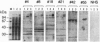Abstract
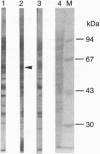
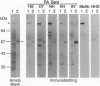
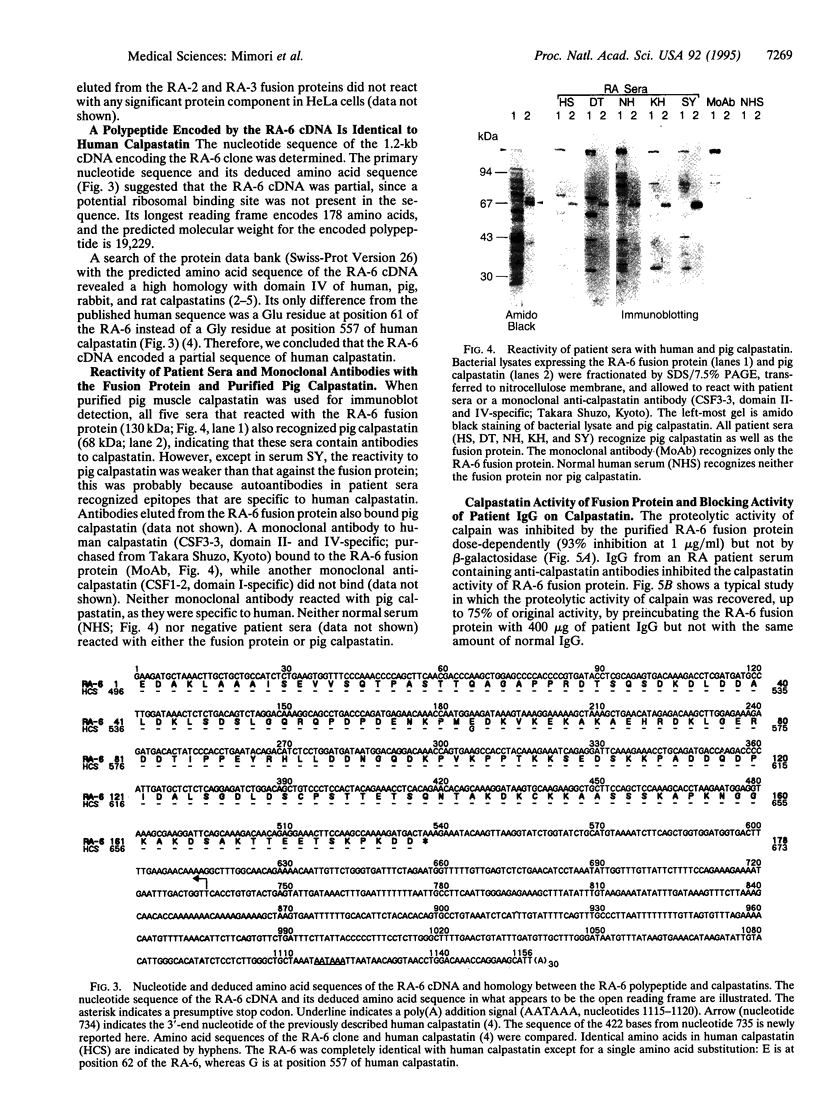
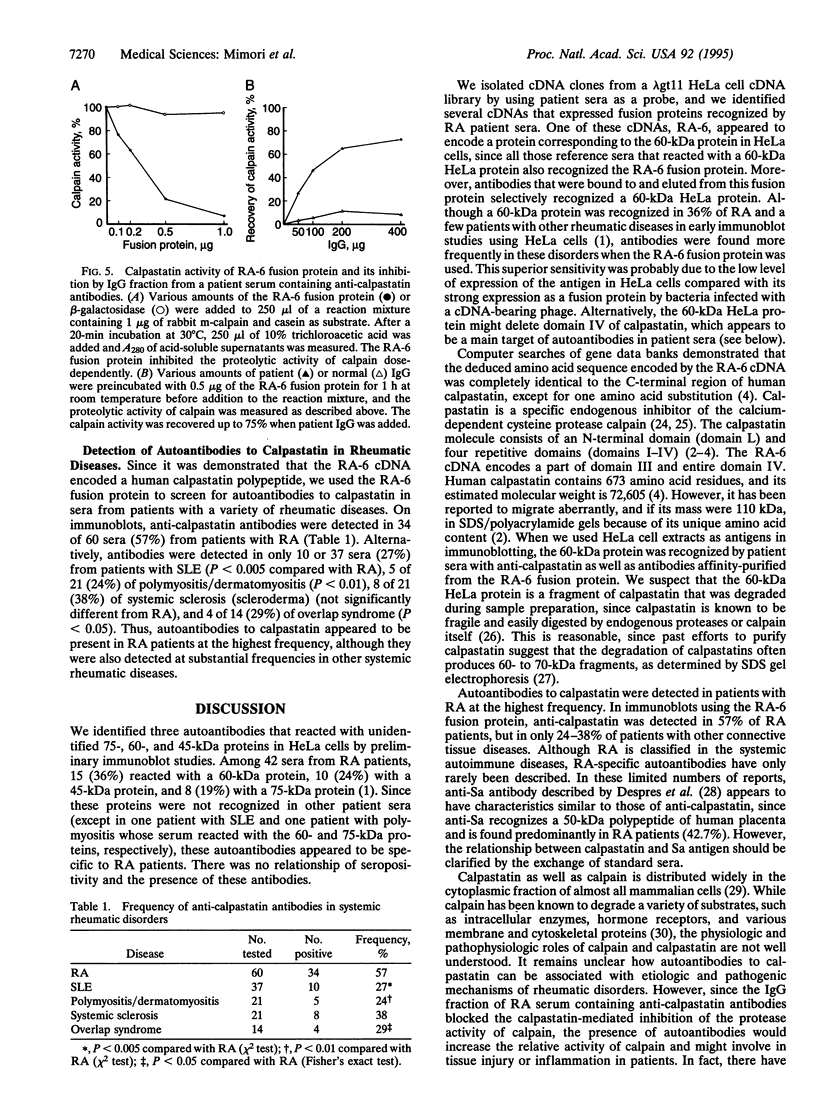
We identified an autoantibody that reacts with calpastatin [an inhibitor protein of the calcium-dependent neutral protease calpain (EC 3.4.22.17)]. In early immunoblot studies, sera from patients with rheumatoid arthritis (RA) recognized unidentified 60-, 45-, and 75-kDa proteins in HeLa cell extracts. To identify these autoantigens, we used patient sera to clone cDNAs from a lambda gt11 expression library. We isolated clones of four genes that expressed fusion proteins recognized by RA sera. The 1.2-kb cDNA insert (termed RA-6) appeared to encode a polypeptide corresponding to the 60-kDa antigen from HeLa cells, since antibodies bound to the RA-6 fusion protein also reacted with a 60-kDa HeLa protein. The deduced amino acid sequence of the RA-6 cDNA was completely identical with the C-terminal 178 amino acids of human calpastatin except for one amino acid substitution. Patient sera that reacted with the RA-6 also bound pig muscle calpastatin, and a monoclonal antibody to human calpastatin recognized the RA-6 fusion protein, confirming the identity of RA-6 with calpastatin. Moreover, the purified RA-6 fusion protein inhibited the proteolytic activity of calpain, and IgG from a serum containing anti-calpastatin antibodies blocked the calpastatin activity of the RA-6 fusion protein. Immunoblots of the RA-6 product detected autoantibodies to calpastatin in 57% of RA patients; this incidence was significantly higher than that observed in other systemic rheumatic diseases, including systemic lupus erythematosus (27%), polymyositis/dermatomyositis (24%), systemic sclerosis (38%), and overlap syndrome (29%). Thus, anti-calpastatin antibodies are present most frequently in patients with RA and may participate in pathogenic mechanisms of rheumatic diseases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Kitahara-Ozawa A., Sugamura K., Lee W. J., Yodoi J., Maki M., Murachi T., Hatanaka M. Expression of calpain II gene in human hematopoietic system cells infected with human T-cell leukemia virus type I. J Biol Chem. 1992 Sep 25;267(27):19373–19378. [PubMed] [Google Scholar]
- Adachi Y., Takano E., Murachi T., Hatanaka M. Distribution and expression of calpastatin in human hematopoietic system cells. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):223–227. [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Asada K., Ishino Y., Shimada M., Shimojo T., Endo M., Kimizuka F., Kato I., Maki M., Hatanaka M., Murachi T. cDNA cloning of human calpastatin: sequence homology among human, pig, and rabbit calpastatins. J Enzyme Inhib. 1989;3(1):49–56. doi: 10.3109/14756368909030363. [DOI] [PubMed] [Google Scholar]
- Després N., Boire G., Lopez-Longo F. J., Ménard H. A. The Sa system: a novel antigen-antibody system specific for rheumatoid arthritis. J Rheumatol. 1994 Jun;21(6):1027–1033. [PubMed] [Google Scholar]
- Emori Y., Kawasaki H., Imajoh S., Imahori K., Suzuki K. Endogenous inhibitor for calcium-dependent cysteine protease contains four internal repeats that could be responsible for its multiple reactive sites. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3590–3594. doi: 10.1073/pnas.84.11.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui I., Tanaka K., Murachi T. Extracellular appearance of calpain and calpastatin in the synovial fluid of the knee joint. Biochem Biophys Res Commun. 1989 Jul 31;162(2):559–566. doi: 10.1016/0006-291x(89)92347-4. [DOI] [PubMed] [Google Scholar]
- Imajoh S., Kawasaki H., Suzuki K. Limited autolysis of calcium-activated neutral protease (CANP): reduction of the Ca2+-requirement is due to the NH2-terminal processing of the large subunit. J Biochem. 1986 Sep;100(3):633–642. doi: 10.1093/oxfordjournals.jbchem.a121755. [DOI] [PubMed] [Google Scholar]
- Inomata M., Nomoto M., Hayashi M., Nakamura M., Imahori K., Kawashima S. Comparison of low and high calcium requiring forms of the calcium-activated neutral protease (CANP) from rabbit skeletal muscle. J Biochem. 1984 Jun;95(6):1661–1670. doi: 10.1093/oxfordjournals.jbchem.a134779. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Ishida S., Emori Y., Suzuki K. Rat calpastatin has diverged primary sequence from other mammalian calpastatins but retains functionally important sequences. Biochim Biophys Acta. 1991 Mar 26;1088(3):436–438. doi: 10.1016/0167-4781(91)90139-d. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Mikawa K., Hashimoto K., Yasuda I., Tanaka S., Tominaga M., Kuroda T., Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain). J Biol Chem. 1989 Mar 5;264(7):4088–4092. [PubMed] [Google Scholar]
- Kitajima I., Yamamoto K., Sato K., Nakajima Y., Nakajima T., Maruyama I., Osame M., Nishioka K. Detection of human T cell lymphotropic virus type I proviral DNA and its gene expression in synovial cells in chronic inflammatory arthropathy. J Clin Invest. 1991 Oct;88(4):1315–1322. doi: 10.1172/JCI115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Yamamoto K., Saido T., Kawasaki H., Oppenheim J. J., Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimatsu M., Higashiyama S., Sato K., Ohkubo I., Sasaki M. Calcium dependent cysteine proteinase is a precursor of a chemotactic factor for neutrophils. Biochem Biophys Res Commun. 1989 Oct 31;164(2):875–882. doi: 10.1016/0006-291x(89)91540-4. [DOI] [PubMed] [Google Scholar]
- Murachi T. Calcium-dependent proteinases and specific inhibitors: calpain and calpastatin. Biochem Soc Symp. 1984;49:149–167. [PubMed] [Google Scholar]
- Murachi T., Tanaka K., Hatanaka M., Murakami T. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv Enzyme Regul. 1980;19:407–424. doi: 10.1016/0065-2571(81)90026-1. [DOI] [PubMed] [Google Scholar]
- Nishiura I., Tanaka K., Yamato S., Murachi T. The occurrence of an inhibitor of Ca2+-dependent neutral protease in rat liver. J Biochem. 1978 Dec;84(6):1657–1659. doi: 10.1093/oxfordjournals.jbchem.a132296. [DOI] [PubMed] [Google Scholar]
- Nojima T., Kamata M., Matsunobu T., Kimura M., Mimori T., Matsumura M., Fujii T., Akizuki M. [Detection of autoantibodies in sera from patients with rheumatoid arthritis]. Ryumachi. 1994 Oct;34(5):871–878. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Damiani G., Salamino F., Sparatore B., Michetti M., Horecker B. L. Effects of a monoclonal anti-calpain antibody on responses of stimulated human neutrophils. Evidence for a role for proteolytically modified protein kinase C. J Biol Chem. 1988 Feb 5;263(4):1915–1919. [PubMed] [Google Scholar]
- Rothfield N., Whitaker D., Bordwell B., Weiner E., Senecal J. L., Earnshaw W. Detection of anticentromere antibodies using cloned autoantigen CENP-B. Arthritis Rheum. 1987 Dec;30(12):1416–1419. doi: 10.1002/art.1780301214. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta K., Yumoto N., Murachi T. Fragmentation of a 70000-dalton calpastatin molecule upon its complex formation with calpain. Biochem Int. 1984 Sep;9(3):327–333. [PubMed] [Google Scholar]
- Suzuki K., Shimizu K., Hamamoto T., Nakagawa Y., Hamakubo T., Yamamuro T. Biochemical demonstration of calpains and calpastatin in osteoarthritic synovial fluid. Arthritis Rheum. 1990 May;33(5):728–732. doi: 10.1002/art.1780330516. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Shimizu K., Hamamoto T., Nakagawa Y., Murachi T., Yamamuro T. Characterization of proteoglycan degradation by calpain. Biochem J. 1992 Aug 1;285(Pt 3):857–862. doi: 10.1042/bj2850857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano E., Kitahara A., Sasaki T., Kannagi R., Murachi T. Two different molecular species of pig calpastatin. Structural and functional relationship between 107 kDa and 68 kDa molecules. Biochem J. 1986 Apr 1;235(1):97–102. doi: 10.1042/bj2350097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano E., Maki M., Mori H., Hatanaka M., Marti T., Titani K., Kannagi R., Ooi T., Murachi T. Pig heart calpastatin: identification of repetitive domain structures and anomalous behavior in polyacrylamide gel electrophoresis. Biochemistry. 1988 Mar 22;27(6):1964–1972. doi: 10.1021/bi00406a024. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Shimizu K., Shimizu K., Suzuki K., Nakagawa Y., Yamamuro T. Calcium-dependent cysteine proteinase (calpain) in human arthritic synovial joints. Arthritis Rheum. 1992 Nov;35(11):1309–1317. doi: 10.1002/art.1780351111. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]