Abstract
Purpose
To determine if intra-patient dose escalation of the multi-targeted kinase inhibitor sorafenib is feasible in patients with advanced pretreated solid malignancies.
Methods
An intra-patient dose escalation scheme starting at 400 mg BID was employed in this prospective trial. Doses were escalated to 600 mg BID for the second cycle and to 800 mg BID for the third cycle in the absence of grade 3+ adverse events. In the event of grade 3+ adverse events during cycle 1, doses were reduced to 400 mg daily through cycle 2. Dose re-escalation for cycle 3 was allowed in the absence of grade 3+ adverse events during cycle 2. Further dose escalation was prohibited. The primary endpoint was the overall percentage of patients tolerating dose escalation to 600 mg BID through cycle 2 or tolerating re-escalation to 400 mg BID through cycle 3.
Results
Fifty eligible patients with various solid tumors and a median of 3 prior therapies were enrolled. Eleven patients (22%) tolerated primary dose escalation or re-escalation. Only 14 patients (28%) completed cycle 1 without dose modification or discontinuing treatment. Seven of 13 patients tolerated primary dose escalation through cycle 2. Four of 5 patients tolerated dose re-escalation through cycle 3. Reasons for escalation failure included tumor progression (42%) and adverse events (26%). Common grade 3+ adverse events included hand-foot skin reaction, hypertension, and hypophosphatemia.
Conclusions
Intra-patient dose escalation and/or re-escalation of sorafenib were not feasible in pretreated solid tumor patients. Sorafenib dose escalation remains an investigational approach.
Keywords: dose escalation, dose re-escalation, dose-toxicity relationship, maximum tolerated dose, sorafenib
INTRODUCTION
Sorafenib is an oral small-molecule multi-kinase inhibitor originally developed as a serine/threonine Raf kinase inhibitor. It also inhibits other kinases including the vascular endothelial growth factor receptor (VEGFR) and the rearranged during transfection (RET) receptor, amongst others [1]. Four separate phase I dose-escalation trials of sorafenib using different dose schedules were performed in refractory advanced solid tumors [2-5]. Based on the development of dose limiting skin toxicity, diarrhea, and fatigue at higher doses, 400 mg twice daily was selected for further study [6]. Common toxicities observed in phase III trials with sorafenib included skin rash, hand-foot skin reaction, hypertension, and diarrhea [7,8]. While the approved dosing schedule of 400 mg orally twice daily is now widely used, it has been suggested that the dose and schedule of sorafenib can still be optimized for individual patients [9].
Many patients taking sorafenib remain on chronic therapy with stable or responding disease and tolerable toxicities. Indeed, many patients do not develop significant toxicity at the approved dose [6-8]. Although there are limited data regarding the cumulative toxicities of prolonged sorafenib therapy, there is anecdotal experience that some patients appear to have diminished toxicity with continued exposure [10]. However, the rate of development of tachyphylaxis to sorafenib's early toxicities is incompletely explored. It is also unclear whether there is clinical value to re-escalating the dose of sorafenib back to its prior level in patients who subsequently tolerate a reduced dose.
In 2007, the first results of an intra-patient sorafenib dose escalation trial in advanced renal cell cancer were presented [11]. Efficacy data were presented for 44 evaluable patients treated with 400 mg twice daily of sorafenib on days 1 through 28, followed by 600 mg twice daily (days 29 through 56) and 800 mg twice daily on days 57 and beyond. Ninety-one percent of patients were escalated to total daily doses of 1,200 mg or 1,600 mg. An unprecedented eight patients (18%) had a complete radiographic response, while 14 patients (32%) had partial responses and another 14 patients had stable disease for 3 months or longer. Similar results were presented from an expanded cohort and in a similar but separate small phase II clinical trial in renal cell carcinoma [12,13]. To date, there are no data exploring the possibility of whether the intra-patient dose escalation of sorafenib increases its efficacy in other solid tumors.
In patients with refractory advanced solid tumors, we designed this study to test the feasibility of 1) escalating the dose of sorafenib in patients who tolerate the FDA-approved dose for four weeks (primary intra-patient dose escalation) and 2) re-escalating the dose back to the original dose of 400 mg twice daily in those who required a dose reduction due to toxicity but tolerated the reduced dose for at least four weeks (dose re-escalation).
PATIENTS AND METHODS
Patients
Patients aged 18 years and older with refractory solid tumors were eligible. Patients with measurable or non-measurable disease on imaging studies performed within 28 days of registration were eligible and were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2. Any number of prior treatments was allowed, as long as treatment was completed at least two weeks prior to registration and all toxicities had resolved to grade 1 or less. Further inclusion criteria included ability to give informed consent, ability to take oral medication, and acceptable end-organ function defined by an absolute neutrophil count ≥ 1,500/mm3, hemoglobin ≥ 9.0g/dl, platelet count ≥ 100,000/mm3, total bilirubin ≤ 1.5 times the upper limit of normal (ULN), ALT and AST ≤ 2.5 times the ULN (≤ 5 × ULN for patients with liver involvement), and creatinine ≤ 1.5 times ULN. Patients on warfarin or low molecular-weight heparin treatment were allowed provided they were on a stable dose and had no evidence of bleeding.
Exclusion criteria included any prior treatment with sorafenib or sunitinib and treatment with bevacizumab within the prior 3 months. Patients were excluded for significant cardiac comorbidities including uncontrolled hypertension defined as systolic blood pressure > 150 mmHg or diastolic blood pressure > 90 mmHg despite optimal medical management, New York Heart Association class II or greater congestive heart failure, recent (within past 6 months) myocardial infarction, unstable angina, or ventricular arrhythmia requiring anti-arrhythmic therapy. Further exclusion criteria included thrombolic or embolic events within the past 6 months and evidence of bleeding diathesis or coagulopathy. Patients with Common Terminology Criteria for Adverse Events (CTCAE) grade 2 or higher bleeding events, major surgery, open biopsy, or significant traumatic injury within the prior 4 weeks were also excluded, as were patients with non-healing wound, ulcer, or bone fracture or clinically serious active infection. Due to the death of a patient from pulmonary hemorrhage, the protocol was amended after 18 patients to exclude non-small cell lung cancer with any component of squamous cell carcinoma.
The study protocol was reviewed and approved by the institutional review board of the University of California, Davis. All patients gave written informed consent before treatment.
Study Procedures
Prior to registration, patients underwent a history and physical examination that including assessment of height, weight, ECOG performance status, vital signs, and a pregnancy test for all females of child-bearing potential. Although measurable disease was not required, radiographic tumor assessment was required within 4 weeks of registration. Tumor measurements were subsequently performed every 2 cycles, and responses were evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) criteria [14]. History and physical examination were performed by the treating physician every 4 weeks. Blood pressure was measured weekly and toxicity was monitored continuously through the trial using the CTCAE version 3.0.
Treatment Plan
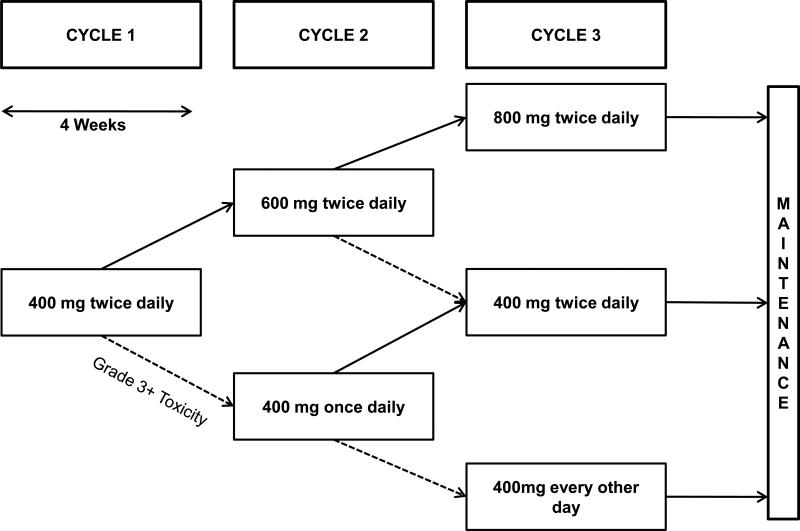
Eligible patients initiated treatment with sorafenib at 400 mg orally twice daily for the first 28-day cycle. Doses were escalated to 600 mg twice daily for the second and 800 mg twice daily for the third cycle if there were no CTCAE grade 3 or higher adverse events in the preceding cycle. If grade 3 or higher toxicity developed at any point in cycle 1, the dose was reduced to 400 mg daily once toxicity had resolved to grade 1 or less and given for the duration of cycle 1 and throughout cycle 2. If no further grade 3 or higher toxicities were observed, the patient was re-escalated to 400 mg twice daily for cycle 3. After cycle 3, no further dose escalation was allowed and dose modification in the maintenance phase followed the package insert. The study design is summarized graphically in Figure 1. Because a secondary intention of the trial was to explore whether intra-patient dose escalation or re-escalation might result in enhanced activity, patients could continue on treatment until progression of target tumor dimensions of up to 100% over baseline or the development of new lesions.
Fig. 1.
Study Schema. Patients were treated at the dose levels described with cycle length defined as four weeks. Dashed arrows refer to dose de-escalation as the result of grade 3 or higher toxicity. Solid arrows refer to dose escalation which was performed during the first 3 cycles if a patient tolerated a dose level for 28 or more days without grade 3 or higher toxicity
Statistical Analysis
The primary endpoint of the trial was an estimate of the overall percentage of patients tolerating a dose escalation to 600 mg twice daily for 28 days plus the percentage tolerating a re-escalation to 400 mg twice daily in cycle 3. We assumed that a dose escalation/re-escalation rate of 70% would be worthy of further study and that a dose escalation/re-escalation rate of 50% or less would not be worthy of further investigation. At a planned sample size of 51 patients and 5% significance, this study had 90% power to differentiate a dose escalation/re-escalation rate of 70% versus 50%. The secondary endpoints of the trial were toxicities, overall response rate (complete or partial response) according to RECIST criteria, and progression free survival (PFS). Numbers and types of toxicities are summarized descriptively. PFS is summarized by life-table based estimates of median times. All patients who received at least one dose of sorafenib were included in these analyses.
RESULTS
Baseline Characteristics
Between December 2008 and October 2009, fifty-one patients with a variety of advanced solid rumors were enrolled. Fifty patients who were eligible to begin treatment and took at least one dose of study drug were included in this analysis. Patients had been treated with a median of 3 prior lines of systemic treatment but had preserved performance status (ECOG 0 or 1 in 84%). Thirty-one (62%) patients were female. The most common tumor types were non-small cell lung cancer (30%) and colorectal cancer (14%). Detailed demographic and clinical characteristics are provided in Table 1.
Table 1.
Patient Demographic and Clinical Characteristics (n = 50)
| Characteristic | n (%) |
|---|---|
| Age, years | |
| Median (range) | 61 (25-88) |
| Prior Lines of Therapy, n | |
| Median (range) | 3 (0-6) |
| ECOG Performance Status | |
| 0 | 14 (28) |
| 1 | 28 (56) |
| 2 | 8 (16) |
| Gender | |
| Female | 31 (62) |
| Male | 19 (38) |
| Race/Ethnicity | |
| White | 36 (72) |
| Asian | 7 (14) |
| Hispanic | 5 (10) |
| Black | 2 (4) |
| Tumor Site | |
| Non-small cell lung cancer | 15 (30) |
| Colorectal Cancer | 7 (14) |
| Head and neck cancer | 4 (8) |
| Pancreatic cancer | 3 (6) |
| Soft tissue sarcoma | 3 (6) |
| Hepatocellular cancer | 2 (4) |
| Differentiated thyroid cancer | 2 (4) |
| Gastric cancer | 2 (4) |
| Adenoid cystic carcinoma | 2 (4) |
| Prostate cancer | 2 (4) |
| Renal cell cancer | 1 (2) |
| Breast cancer | 1 (2) |
| Testicular cancer | 1 (2) |
| Mesothelioma | 1 (2) |
| Bladder cancer | 1 (2) |
| Melanoma | 1 (2) |
| Thymic carcinoma | 1 (2) |
| Ovarian cancer | 1 (2) |
Dose Escalation
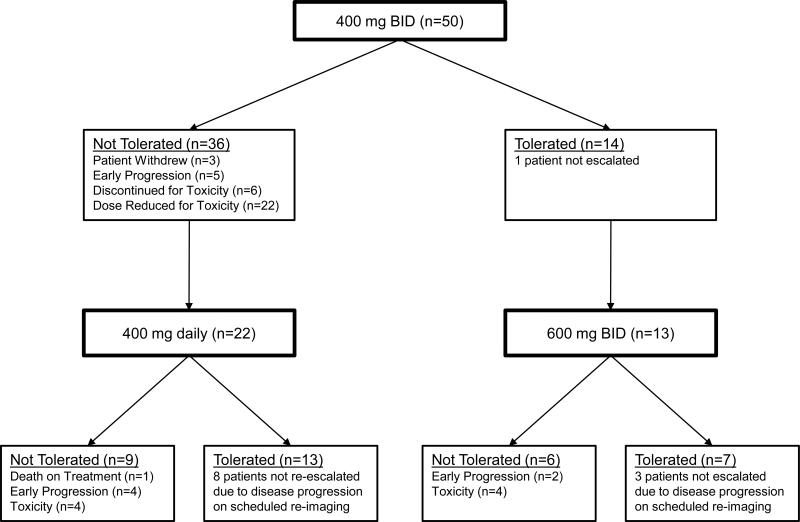
The primary endpoint of the trial was reached by eleven patients (22%). Completion and tolerance for each treatment cohort is summarized in Table 2. During cycle 1 at a dose of 400 mg twice daily, 28 patients required a dose reduction or treatment discontinuation for grade 3 or higher toxicity, 5 patients developed early progression and 3 patients withdrew (Figure 2). Only fourteen patients (28%) were able to complete cycle 1 without the development of dose modifying toxicity or discontinuing study treatment. Seven of thirteen patients (54%) who were treated at an escalated dose of sorafenib (600 mg twice daily) in cycle 2 tolerated it for 4 weeks. All four patients who were subsequently treated with 800 mg twice daily in cycle 3 tolerated that dose.
Table 2.
Dose Escalation Results by Treatment Cohort
| Cohort Total |
Tolerated |
|
|---|---|---|
| Treatment Cohort | n | n (row %) |
| Cycle 1: 400 mg twice daily | 50 | 14 (28%) |
| Cycle 2: 600 mg twice daily | 13 | 7 (54%)1 |
| Cycle 2: 400 mg once daily | 22 | 13 (59%) |
| Cycle 3: 800 mg twice daily | 4 | 4 (100%) |
| Cycle 3: 400 mg twice daily | 5 | 4 (80%)1 |
Tolerance of treatment by patients in these cohorts was the primary endpoint of this trial.
Fig. 2.
Flow of patients through the first two cycles of treatment on the study. Reasons for treatment failure at a given dose level are noted
Fifty-nine percent of the 22 patients treated at the reduced dose of 400 mg daily during cycle 2 completed this cycle of treatment without further grade 3 or higher toxicity or early progression. However, only 5 of these 13 patients were subsequently re-escalated to 400 mg twice daily in cycle 3. The primary reason for this lower rate is disease progression observed on repeat imaging performed after cycle 2. Nevertheless, 4 of these 5 (80%) patients tolerated dose re-escalation back to the 400 mg twice daily dose level.
For the entire trial population, the most common reasons for failure of protocol treatment were progression (42%) and adverse events (26%) (Table 3). Seven of the 18 patients (39%) who attempted dose escalation or re-escalation did not tolerate it, primarily due to adverse events at the escalated doses.
Table 3.
Reasons for Escalation Failure
| Entire Population (n=50) |
Escalation Attempted (n=18) |
|
|---|---|---|
| Category | n (%) | n (%) |
| Disease Progression | 21 (42) | 2 (11) |
| Adverse Events (Total) | 13 (26) | 5 (28) |
| Adverse Events at Escalated Dose | 5 (10) | 5 (28) |
| Withdrawal | 5 (10) | 0 (0) |
| Overall | 39 (78) | 7 (39) |
Efficacy
Treatment efficacy was exploratory in this mixed population of advanced solid tumors. Of the 34 patients who were evaluable for treatment response by RECIST, no responses were observed. Two patients with head and neck squamous cell cancers had evidence of tumor necrosis by radiographic studies and by physical examination. The best overall response was stable disease in 38% of evaluable patients. The development of new lesions was the reason for progression in 11 patients. The median progression free survival in this mixed refractory solid tumor cohort was 4.7 months (95% C.I. 4.3 – 6.1 months).
Toxicity
All fifty treated patients were eligible for toxicity assessment. Toxicity data are summarized in Table 4. Grade 3 or higher adverse events were observed in 64% of the patients. The principal grade 3 or higher non-hematologic treatment-related toxicities were hand-foot skin reaction in eleven patients (22%), hypophosphatemia in four patients (8%), hypertension in four patients (8%), and anorexia in three patients (6%). Common grade 1 or 2 non hematologic treatment-related toxicities were fatigue (34%), hand-foot skin reaction (32%), rash (28%), hypertension (26%), diarrhea (26%) and anorexia (20%). Hematologic toxicities were generally mild. A possibly attributable grade 5 pulmonary hemorrhage developed during cycle 2 of treatment in one patient with extensive endobronchial metastasis from adenoid cystic carcinoma being treated at 400 mg daily.
Table 4.
Major Treatment-Related Toxicities.
| Any Grade1 |
Grade 3-52 |
|||
|---|---|---|---|---|
| Adverse Event | N | % | N | % |
| Any | 42 | 84 | 32 | 64 |
| Blood/Bone Marrow | ||||
| Hemoglobin | 10 | 20 | 0 | 0 |
| Leukopenia | 7 | 14 | 0 | 0 |
| Lymphopenia | 11 | 22 | 3 | 6 |
| Thrombocytopenia | 3 | 6 | 0 | 0 |
| Cardiac | ||||
| Hypertension | 13 | 26 | 4 | 8 |
| Constitutional | ||||
| Fever | 5 | 10 | 0 | 0 |
| Fatigue | 17 | 34 | 1 | 2 |
| Weight Loss | 7 | 14 | 0 | 0 |
| Dermatologic/Skin | ||||
| Dry Skin | 5 | 10 | 0 | 0 |
| Hand-foot skin reaction | 16 | 32 | 11 | 22 |
| Pruritis | 3 | 6 | 0 | 0 |
| Rash | 14 | 28 | 2 | 4 |
| Gastrointestinal | ||||
| Anorexia | 10 | 20 | 3 | 6 |
| Constipation | 4 | 8 | 1 | 2 |
| Diarrhea | 13 | 26 | 2 | 4 |
| Mucositis | 6 | 12 | 1 | 2 |
| Nausea | 8 | 16 | 1 | 2 |
| Hemorrhage/Bleeding | ||||
| Pulmonary/Upper | ||||
| Respiratory | 2 | 4 | 2 | 4 |
| Lymphatics | ||||
| Edema | 3 | 6 | 0 | 0 |
| Metabolic/Laboratory | ||||
| Hypoalbuminemia | 4 | 8 | 2 | 4 |
| Alkaline phosphatase | 7 | 14 | 0 | 0 |
| AST | 5 | 10 | 0 | 0 |
| Bicarbonate, serum – low | 4 | 8 | 0 | 0 |
| Hyperbilirubinemia | 3 | 6 | 0 | 0 |
| Hyperkalemia | 3 | 6 | 1 | 2 |
| Hypokalemia | 7 | 14 | 2 | 4 |
| Hyponatremia | 5 | 10 | 1 | 2 |
| Hypophosphatemia | 9 | 18 | 4 | 8 |
| Musculoskeletal/Soft Tissue | ||||
| Muscle Weakness | 2 | 4 | 2 | 4 |
| Neurology | ||||
| Mood alteration | 4 | 8 | 0 | 0 |
| Pain | ||||
| Abdomen | 5 | 10 | 0 | 0 |
| Headache | 3 | 6 | 0 | 0 |
| Musculoskeletal | 5 | 10 | 1 | 2 |
| Other | 5 | 10 | 2 | 4 |
| Pulmonary/Upper Respiratory | ||||
| Voice changes | 3 | 6 | 0 | 0 |
Toxicities of any grade occurring in 3 or more individuals
Grade 3-5 toxicities occurring in 2 or more individuals
DISCUSSION
In this study of patients with heavily pre-treated advanced solid tumors, primary intra-patient dose escalation or dose re-escalation following a dose reduction was not feasible for the majority of patients. We observed substantial toxicity at the recommended dose and this severely limited the population eligible for dose escalation. Due to a combination of rapid disease progression and toxicities at standard doses, a very small percentage of patients enrolled were eligible for dose escalation or re-escalation. However, primary intra-patient dose escalation or dose re-escalation was successful in over half of those in whom it was attempted. Further research into the determinants of sorafenib-associated toxicities is needed to allow for the identification of patients who may potentially benefit from this strategy.
The spectrum of toxicities associated with sorafenib observed on this trial was generally consistent with those seen with the use of sorafenib in other settings; however, the frequency of grade 3 or higher toxicities was increased. For example, grade 3 hand-foot skin reactions were observed in 22% of patients in this study compared with 12% of patients in phase III TARGETs study of sorafenib in advanced RCC and 8% in the phase III SHARP trial in advanced HCC [7,8]. The high rate of severe toxicity at the recommended dose of sorafenib observed in our trial may be related to the fact that these patients had been heavily treated prior to enrollment into this trial. It is also consistent with several observational studies of sorafenib-associated toxicities in general clinical practice. Of 58 patients with advanced RCC treated in an expanded access nonrandomized treatment protocol at the Princess Margaret Hospital for a median of 7 months, CTCAE grade 3 adverse events were observed in 64% of patients with 62% requiring interruption of sorafenib dosing for toxicity [15]. Additionally, in a retrospective analysis of 24 unselected patients treated with sorafenib at the Medical College of Georgia, treatment interruption was required by 63% at a median of 2 weeks of initiation of treatment [16]. However, 38% of these sorafenib-treated patients in that cohort were successfully re-escalated to the starting dose at a median of 7 weeks from starting treatment.
Our results differ from preliminary results of intra-patient sorafenib dose escalation trials in patients with metastatic renal cell cancer [11-13]. The more homogeneous patient populations treated in those trials had been treated with a maximum of one prior regimen and tolerance of the FDA-approved regimen was excellent. While untreated patients with renal cell or hepatocellular carcinoma were eligible for this study, the majority of enrolled patients in this current trial had advanced solid tumors with several lines of previous treatment. Although we selected patients with preserved performance status and organ function and did not observe a difference in tolerance based on number of prior treatment regimens (data not shown), reduced tolerance to sorafenib as a result of prior therapy could explain the higher rates of toxicity observed in this trial. Moreover, the low tolerance of the starting dose of sorafenib in this trial limits comparison to prior dose escalation trials in metastatic renal cell cancer.
There are several other limitations of our study design. Drug levels were not measured as part of this study and therefore could not be correlated to drug tolerance; however, a clear relationship between drug exposure, dose, and drug-related adverse events was not observed in sorafenib's phase I testing [6,17]. Furthermore, while patients could continue with escalation or re-escalation with evidence of progression (up to 100% tumor growth, but no new lesions), the number of patients eligible for dose re-escalation was diminished by progression beyond these bounds. The efficacy of sorafenib as a single agent in this unselected population was minimal.
As in prior intra-patient dose escalation trials, we chose to start sorafenib at the current recommended dose, but in this trial we observed unexpected rates of toxicity during the first cycle of treatment. Whether initiating escalation after starting at a lower dose or waiting a longer period of time prior to escalation would be components of a more effective strategy is not known. Of note, a significant fraction of patients tolerated the higher dose when intra-patient dose escalation or re-escalation to a higher dose was attempted. Indeed, several patients tolerated the highest dose of sorafenib on this trial (1600 mg per day) without significant adverse events. Future studies should measure serum drug concentrations and search for biomarkers to select patients for dose escalation strategies. Our study does not exclude the possibility of success and benefit for this approach in carefully selected patients.
In conclusion, we did not meet our definition of feasibility for sorafenib dose escalation or dose re-escalation in this population of patients with refractory advanced solid tumors. The toxicities observed at standard doses in fit but pretreated patients were substantial. Future studies of this strategy should seek selection biomarkers and alternative study populations. Intra-patient sorafenib dose escalation and re-escalation after a toxicity-related dose reduction remain experimental approaches.
Acknowledgements
Research support provided by Bayer Healthcare/Onyx Pharmaceuticals and UC Davis Cancer Center Support Grant, P30CA093373-06. Study Identifier: NCT00810394. Presented in part at the 2010 ASCO Annual Meeting (Abstract #3055)
Footnotes
Disclosure: Dr. Lara received research funding from Bayer Healthcare and Onyx Pharmaceuticals for the conduct of this trial. There is no other conflict of interest disclosure.
REFERENCES
- 1.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. doi:64/19/7099 [pii] 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 2.Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, de Valeriola D, Strumberg D, Brendel E, Haase CG, Schwartz B, Piccart M. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92(10):1855–1861. doi: 10.1038/sj.bjc.6602584. doi:6602584 [pii] 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11(15):5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. doi:11/15/5472 [pii] 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 4.Moore M, Hirte HW, Siu L, Oza A, Hotte SJ, Petrenciuc O, Cihon F, Lathia C, Schwartz B. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005;16(10):1688–1694. doi: 10.1093/annonc/mdi310. doi:mdi310 [pii] 10.1093/annonc/mdi310. [DOI] [PubMed] [Google Scholar]
- 5.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, Schwartz B, Awada A, Voigtmann R, Scheulen ME, Seeber S. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23(5):965–972. doi: 10.1200/JCO.2005.06.124. doi:JCO.2005.06.124 [pii] 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 6.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, Hirte HW, Eder JP, Lenz HJ, Schwartz B. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12(4):426–437. doi: 10.1634/theoncologist.12-4-426. doi:12/4/426 [pii] 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. doi:356/2/125 [pii] 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. doi:359/4/378 [pii] 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI. Vascular endothelial growth factor-targeted therapy in renal cell carcinoma: current status and future directions. Clin Cancer Res. 2007;13(4):1098–1106. doi: 10.1158/1078-0432.CCR-06-1989. doi:13/4/1098 [pii] 10.1158/1078-0432.CCR-06-1989. [DOI] [PubMed] [Google Scholar]
- 10.Flaherty KT, Brose MS. Sorafenib-Related Hand-Foot Skin Reaction Improves, Not Worsens, with Continued Treatment. Clin Cancer Res. 2009;15(24):7749. doi: 10.1158/1078-0432.CCR-09-1190. doi:15/24/7749 [pii] 10.1158/1078-0432.CCR-09-1190. [DOI] [PubMed] [Google Scholar]
- 11.Amato RJ, Harris P, Dalton M, Khan M, Alter R, Zhai Q, Brady JR, Jac J, Hauke R, Srinivas S. A phase II trial of intra-patient dose-escalated sorafenib in patients (pts) with metastatic renal cell cancer (MRCC). J Clin Oncol. 2007;25(20 suppl) June abstr 5026. [Google Scholar]
- 12.Amato RJ, Jac J, Harris P, Dalton M, Saxena S, Monzon F, Zhai J, Brady JR, Willis JP. A phase II trial of intra-patient dose escalated-sorafenib in patients (pts) with metastatic renal cell cancer (MRCC) J Clin Oncol. 2008;26(20 suppl) May abstr 5122. [Google Scholar]
- 13.Srinivas S, Harshman L, Hauke R. Sorafenib monotherapy in patients with treatment-naive metastatic renal cell cancer: preliminary results of a phase II intra-patient dose-escalation study. J Clin Oncol. 2009;27(suppl) abstr e14564. [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Riechelmann RP, Chin S, Wang L, Tannock IF, Berthold DR, Moore MJ, Knox JJ. Sorafenib for metastatic renal cancer: the Princess Margaret experience. Am J Clin Oncol. 2008;31(2):182–187. doi: 10.1097/COC.0b013e3181574084. doi:10.1097/COC.0b013e318157408400000421-200804000-00012 [pii] [DOI] [PubMed] [Google Scholar]
- 16.La Vine DB, Coleman TA, Davis CH, Carbonell CE, Davis WB. Frequent dose interruptions are required for patients receiving oral kinase inhibitor therapy for advanced renal cell carcinoma. Am J Clin Oncol. 2010;33(3):217–220. doi: 10.1097/COC.0b013e3181a650a6. doi:10.1097/COC.0b013e3181a650a6. [DOI] [PubMed] [Google Scholar]
- 17.Miller AA, Murry DJ, Owzar K, Hollis DR, Kennedy EB, Abou-Alfa G, Desai A, Hwang J, Villalona-Calero MA, Dees EC, Lewis LD, Fakih MG, Edelman MJ, Millard F, Frank RC, Hohl RJ, Ratain MJ. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2009;27(11):1800–1805. doi: 10.1200/JCO.2008.20.0931. doi:JCO.2008.20.0931 [pii] 10.1200/JCO.2008.20.0931. [DOI] [PMC free article] [PubMed] [Google Scholar]