAE mice (p = 0.07).
Fig. 3.
Effects of ELKO and EAE on paracellular permeability of the BSCB. (A) The spinal cord uptake of sodium (Na) fluorescein 30 min after iv injection was higher in EAE mice than in CFA mice. The increase was less pronounced in the ELKO with EAE than in the WT group with EAE. (B) The brain uptake was also higher in EAE than CFA groups, and showed a trend of difference (p = 0.07) between the WT and ELKO groups with EAE. *p < 0.05; **p < 0.01.
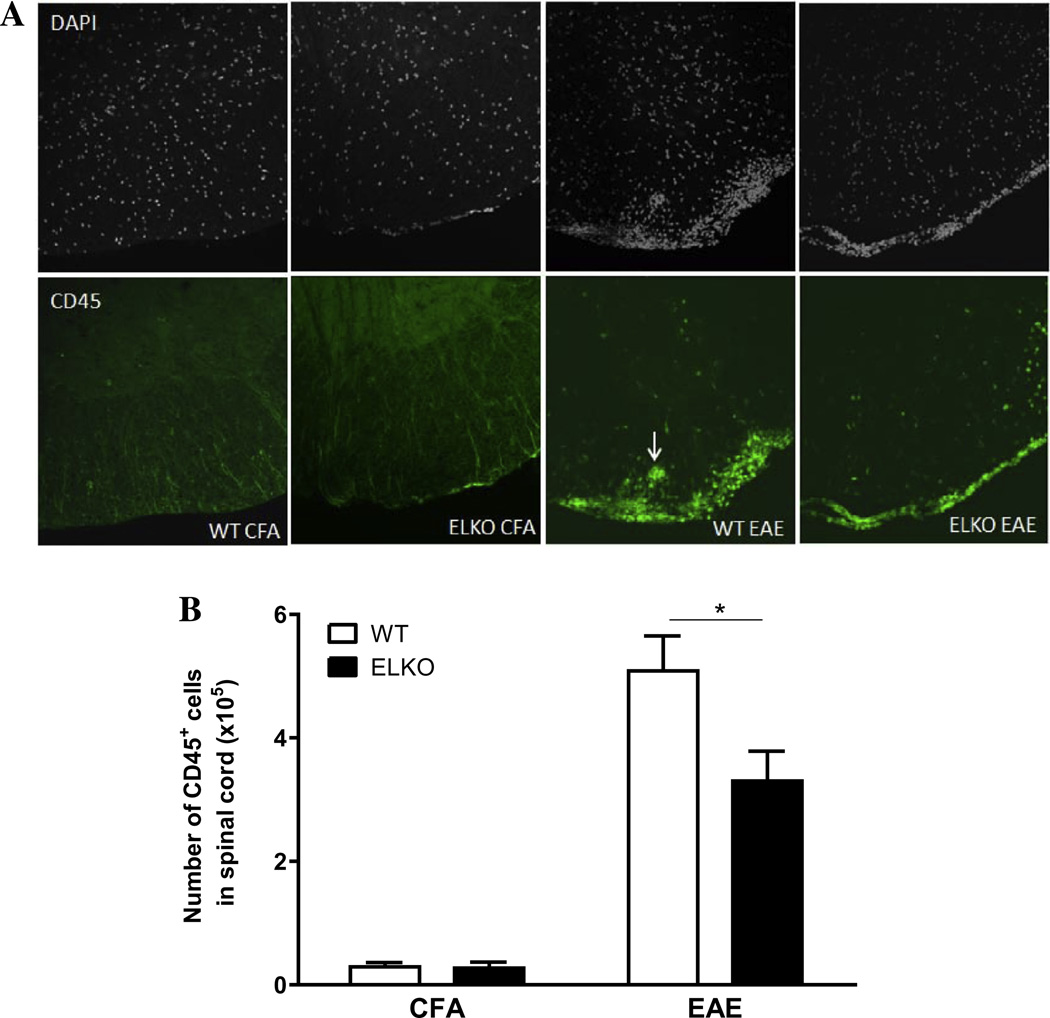
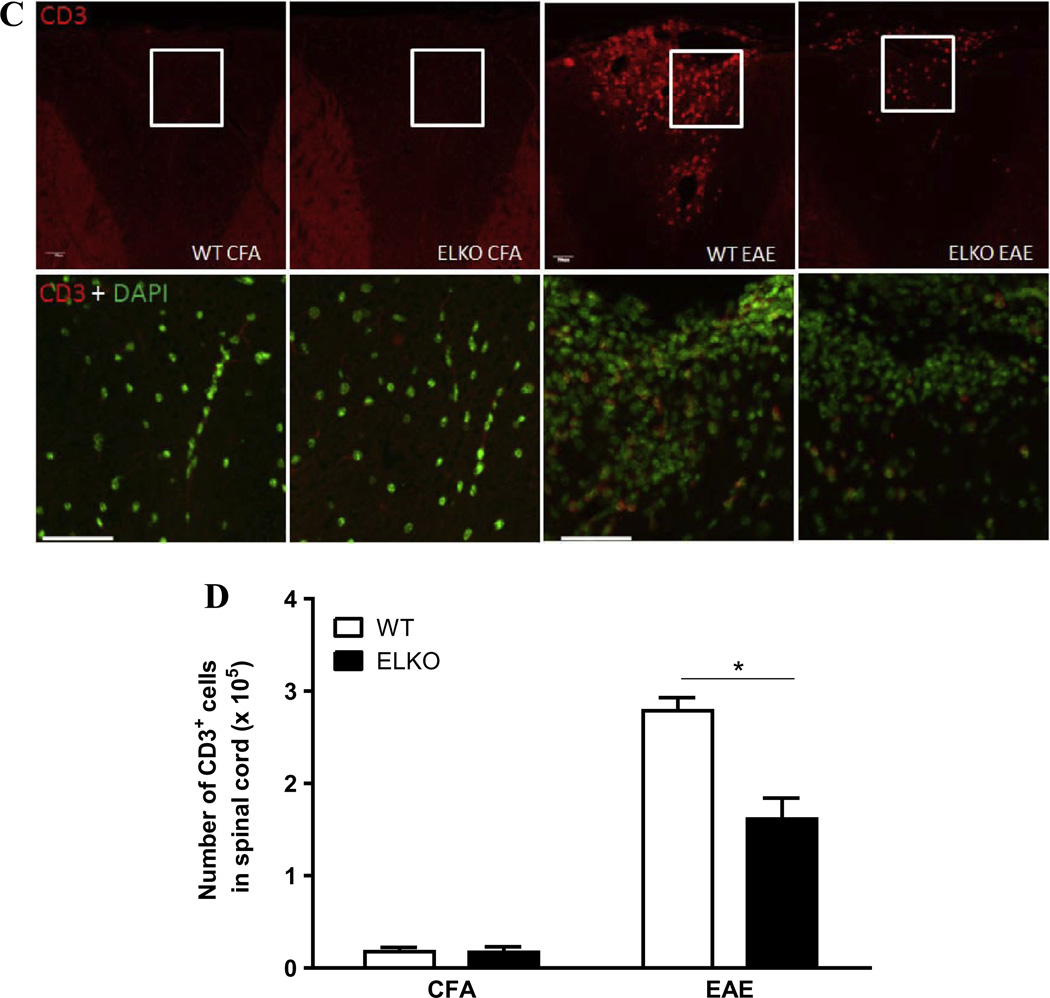
The higher BSCB permeability corresponded with more CD45+ cells associated with the spinal cord in the WT EAE mice (Fig. 4A). In the ventral horn of cervical spinal cord from four groups of mice studied on day 17 after CFA treatment or EAE induction, the WT mice appeared to have more cellularity shown by DAPI nuclear staining. CD45-immunopositive cells were mainly associated with meninges. There were more CD45+ cells in WT with EAE than ELKO with EAE, and some presented as a perivascular cuff or were already associated with spinal cord parenchyma. The ELKO section had apparently less infiltration. Complementary flow cytometry analysis and two-way ANOVA showed that there was a large effect of EAE [F(1,8) = 107.4, p < 0.00001], though strain effect [F(1,8) = 5.7, p < 0.05] and interaction (p < 0.05) were also significant. Post-hoc analysis confirmed a reduction (p < 0.05) of the number of CD45+ cell in the ELKO mice compared with their WT counterparts on day 17 after EAE induction (Fig. 4B).
Fig. 4.
Leukocyte accumulation in the spinal cord was seen by immunofluorescent staining of CD45 (marker for leukocytes) and CD3 (marker for T cells) against a background of DAPI staining to demarcate all nucleated cells (both infiltrated and CNS residential cells). There was increased cellularity in the WT mice, both for CD45 in the ventral cervical spinal cord (A) and CD3 in the dorsal cervical spinal cord (C). There was also perivascular cuffing in the WT with EAE (arrow). The difference in the number of infiltrated cells in the whole spinal cord was quantified by flow cytometry of cells recovered after vascular washout, homogenization, and Percoll gradient isolation. The number of CD45+ cells (B) and CD3+ cells (D) both showed less increase in ELKO mice with EAE than WT mice with EAE (n = 3/group). *p < 0.05.
Consistent with the patterns of CD45 immunostaining, CD3+ cells also showed more infiltration in the WT EAE group than the ELKO EAE group, whereas the CFA-treated WT and ELKO mice did not have many CD3 + cells associated with the cervical spinal cord on day 17 (Fig. 4C). Among the infiltrated cells demarcated by DAPI staining in the cluster shown in the dorsal cervical spinal cord, there were fewer CD3+ cells in ELKO than WT sections. Flow cytometry quantification supports a difference between the WT and ELKO groups (Fig. 4D). There was an effect of strain [F(1,7) =16.9, p <0.005] as well as an effect of EAE [F(1,7) = 199.1, p < 0.0001] and a significant interaction.
3.4. Profiles of cells in peripheral immune organs and those infiltrating the spinal cord
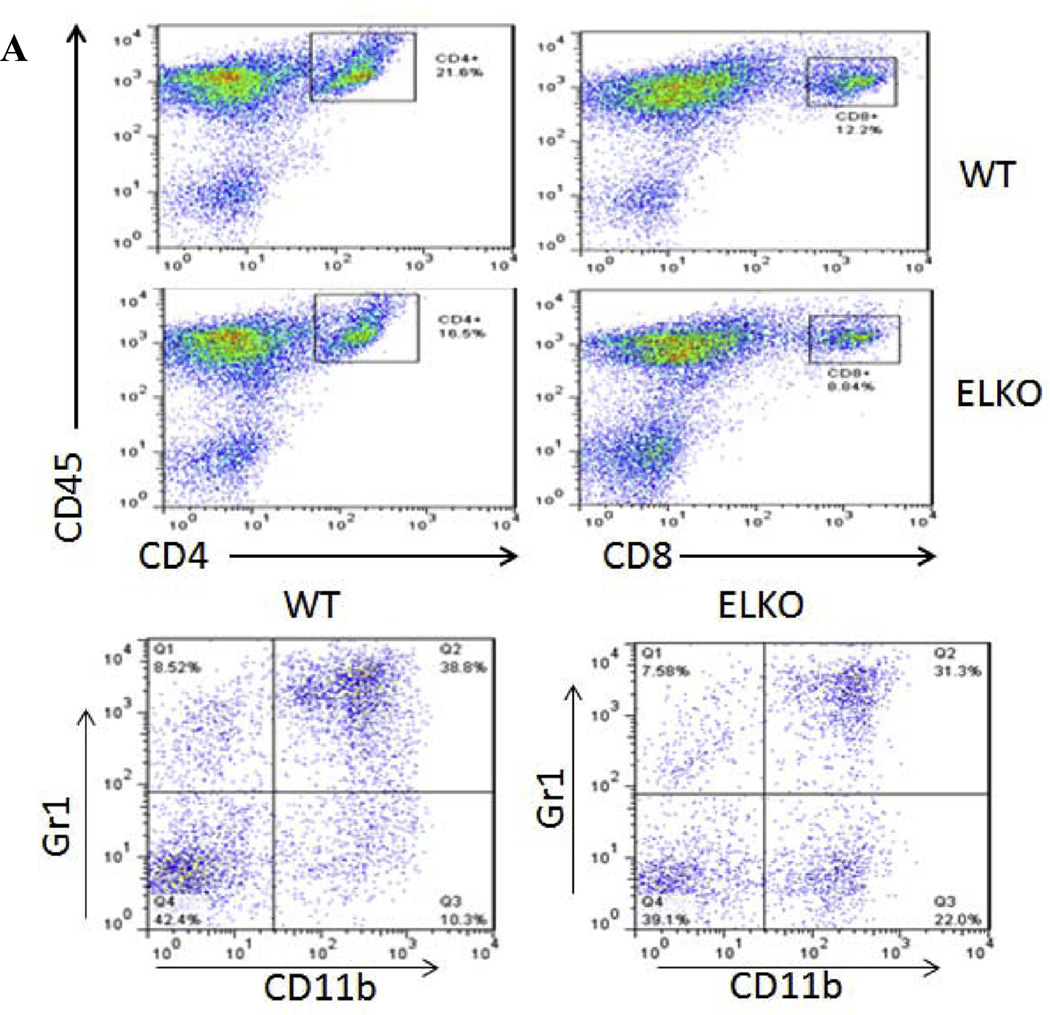
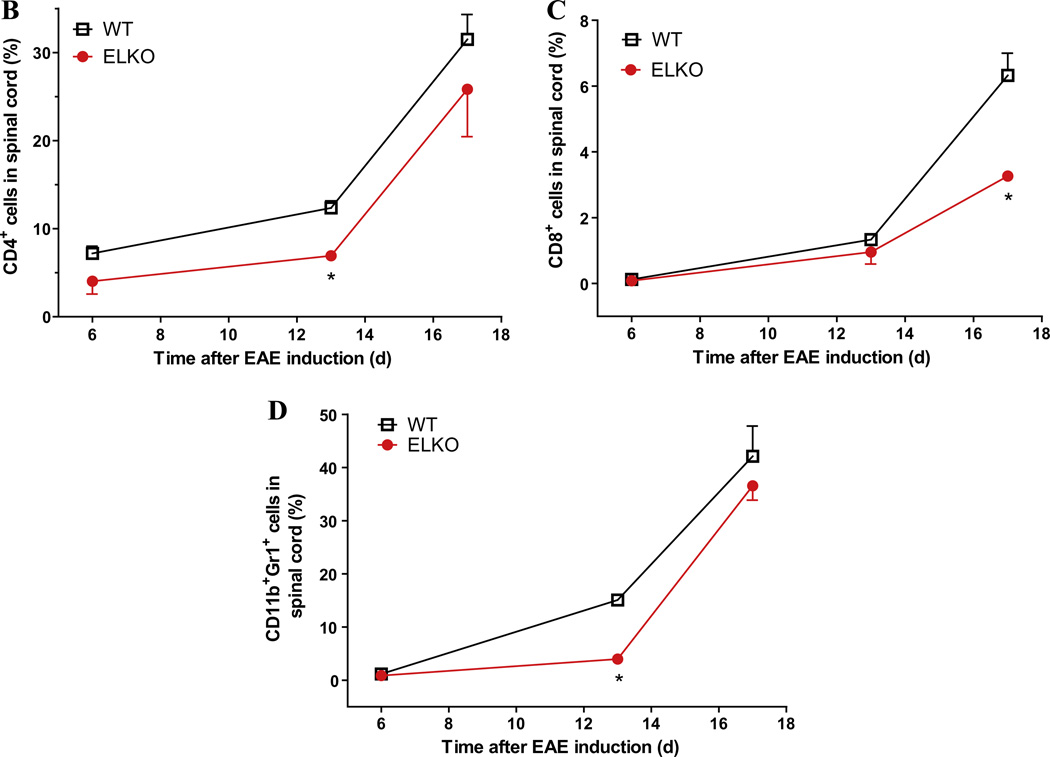
To determine the evolving changes of immune cell profile during the course of EAE, 2 groups of mice were studied: WT or ELKO mice treated with EAE. In each group, three time points were chosen for the study (n = 3 mice/time point): days 6 (asymptomatic phase), 13 (disease onset), and 17 (peak) after immunization. Three populations of cells that had infiltrated into the spinal cord were analyzed by flow cytometry: CD4+ T cells, CD8+ T cells, and CD11b+Gr1+ granulocytes. The FACS profile and gating strategy are shown in Fig. 5A for leukocytes isolated from the spinal cord of WT and ELKO mice on day 17 of EAE. For CD4+ cells, there was a significant effect of time [F(2,11) = 40.4, p < 0.0001] but not strain, although post hoc tests showed that ELKO mice had a lower percentage of CD4+ cells on day 13 (p < 0.05; Fig. 5B). The percentage of CD8+ T cells was very low in both WT and ELKO mice on day 6. There was minimal increase on day 13 and a somewhat greater increase on day 17. This resulted in a significant effect of strain [F(1,9) = 14.1, p < 0.005], time [F(2,9) = 89.8, p < 0.0001], and their interaction. The percentage of CD8+ cells was higher in WT than ELKO mice on day 17 (p < 0.05, Fig. 5C). The percentage of CD11b+Gr1+ granulocytes was also very low in the spinal cord of both WT and ELKO mice on day 6. There was no increase in the ELKO mice on day 13 but a significant increase in the WT mice on day 13, and both groups showed elevation on day 17 (p < 0.05). This contributed to significant effects of both strain [F(1,10) = 5.1, p < 0.05] and time [F(2,10) = 97,6, p < 0.0001] with a lack of interaction (Fig. 5D).
Fig. 5.
Flow cytometric analysis of leukocytes recovered from the spinal cord of WT or ELKO mice with EAE. (A) Flow cytometry profiles of the cell surface markers and gating strategy. (B) The percent of CD4+ T cells among all cells analyzed in the same sample showed an increase over time, and was less on day 13 in the ELKO mice with EAE than in the WT mice with EAE. (C) The percent of CD8+ T cells increased over time in both WT and ELKO mice, but the elevation was less pronounced in the ELKO group than in the WT group on day 17 of EAE. (D) In WT mice with EAE, the percentage of CD11b+Gr1+ granulocytes increased over time. In ELKO mice with EAE, the increase was not present on day 13 but became apparent on day 17. At this time, the level remained lower than that in WT mice with EAE (n = 3/time point in each group). *p < 0.05 when WT and ELKO were compared on the same day of EAE.
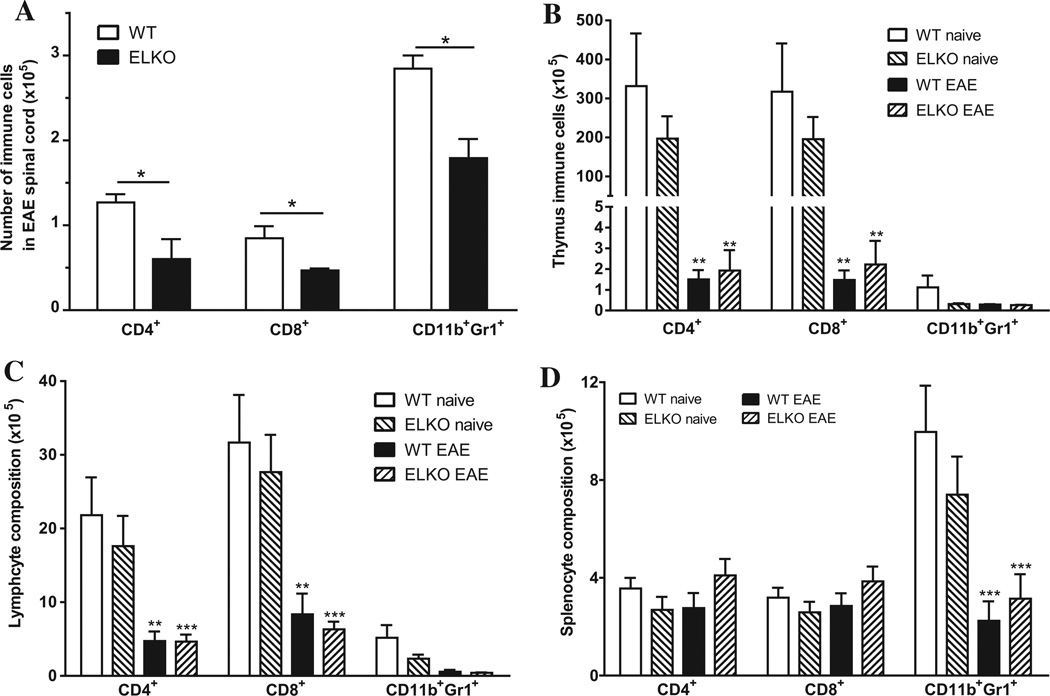
Subsequent validation focused on 4 groups of mice: WT or ELKO mice on day 17 after EAE induction, or their littermates left untreated (naïve) and studied on the same day (n = 3/group). Naïve mice were used as controls for this study to best detect potential differences in the immune profile possibly resulting from the embryonic ELKO mutation. The spinal cord from naïve mice did recover some infiltrating leukocytes. Fig. 6A shows that both WT and ELKO mice with EAE had substantial infiltration of CD4, CD8 T cells, and CD11b+Gr1+ granulocytes. Two-way ANOVA showed that both strain [F(1,12) = 27.4, p < 0.005] and cell type [F(2,12) = 59.1, p < 0.005] affected the outcome; post hoc tests confirmed significant reduction of the number of all three types of cells in the ELKO group compared with the WT mice with EAE. By contrast, there was no difference between the WT and ELKO groups in peripheral immune organs, either in naïve mice or EAE induction. In the thymus, there was a reduction of CD4 and CD8 T cells (p < 0.01 for each) as a result of EAE (Fig. 6B). The lymph nodes also showed reduction of CD4 (p < 0.01) and CD8 (p < 0.001) (Fig. 6C). The number of CD11b+Gr1+ granulocytes was not affected in either thymus or lymph nodes, but was decreased in splenocytes from the EAE mice in comparison with respective naïve controls (p < 0.01, Fig. 6D). Altogether, the results indicate that the difference in infiltrated cells between the ELKO and WT spinal cord was not caused by altered peripheral immune cell composition.
Fig. 6.
Effect of ELKO and EAE on the number of three major cell populations. (A) Among cells recovered from spinal cord 17 days after EAE induction, there were fewer CD4+, CD8+, and CD11b+Gr1+ cells in the ELKO group than in the WT group. (B) In the thymus, EAE (day 17 after induction) reduced the number of CD4+ and CD8+ T cells without affecting the CD11b+Gr1+ granulocytes. There was no difference between ELKO and WT, either in naïve or EAE mice. (C) The lymph nodes showed the same pattern of changes as the thymus. (D) Neither ELKO nor EAE affected the number of CD4+ and CD8+ T cells in the spleen. However, the number of CD11b+Gr1+ granulocytes was decreased by EAE, though ELKO had no additional effect (n = 3/group). *p < 0.05; **p < 0.01; ***p < 0.005.
3.5. ELKO mice show less elevation of the mRNA of selective chemokines and cytokines in enriched spinal cord microvessels
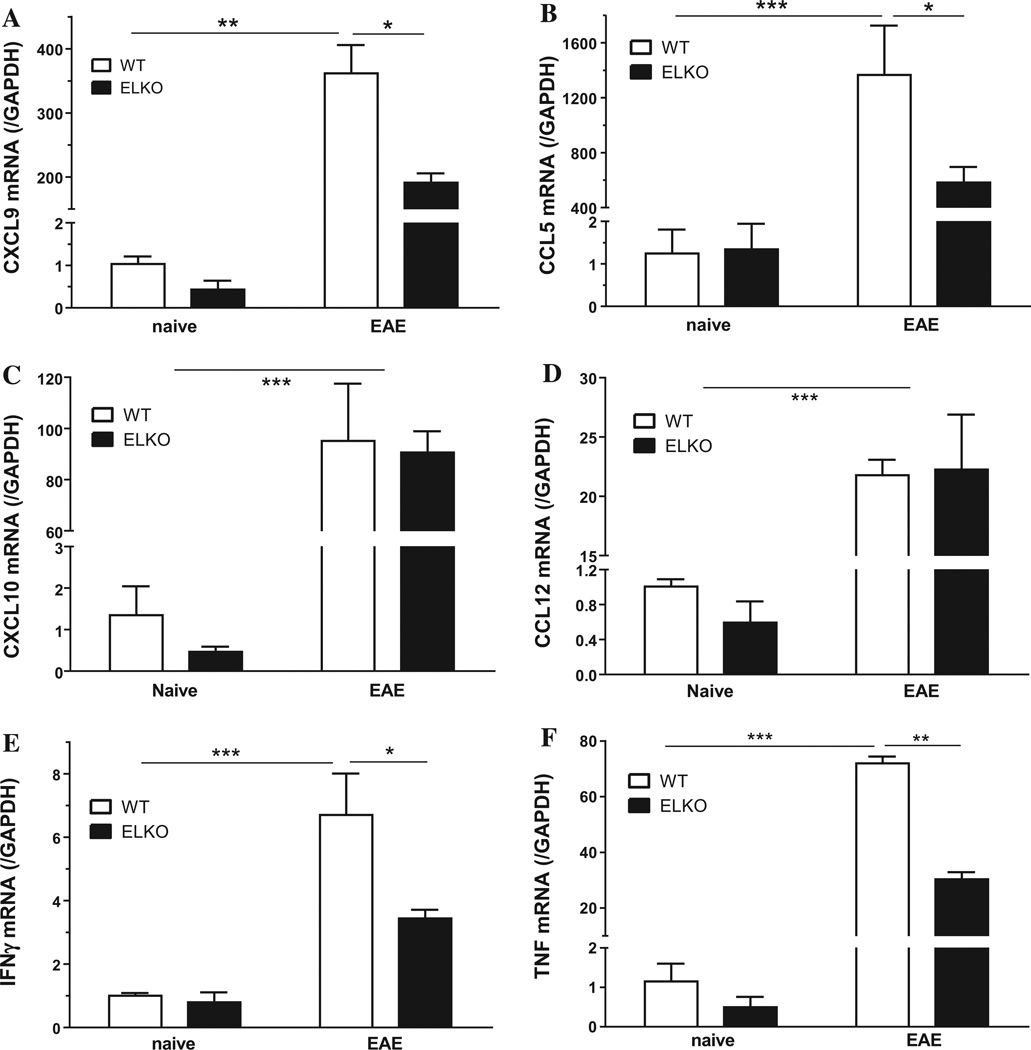
The type of leukocytes infiltrated into the spinal cord may be associated with upregulation of specific chemokines for the particular subtype of inflammatory cells. Therefore we compared four groups of enriched spinal cord microvessels: WT or ELKO mice, either on day 17 after EAE induction or their naive littermates studied at the same day (n = 3/group). Two-way ANOVA showed effects of both strain [F(1,7) = 26.6, p < 0.005] and EAE [F(1,7) = 275.8, p < 0.0001] on C-X-C motif ligand 9 (CXCL9). Similar effects were seen for C–C motif ligand 5 (CCL5, or RANTES), TNF, and IFNγ, but only an EAE effect on CXCL10 and CCL12. Post-hoc analysis showed there was less increase of mRNA in the ELKO EAE mice than the WT EAE mice for CXCL9 (Fig. 7A) and CCL5 (Fig. 7B). By contrast, there was no difference between the WT and ELKO EAE groups in the mRNA expression of CXCL10 (Fig. 7C) and CCL12 (Fig. 7D).
Fig. 7.
Effects of both EAE and the ELKO mutation on chemokine and cytokine mRNA expression in enriched spinal cord microvessels. (A) CXCL9 and (B) CCL5 were increased on day 17 after EAE induction in comparison with naïve controls. The increase in the ELKO group with EAE was less pronounced than that in the WT group with EAE. (C) CXCL10 and (D) CCL12 were increased by EAE but there was no difference between WT and ELKO groups. (E) IFNγ and (F) TNF showed upregulation in EAE microvessels; the increase was more in WT group and less pronounced in the ELKO group (n = 3/group). *p < 0.05; **p < 0.01; ***p < 0.005.
Besides chemokines, cell adhesion molecules can also be upregulated in EAE and facilitate the infiltration of inflammatory cells across the BBB and BSCB. Both inducible cell adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM) had increased mRNA expression as a result of EAE (p < 0.01), but there was no difference between the WT and ELKO mice (data not shown).
Proinflammatory cytokines also play essential roles in EAE progression. In enriched spinal cord microvessels studied on day 17, both EAE and strain had significant effects on the mRNA for interferon (IFN)-γ (Fig. 7E). In WT mice, there was an increase of IFN-γ mRNA by EAE in comparison with the naïve group (p < 0.005). The change was less pronounced in the ELKO mice, which showed a lower level of IFN-γ mRNA than WT mice after EAE (p < 0.05). Similarly, TNF mRNA was increased by EAE, and the increase in WT mice was higher than in ELKO mice with EAE (p < 0.01) as well as naïve WT controls (p < 0.005) (Fig. 7F).
3.6. Effect of ELKO on tight junction protein expression after EAE
Four groups of mice were studied (n = 3/group): WT and ELKO mice on day 17 after EAE induction, or their littermates studied on the same day (naïve control). Two-way ANOVA showed that both strain and EAE affected the mRNA expression of tight junction proteins in enriched spinal cord microvessels. There was a significant effect of EAE on mRNA of most TJ proteins measured from spinal cord microvessels, including TJ-associated adaptor proteins zona occludin (ZO)-1 (Fig. 8A), major TJ complex components occludin (Fig. 8B), claudin-1 (Fig. 8C), claudin-2 (Fig. 8D), and claudin-5 (Fig. 8F). However, claudin-3 was unchanged (Fig. 8E). For ZO-1 and occludin, a significant strain effect was also present. In WT mice, EAE induced a significant decrease of mRNA for all of these tight junction proteins (p < 0.005). In ELKO mice, there was also a reduction, but the levels of ZO-1 and occludin remained higher in ELKO mice with EAE than in WT mice with EAE (p < 0.05). Such strain difference was not seen for claudin-1, claudin-2, claudin-3, and claudin-5.
Fig. 8.
Differential effects of EAE and ELKO mutation were seen on mRNA expression of tight junction proteins in spinal cord microvessels. ZO-1 (A) and occludin (B) were decreased by EAE on day 17. The reduction was less severe in the ELKO than WT mice with EAE. Claudin-1 (C), claudin-2 (D), and claudin-5 (F) showed an effect of EAE but not of the ELKO mutation by 2-way ANOVA. Claudin-3 (E) did not show a difference induced by EAE or ELKO. *p < 0.05 for post hoc comparison between ELKO EAE and WT EAE.
4. Discussion
Leptin receptors in the endothelial cells of the BBB and BSCB are known to mediate leptin transport from blood to the CNS. However, the roles of endothelial leptin signaling in EAE progression have not been addressed. Results from this study show that leptin receptors at the BSCB show dynamic changes with an initial elevation preceding EAE onset and subsequent attenuation. In ELKO mice lacking endothelial leptin signaling, there was less disease burden, impairment of BSCB function, leukocyte infiltration, and inflammation at the level of BSCB. The results indicate that endothelial leptin signaling contributes to the worsening of EAE.
To determine the endothelial specific effect of leptin signaling in response to EAE, we used ELKO mice derived from two generations of cross-breeding of Tie2-Cre mice and ObR-floxed mice (Hsuchou et al., 2011). The Tie2 promoter also drives the expression of hematopoietic cells in the embryonic stage (Koni et al., 2001), and has been widely used in both vascular endothelial studies and immunological studies (Kondo et al., 2004; Bao et al., 2010; Tang et al., 2010). Although endothelial expression is less exclusive than the strategy of using an inducible Tie2-Cre transgenic mouse line (Constien et al., 2001; Forde et al., 2002), there is no perfect strategy for endothelial gene targeting in EAE studies. This is because estrogen and inflammation are both critical factors in EAE pathology; tamoxifen, doxycycline, and other related inducing agents provoke gene expression that can affect the course of EAE, making the design of control experiments extremely tricky and difficult. Since we focused on the BSCB and used the complementary approach of ex-vivo biochemical assays, we felt the most suitable approach was to use Tie2-Cre mice to drive the excision of ObR. The ObR-floxed mice from the Chua lab (McMinn et al., 2004) have loxP sites flanking exon17 of the leptin receptor which encodes the membrane juxtapositional cytoplasmic domain. As a result, the ELKO mice produce a membrane-bound mutant ObR without signaling properties, along with a soluble leptin receptor in endothelia, whereas ObR expression in CNS cells is not affected. The specificity of ELKO has been verified by BBB transport assays (Hsuchou et al., 2011) and studies with isolated microvessels (Pan et al., 2012), and the metabolic phenotype of the ELKO mice has also been characterized (Pan et al., 2012). Flow cytometric analysis ruled out differences in peripheral immune profile in the WT and ELKO mice either before or after EAE induction (Fig. 6). The result suggests that ELKO mice serve as an appropriate model for the EAE and BSCB studies. However, adoptive transfer of T cells or bone marrow chimeras would be the best approach to unequivocally rule out the potential contribution of leukocyte ObR to EAE pathogenesis.
In wildtype mice, a blood leptin surge (transient hyperleptinemia) has been shown to precede the onset of EAE and correlate with the development of pathogenic T cell response (Sanna et al., 2003). Leptin potentiates (Matarese et al., 2001b, 2005), whereas anti-leptin treatment attenuates (De Rosa et al., 2006) the autoimmune response in EAE. The persistence of hyperleptinemia would therefore predict a worsening of disease. However, ELKO mice show lower EAE scores, coinciding with attenuated immune cell infiltration and inflammatory response. The hyperleptinemia in the ELKO mice is caused by reduced tissue uptake and degradation, in association with elevated soluble leptin receptor (Hsuchou et al., 2013a). Since hyperleptinemia in the ELKO mice did not translate into an exaggerated autoimmune response, the results suggest that the response at the BBB and within the CNS is more important for determination of outcome after EAE challenge. Since persistent hyperleptinemia in the ELKO mice lacks the predicted consequence of worsening of EAE, the results strongly support a critical role of BBB and BSCB endothelial leptin signaling in promoting EAE.
Impaired function of the BBB and BSCB is a key feature in EAE, and it correlates with disease severity (Fabis et al., 2007; Bennett et al., 2010). The EAE scores for the ELKO mice were lower than for the WT mice during the peak of the disease, although there was no change in disease incidence or in the onset or resolution of the symptoms. The reduction of score shows a reduced disease burden. We have shown that leptin concentrations are higher in the ELKO mice (Hsuchou et al., 2013a). This pattern remained the same after EAE (Fig. 2B). Thus, downregulation of leptin receptors at the BSCB and the increase of serum-antagonizing sObR might provide partial compensation to overcome the inflammatory effects of leptin. Leptin has diverse immune regulatory effects on peripheral immune cells (La Cava and Matarese, 2004; Matarese et al., 2010), and the changes of peripheral immune activation may alter CNS neuropathology. Since ELKO mice had a similar time course of disease as WT mice after EAE induction, this suggests that endothelial leptin signaling plays a role in the initial increase of BSCB permeability and leukocyte migration into the CNS, rather than at the stage of resolution of EAE.
Since the CFA control group contains pertussis toxin, it is possible that the permeability of the BSCB in these mice could have been higher than in naïve controls. Correlating with an increased paracellular permeability of the BSCB shown by sodium fluorescein extravasation, there was an increase of cell accumulation in venules, pia matter, and spinal cord parenchyma, particularly in the peripheral white matter of both ventral and dorsal horns. Many of the DAPI (+) exogenous cells (not intrinsic to spinal cord) were CD45+ leukocytes, including CD3+ T cells. The difference of cell infiltration between the ELKO and WT groups seen by immunohistochemistry was further confirmed by flow cytometry. There were differences in immune cell profiles among leukocytes recovered from spinal cord homogenates collected after vascular washout to remove the loosely adherent cells in large vessels. In comparison with WT mice, there was a reduction in the percentage of CD4+ T cells and CD11b+Gr1+ granulocytes (mainly neutrophils) in ELKO mice on day 13 of EAE. On day 17, the differences of both cells were no longer present, but CD8+ T cells showed a lower percentage in ELKO mice. There was also a reduction of the total number of infiltrated CD4+ and CD8+ T cells. Since a perfusion procedure was applied to clear the vascular space in both IHC and flow cytometry studies, the increase mainly reflects those cells that had already infiltrated the BSCB and reached spinal cord parenchyma.
As expected, EAE increased the expression of chemokines, proinflammatory cytokines, and cell adhesion molecules measured in enriched spinal cord microvessels, and reduced the mRNA for immune regulatory cytokines and TJ proteins. Cerebral microvessels are a major source of production of chemokines and cytokines in inflammatory states (Tripathy et al., 2010). In ELKO mice, however, selective attenuation of the changes was seen for many of these molecules. The smaller elevation of CCL5 and CXCL9 correlated with reduced recruitment of T cells and granulocytes. The smaller increase of IFNγ and TNF in the microvessels of ELKO mice indicates a lower level of inflammation, and the smaller reduction of ZO-1 and occludin correlated with better preserved paracellular permeability of the BSCB. We did not detect an effect of ELKO on the mRNA expression of claudin-1 or claudin-2. The mRNAs for claudin-1 and −2 were detected, contrary to a previous report that claudin-1 is not present in normal BBB though its overexpression in endothelia is shown to provide partial protection against EAE (Pfeiffer et al., 2011). Possible explanations include a non-endothelial source of claudin-1 in the enriched microvessel preparation, and its inducible expression by EAE. For example, astrocytes also express tight junction proteins including claudin-1 and occludin. Overall, differential regulation of components of the tight junction and adherent junction complex is seen in different disease processes (Hawkins et al., 2004; Huber et al., 2001; Liebner et al., 2000a). Since peripheral immune profiles did not change, the attenuated inflammatory changes and better preserved BSCB permeability were the major causes of the lower cell infiltration and EAE scores in ELKO mice with EAE.
Endothelial ObR serve as both transporting and signaling receptors. ObRa is more abundant than ObRb at the BBB level (Hileman et al., 2002; Pan et al., 2008b); thus, leptin-induced signaling is probably driven by STAT1 and MAPK activation rather than by STAT3. The ELKO mice still express a mutant, membrane-bound receptor with a very short cytoplasmic tail (that does not possess signaling domains). Thus, transport functions persist, as shown in our previous experiments (Hsuchou et al., 2011). The main difference between ELKO and WT lies in an absence of endothelial signaling. This appears to have a direct effect of TJ protein expression and function.
In summary, this is the first report that endothelial leptin signaling modulates the permeability of the BSCB, infiltration of leukocytes, and key molecules controlling BSCB function. The novel evidence from ELKO mice shows that endothelial leptin signaling enhances inflammation and BSCB dysfunction during EAE.
Acknowledgment
Grant support was provided by NIH (DK54880 and DK62249 to AJK, and NS62291 to WP). The ObR-floxed mice used for endothelial excision of exon 17 originated from Dr. Streamson Chua Jr. (Albert Einstein Medical College) and was cross-bred to pure C57 strain background by Dr. Silvana Obici’s lab (University of Vermont). Cryosectioning and flow cytometry analysis were performed in the Cell Biology and Bioimaging core facility with a service charge.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim. Biophys. Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Bao X, Moseman EA, Saito H, Petryniak B, Thiriot A, Hatakeyama S, Ito Y, Kawashima H, Yamaguchi Y, Lowe JB, von Andrian UH, Fukuda M. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity. 2010;33:817–829. doi: 10.1016/j.immuni.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Basivireddy J, Kollar A, Biron KE, Reickmann P, Jefferies WA, McQuaid S. Blood-brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. J. Neuroimmunol. 2010;229:180–191. doi: 10.1016/j.jneuroim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Constien R, Forde A, Liliensiek B, Grone HJ, Nawroth P, Hammerling G, Arnold B. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis. 2001;30:36–44. doi: 10.1002/gene.1030. [DOI] [PubMed] [Google Scholar]
- De Rosa V, Procaccini C, La CA, Chieffi P, Nicoletti GF, Fontana S, Zappacosta S, Matarese G. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J. Clin. Invest. 2006;116:447–455. doi: 10.1172/JCI26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Spuch C, Antequera D, Rodal I, de Yebenes JG, Molina JA, Bermejo F, Carro E. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol. Aging. 2008;29:902–912. doi: 10.1016/j.neurobiolaging.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Fabis MJ, Scott GS, Kean RB, Koprowski H, Hooper DC. Loss of blood–brain barrier integrity in the spinal cord is common to experimental allergic encephalomyelitis in knockout mouse models. Proc. Natl. Acad. Sci. USA. 2007;104:5656–5661. doi: 10.1073/pnas.0701252104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde A, Constien R, Grone HJ, Hammerling G, Arnold B. Temporal Cre-mediated recombination exclusively in endothelial cells using Tie2 regulatory elements. Genesis. 2002;33:191–197. doi: 10.1002/gene.10117. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood–brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Hileman SM, Pierroz DD, Masuzaki H, Bjorbaek C, El Haschimi K, Banks WA, Flier JS. Characterization of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–783. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009a;132:889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Jayaram B, Kastin AJ, Wang Y, Ouyang S, Pan W. Endothelial cell leptin receptor mutant mice have hyperleptinemia and reduced tissue uptake. J. Cell. Physiol. 2013a;228:1610–1616. doi: 10.1002/jcp.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Kastin AJ, Tu H, Markadakis EN, Stone KP, Wang Y, Heymsfield SB, Chua SC, Jr., Obici S, Magrisso IJ, Pan W. Effects of cell type-specific leptin receptor mutation on leptin transport across the BBB. Peptides. 2011;32:1392–1399. doi: 10.1016/j.peptides.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Mishra PK, Kastin AJ, Wu X, Wang Y, Ouyang S, Pan W. Saturable leptin transport across the BBB persists in EAE mice. J. Mol. Neurosci. 2013b;51:364–370. doi: 10.1007/s12031-013-9993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, Wu X, Kastin AJ. Cessation of blood-to-brain influx of interleukin-15 during development of EAE. J. Cereb. Blood Flow Metab. 2009b;29:1568–1578. doi: 10.1038/jcbfm.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- Juhler M, Barry DI, Offner H, Konat G, Klinken L, Paulson OB. Blood–brain and blood–spinal cord barrier permeability during the course of experimental allergic encephalomyelitis in the rat. Brain Res. 1984;302:347–355. doi: 10.1016/0006-8993(84)90249-x. [DOI] [PubMed] [Google Scholar]
- Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J. Pathol. 2003;201:319–327. doi: 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- Kondo T, Hafezi-Moghadam A, Thomas K, Wagner DD, Kahn CR. Mice lacking insulin or insulin-like growth factor 1 receptors in vascular endothelial cells maintain normal blood–brain barrier. Biochem. Biophys. Res. Commun. 2004;317:315–320. doi: 10.1016/j.bbrc.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J. Exp. Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrimbux D, Gaffen Z, Farrell CL, Martin D, Thomas SA. The involvement of the blood–brain and the blood–cerebrospinal fluid barriers in the distribution of leptin into and out of the rat brain. Neuroscience. 2004;123:527–536. doi: 10.1016/j.neuroscience.2003.08.061. [DOI] [PubMed] [Google Scholar]
- La Cava A, Matarese G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000a;100:323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- Liebner S, Kniesel U, Kalbacher H, Wolburg H. Correlation of tight junction morphology with the expression of tight junction proteins in blood–brain barrier endothelial cells. Eur. J. Cell Biol. 2000b;79:707–717. doi: 10.1078/0171-9335-00101. [DOI] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Matarese G, Carrieri PB, La CA, Perna F, Sanna V, De RV, Aufiero D, Fontana S, Zappacosta S. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Carrieri PB, Montella S, De RV, La CA. Leptin as a metabolic link to multiple sclerosis. Nat. Rev. Neurol. 2010;6:455–461. doi: 10.1038/nrneurol.2010.89. [DOI] [PubMed] [Google Scholar]
- Matarese G, Di GA, Sanna V, Lord GM, Howard JK, Di TA, Bloom SR, Lechler RI, Zappacosta S, Fontana S. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J. Immunol. 2001a;166:5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- Matarese G, Sanna V, Di Giacomo A, Lord GM, Howard JK, Blood SR, Lechler RI, Fontana S, Zappacosta S. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur. J. Immunol. 2001b;31:1324–1332. doi: 10.1002/1521-4141(200105)31:5<1324::AID-IMMU1324>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Matarese G, Sanna V, Di Giacomo A, Lord GM, Howard JK, Bloom SR, Lechler RI, Fontana S, Zappacosta S. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur. J. Immunol. 2001c;31:1324–1332. doi: 10.1002/1521-4141(200105)31:5<1324::AID-IMMU1324>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Dragatsis I, Dietrich P, Ludwig T, Eiden S, Chua SC., Jr. An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm. Genome. 2004;15:677–685. doi: 10.1007/s00335-004-2340-1. [DOI] [PubMed] [Google Scholar]
- Mishra PK, Hsuchou H, Ouyang S, Kastin AJ, Wu X, Pan W. Loss of astrocytic leptin signaling worsens experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2013;34:98–107. doi: 10.1016/j.bbi.2013.07.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang S, Hsuchou H, Kastin AJ, Wang Y, Yu C, Pan W. Diet-induced obesity suppresses expression of many proteins at the blood–brain barrier. J. Cereb. Blood Flow Metab. 2014;34:43–51. doi: 10.1038/jcbfm.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Banks WA, Kennedy MK, Gutierrez EG, Kastin AJ. Differential permeability of the BBB in acute EAE: enhanced transport of TNF-α. Am. J. Physiol. 1996;271:E636–E642. doi: 10.1152/ajpendo.1996.271.4.E636. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Cornelissen-Guillaume GG, Jayaram B, Wang Y, Tu H, Halberg F, Wu X, Chua SC, Jr., Kastin AJ. Endothelial leptin receptor mutation provides partial resistance to diet-induced obesity. J. Appl. Physiol. 2012;112:1410–1418. doi: 10.1152/japplphysiol.00590.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology. 2008a;149:2798–2806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Tu H, Kastin AJ. Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology. 2008b;149:877–885. doi: 10.1210/en.2007-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Diurnal variation of leptin entry from blood to brain involving partial saturation of the transport system. Life Sci. 2001;68:2705–2714. doi: 10.1016/s0024-3205(01)01085-2. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Evolution of neuropeptide concepts illustrated by MIF-1 and MSH. In: Shioda S, Homma I, Kato N, editors. Transmitters and Modulators in Health and Disease. Tokyo: Springer; 2009. pp. 3–17. [Google Scholar]
- Pan W, Stone KP, Hsuchou H, Manda VK, Zhang Y, Kastin AJ. Cytokine signaling modulates blood–brain barrier function. Curr. Pharm. Des. 2011;17:3729–3740. doi: 10.2174/138161211798220918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Yu C, Hsuhou H, Khan RS, Kastin AJ. Cerebral microvascular IL15 is a novel mediator of TNF action. J. Neurochem. 2009;111:819–827. doi: 10.1111/j.1471-4159.2009.06371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F, Schafer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood–brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–614. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino PA, Cardona AE. Isolation of brain and spinal cord mononuclear cells using percoll gradients. J. Vis. Exp. 2011 doi: 10.3791/2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna V, Di GA, La CA, Lechler RI, Fontana S, Zappacosta S, Matarese G. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J. Clin. Invest. 2003;111:241–250. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Harrington A, Yang X, Friesel RE, Liaw L. The contribution of the Tie2+ lineage to primitive and definitive hematopoietic cells. Genesis. 2010;48:563–567. doi: 10.1002/dvg.20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D, Yin X, Sanchez A, Luo J, Martinez J, Grammas P. Cerebrovascular expression of proteins related to inflammation, oxidative stress and neurotoxicity is altered with aging. J. Neuroinflammation. 2010;7:63. doi: 10.1186/1742-2094-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J. Cell. Physiol. 2008;214:301–305. doi: 10.1002/jcp.21195. [DOI] [PubMed] [Google Scholar]
- van Dongen JJ, Krissansen GW, Wolvers-Tettero IL, Comans-Bitter WM, Adriaansen HJ, Hooijkaas H, van Wering ER, Terhorst C. Cytoplasmic expression of the CD3 antigen as a diagnostic marker for immature T-cell malignancies. Blood. 1988;71:603–612. [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood–brain barrier: development, composition and regulation. Vascul. Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote E-H, Risau W, Engelhardt B. Localization of claudin-3 in tight junctions of the blood–brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- Wu X, Mishra PK, Hsuchou H, Kastin AJ, Pan W. Upregulation of astrocytic leptin receptor in mice with experimental autoimmune encephalomyelitis. J. Mol. Neurosci. 2013;49:446–456. doi: 10.1007/s12031-012-9825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Pan W, He Y, Hsuchou H, Kastin AJ. Cerebral interleukin-15 shows upregulation and beneficial effects in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2010;223:65–72. doi: 10.1016/j.jneuroim.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]