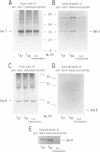
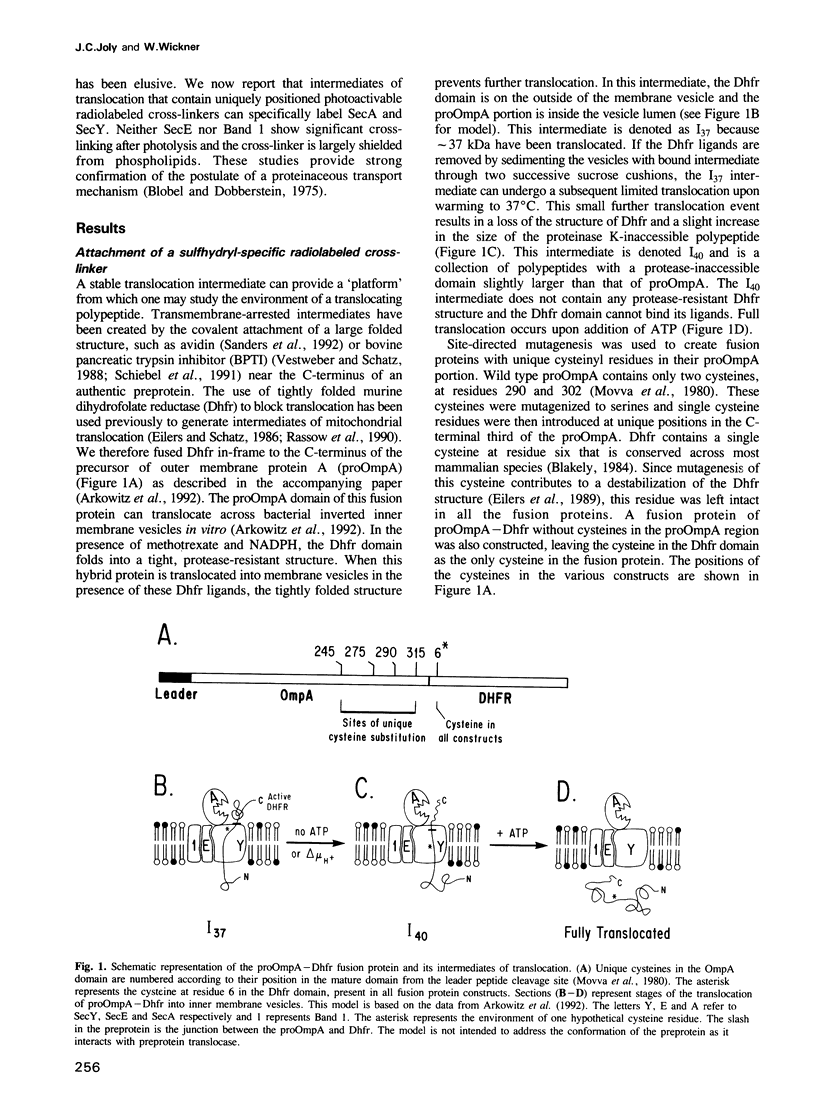
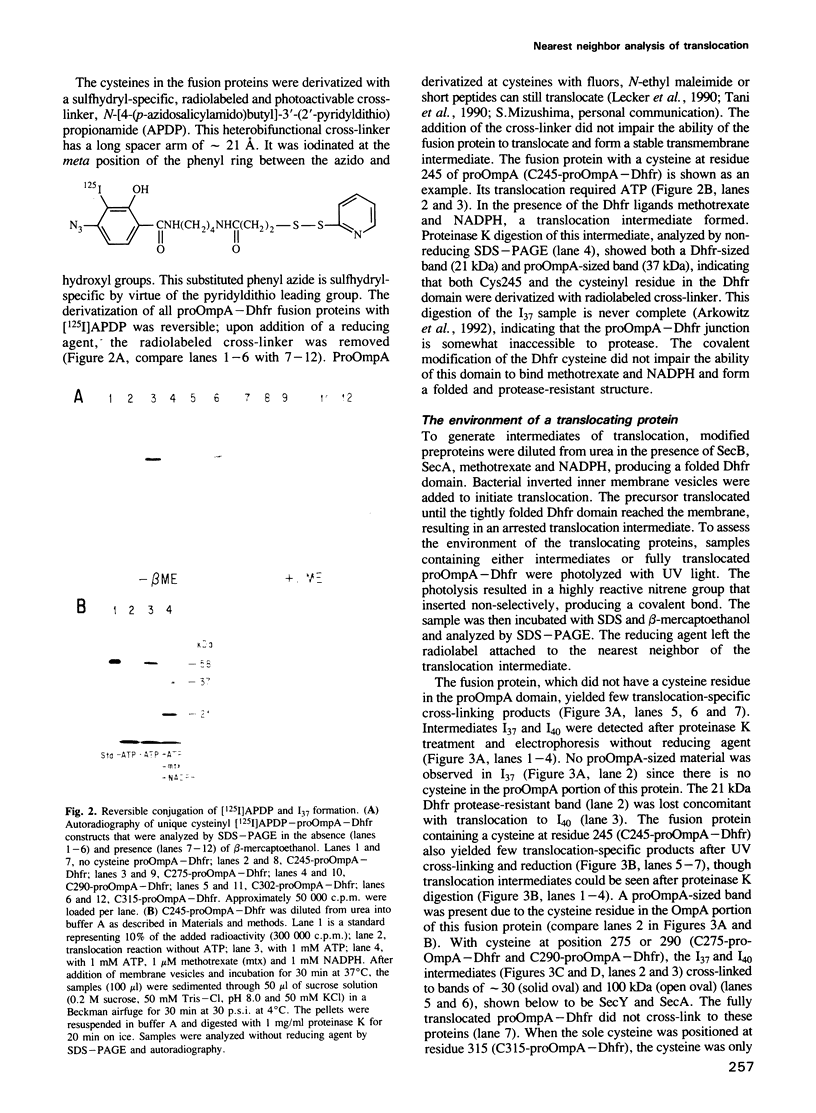
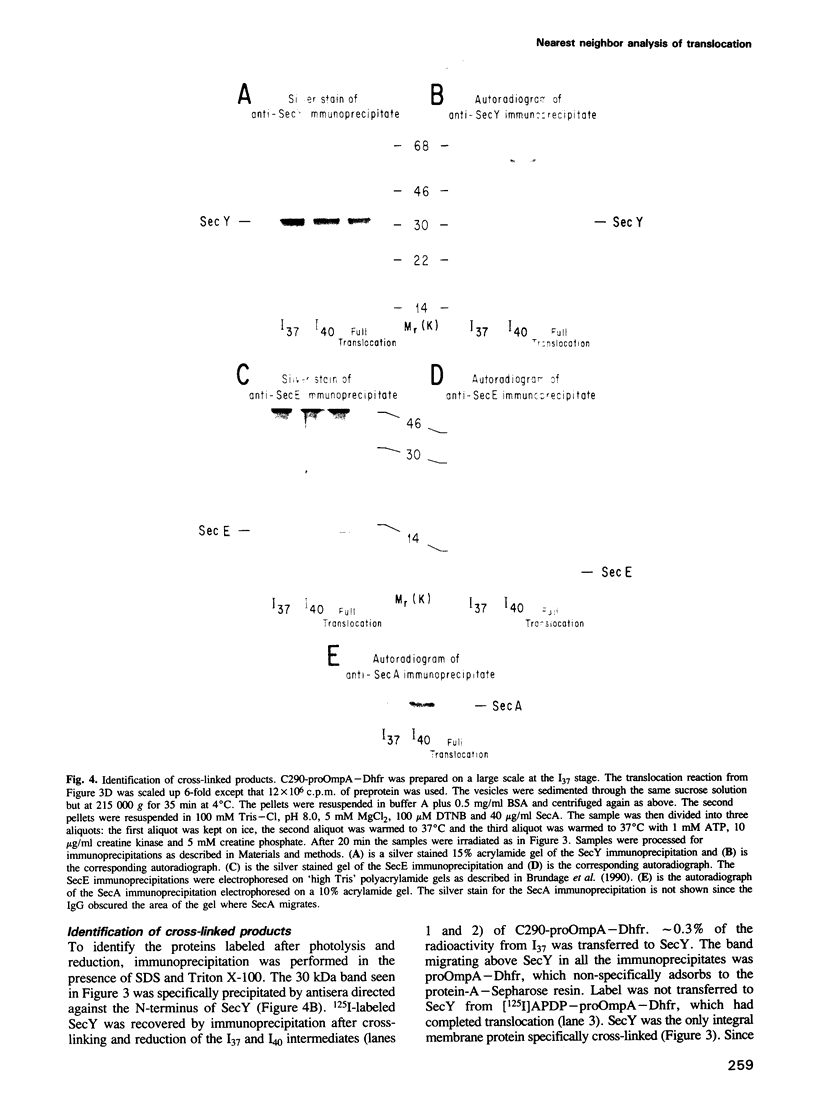
Abstract
To study the environment of a preprotein as it crosses the plasma membrane of Escherichia coli, unique cysteinyl residues were introduced into proOmpA and the genes for these mutant preproteins were fused to the gene of dihydrofolate reductase (Dhfr). A photoactivable, radiolabeled and reducible cross-linker was then attached to the unique cysteinyl residue of each purified protein. Partially translocated polypeptides were generated and arrested in their membrane transit by the folded structure of the dihydrofolate reductase domain. After photolysis to label their nearest neighbors and reduction of the disulfide bond between proOmpA-Dhfr and the cross-linker, radiolabeled cross-linker was selectively recovered with the SecA and SecY subunits of preprotein translocase. Strikingly, neither the SecE nor Band 1 subunits were cross-linked to any of the constructs and the membrane phospholipids were almost entirely shielded from cross-linking. The fact that SecY and SecA are the only membrane proteins cross-linked to the translocating chains suggests that they may form an entirely proteinaceous pathway through which secreted proteins pass during membrane transit.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbach C., Marti T., Khorana H. G., Hubbell W. L. Transmembrane protein structure: spin labeling of bacteriorhodopsin mutants. Science. 1990 Jun 1;248(4959):1088–1092. doi: 10.1126/science.2160734. [DOI] [PubMed] [Google Scholar]
- Arkowitz R. A., Joly J. C., Wickner W. Translocation can drive the unfolding of a preprotein domain. EMBO J. 1993 Jan;12(1):243–253. doi: 10.1002/j.1460-2075.1993.tb05650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bayley H., Knowles J. R. Photogenerated reagents for membrane labeling. 1. Phenylnitrene formed within the lipid bilayer. Biochemistry. 1978 Jun 13;17(12):2414–2419. doi: 10.1021/bi00605a025. [DOI] [PubMed] [Google Scholar]
- Bieker K. L., Phillips G. J., Silhavy T. J. The sec and prl genes of Escherichia coli. J Bioenerg Biomembr. 1990 Jun;22(3):291–310. doi: 10.1007/BF00763169. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Stern L. J., Chao B. H., Khorana H. G. Structure-function studies on bacteriorhodopsin. IV. Purification and renaturation of bacterio-opsin polypeptide expressed in Escherichia coli. J Biol Chem. 1987 Jul 5;262(19):9271–9276. [PubMed] [Google Scholar]
- Brundage L., Fimmel C. J., Mizushima S., Wickner W. SecY, SecE, and band 1 form the membrane-embedded domain of Escherichia coli preprotein translocase. J Biol Chem. 1992 Feb 25;267(6):4166–4170. [PubMed] [Google Scholar]
- Brundage L., Hendrick J. P., Schiebel E., Driessen A. J., Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990 Aug 24;62(4):649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Blobel G., Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. doi: 10.1073/pnas.75.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke E., Guthrie B., Lecker S., Lill R., Wickner W. ProOmpA is stabilized for membrane translocation by either purified E. coli trigger factor or canine signal recognition particle. Cell. 1988 Sep 23;54(7):1003–1011. doi: 10.1016/0092-8674(88)90115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K., Lill R., Crooke E., Rice M., Moore K., Wickner W., Oliver D. SecA protein, a peripheral protein of the Escherichia coli plasma membrane, is essential for the functional binding and translocation of proOmpA. EMBO J. 1989 Mar;8(3):955–959. doi: 10.1002/j.1460-2075.1989.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K., Wickner W. Specific recognition of the leader region of precursor proteins is required for the activation of translocation ATPase of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8630–8634. doi: 10.1073/pnas.86.22.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Endo T., Schatz G. Adriamycin, a drug interacting with acidic phospholipids, blocks import of precursor proteins by isolated yeast mitochondria. J Biol Chem. 1989 Feb 15;264(5):2945–2950. [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Fikes J. D., Bassford P. J., Jr Novel secA alleles improve export of maltose-binding protein synthesized with a defective signal peptide. J Bacteriol. 1989 Jan;171(1):402–409. doi: 10.1128/jb.171.1.402-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitsch S. L., Khorana H. G. Structural studies on transmembrane proteins. 1. Model study using bacteriorhodopsin mutants containing single cysteine residues. Biochemistry. 1989 Sep 19;28(19):7800–7805. doi: 10.1021/bi00445a041. [DOI] [PubMed] [Google Scholar]
- Gardel C., Johnson K., Jacq A., Beckwith J. The secD locus of E.coli codes for two membrane proteins required for protein export. EMBO J. 1990 Oct;9(10):3209–3216. doi: 10.1002/j.1460-2075.1990.tb07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B. L., Wickner W. M13 procoat inserts into liposomes in the absence of other membrane proteins. J Biol Chem. 1985 Oct 25;260(24):13281–13285. [PubMed] [Google Scholar]
- Görlich D., Hartmann E., Prehn S., Rapoport T. A. A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature. 1992 May 7;357(6373):47–52. doi: 10.1038/357047a0. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Lecker S., Schiebel E., Hendrick J. P., Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990 Oct 19;63(2):269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Wickner W. SecA protein needs both acidic phospholipids and SecY/E protein for functional high-affinity binding to the Escherichia coli plasma membrane. J Biol Chem. 1991 Dec 25;266(36):24596–24600. [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. S., Bayley H., Liao M. J., London E., Khorana H. G. Refolding of an integral membrane protein. Denaturation, renaturation, and reconstitution of intact bacteriorhodopsin and two proteolytic fragments. J Biol Chem. 1981 Apr 25;256(8):3802–3809. [PubMed] [Google Scholar]
- Ito K., Date T., Wickner W. Synthesis, assembly into the cytoplasmic membrane, and proteolytic processing of the precursor of coliphage M13 coat protein. J Biol Chem. 1980 Mar 10;255(5):2123–2130. [PubMed] [Google Scholar]
- Killian J. A., de Jong A. M., Bijvelt J., Verkleij A. J., de Kruijff B. Induction of non-bilayer lipid structures by functional signal peptides. EMBO J. 1990 Mar;9(3):815–819. doi: 10.1002/j.1460-2075.1990.tb08178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. In vivo evidence for the role of the epsilon subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J Bacteriol. 1984 Dec;160(3):1055–1060. doi: 10.1128/jb.160.3.1055-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985 Jul;163(1):267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A. Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5320–5324. doi: 10.1073/pnas.86.14.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker S. H., Driessen A. J., Wickner W. ProOmpA contains secondary and tertiary structure prior to translocation and is shielded from aggregation by association with SecB protein. EMBO J. 1990 Jul;9(7):2309–2314. doi: 10.1002/j.1460-2075.1990.tb07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker S., Lill R., Ziegelhoffer T., Georgopoulos C., Bassford P. J., Jr, Kumamoto C. A., Wickner W. Three pure chaperone proteins of Escherichia coli--SecB, trigger factor and GroEL--form soluble complexes with precursor proteins in vitro. EMBO J. 1989 Sep;8(9):2703–2709. doi: 10.1002/j.1460-2075.1989.tb08411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R., Dowhan W., Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990 Jan 26;60(2):271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- McKnight C. J., Stradley S. J., Jones J. D., Gierasch L. M. Conformational and membrane-binding properties of a signal sequence are largely unaltered by its adjacent mature region. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5799–5803. doi: 10.1073/pnas.88.13.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movva N. R., Nakamura K., Inouye M. Gene structure of the OmpA protein, a major surface protein of Escherichia coli required for cell-cell interaction. J Mol Biol. 1980 Nov 5;143(3):317–328. doi: 10.1016/0022-2836(80)90193-x. [DOI] [PubMed] [Google Scholar]
- Müller G., Zimmermann R. Import of honeybee prepromelittin into the endoplasmic reticulum: structural basis for independence of SRP and docking protein. EMBO J. 1987 Jul;6(7):2099–2107. doi: 10.1002/j.1460-2075.1987.tb02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsch A., Wiedmann M., Rapoport T. A. Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell. 1992 Apr 17;69(2):343–352. doi: 10.1016/0092-8674(92)90414-8. [DOI] [PubMed] [Google Scholar]
- Nishiyama K., Mizushima S., Tokuda H. The carboxyl-terminal region of SecE interacts with SecY and is functional in the reconstitution of protein translocation activity in Escherichia coli. J Biol Chem. 1992 Apr 5;267(10):7170–7176. [PubMed] [Google Scholar]
- Ohno-Iwashita Y., Wickner W. Reconstitution of rapid and asymmetric assembly of M13 procoat protein into liposomes which have bacterial leader peptidase. J Biol Chem. 1983 Feb 10;258(3):1895–1900. [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Molecular genetics of membrane phospholipid synthesis. Annu Rev Genet. 1986;20:253–295. doi: 10.1146/annurev.ge.20.120186.001345. [DOI] [PubMed] [Google Scholar]
- Rassow J., Hartl F. U., Guiard B., Pfanner N., Neupert W. Polypeptides traverse the mitochondrial envelope in an extended state. FEBS Lett. 1990 Nov 26;275(1-2):190–194. doi: 10.1016/0014-5793(90)81469-5. [DOI] [PubMed] [Google Scholar]
- Rietveld A., Ponjee G. A., Schiffers P., Jordi W., van de Coolwijk P. J., Demel R. A., Marsh D., de Kruijff B. Investigations on the insertion of the mitochondrial precursor protein apocytochrome c into model membranes. Biochim Biophys Acta. 1985 Sep 10;818(3):398–409. doi: 10.1016/0005-2736(85)90015-x. [DOI] [PubMed] [Google Scholar]
- Riggs P. D., Derman A. I., Beckwith J. A mutation affecting the regulation of a secA-lacZ fusion defines a new sec gene. Genetics. 1988 Apr;118(4):571–579. doi: 10.1093/genetics/118.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Kennedy E. P. Asymmetrical distribution of phospholipids in the membrane of Bacillus megaterium. J Mol Biol. 1977 Mar 5;110(3):603–618. doi: 10.1016/s0022-2836(77)80114-9. [DOI] [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Bieker K. L., Ottemann K. M., Silhavy T. J., Beckwith J. One of three transmembrane stretches is sufficient for the functioning of the SecE protein, a membrane component of the E. coli secretion machinery. EMBO J. 1991 Jul;10(7):1749–1757. doi: 10.1002/j.1460-2075.1991.tb07699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Riggs P. D., Jacq A., Fath M. J., Beckwith J. The secE gene encodes an integral membrane protein required for protein export in Escherichia coli. Genes Dev. 1989 Jul;3(7):1035–1044. doi: 10.1101/gad.3.7.1035. [DOI] [PubMed] [Google Scholar]
- Schiebel E., Driessen A. J., Hartl F. U., Wickner W. Delta mu H+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991 Mar 8;64(5):927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [PubMed] [Google Scholar]
- Simon S. M., Blobel G. Signal peptides open protein-conducting channels in E. coli. Cell. 1992 May 15;69(4):677–684. doi: 10.1016/0092-8674(92)90231-z. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Blobel G., Zimmerberg J. Large aqueous channels in membrane vesicles derived from the rough endoplasmic reticulum of canine pancreas or the plasma membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6176–6180. doi: 10.1073/pnas.86.16.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stader J., Gansheroff L. J., Silhavy T. J. New suppressors of signal-sequence mutations, prlG, are linked tightly to the secE gene of Escherichia coli. Genes Dev. 1989 Jul;3(7):1045–1052. doi: 10.1101/gad.3.7.1045. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Wiedmann M., Schlossmann J., Keil P., Neupert W., Pfanner N. Mapping of the protein import machinery in the mitochondrial outer membrane by crosslinking of translocation intermediates. Nature. 1992 Jan 2;355(6355):84–87. doi: 10.1038/355084a0. [DOI] [PubMed] [Google Scholar]
- Tani K., Tokuda H., Mizushima S. Translocation of ProOmpA possessing an intramolecular disulfide bridge into membrane vesicles of Escherichia coli. Effect of membrane energization. J Biol Chem. 1990 Oct 5;265(28):17341–17347. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Müller-Eberhard H. J., Podack E. R. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982 Aug 5;298(5874):534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Brunner J., Baker A., Schatz G. A 42K outer-membrane protein is a component of the yeast mitochondrial protein import site. Nature. 1989 Sep 21;341(6239):205–209. doi: 10.1038/341205a0. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. A chimeric mitochondrial precursor protein with internal disulfide bridges blocks import of authentic precursors into mitochondria and allows quantitation of import sites. J Cell Biol. 1988 Dec;107(6 Pt 1):2037–2043. doi: 10.1083/jcb.107.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Watts C., Silver P., Wickner W. Membrane assembly from purified components. II. Assembly of M13 procoat into liposomes reconstituted with purified leader peptidase. Cell. 1981 Aug;25(2):347–353. doi: 10.1016/0092-8674(81)90053-2. [DOI] [PubMed] [Google Scholar]
- Weiss J. B., Ray P. H., Bassford P. J., Jr Purified secB protein of Escherichia coli retards folding and promotes membrane translocation of the maltose-binding protein in vitro. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8978–8982. doi: 10.1073/pnas.85.23.8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Vrije T., de Swart R. L., Dowhan W., Tommassen J., de Kruijff B. Phosphatidylglycerol is involved in protein translocation across Escherichia coli inner membranes. Nature. 1988 Jul 14;334(6178):173–175. doi: 10.1038/334173a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]