Abstract
Tinea versicolor (TV) is a common cutaneous fungal infection characterized by superficial scaling and a mild disturbance of skin pigmentation. It typically affects the chest, upper back, and shoulders. However, involvement of more unusual regions of the body such as the face and scalp, arms and legs, intertriginous sites, genitalia, areolae, and palms and soles has been reported. This report details two such cases observed at our institution: a 32-year-old woman with involvement of the popliteal fossa and a 16-year-old boy with involvement of the groin. The clinician must be aware of these variations in location and perform the appropriate diagnostic workup when lesions have the characteristic morphology of TV despite an unusual location. The etiology, pathophysiology, and epidemiology of TV are reviewed and current literature describing other instances of TV in uncommon locations is discussed.
Keywords: tinea versicolor, pityriasis versicolor, Malassezia, groin, flexures
Introduction
Tinea versicolor, also known as pityriasis versicolor, is a common cutaneous fungal infection characterized by superficial scaling and a mild disturbance of skin pigmentation. It classically presents as round to oval macules that can be hypopigmented, hyperpigmented, or erythematous (hence the name versicolor) and typically affects the chest, upper back, and shoulders. However, involvement of more unusual regions of the body such as the face and scalp, arms and legs, intertriginous sites, genitalia, areolae, and palms and soles [1–6] has been reported. This report details two cases observed at our institution in which the infection occurred in uncommon distributions.
Cases
Case 1
A 32-year-old Asian woman presented with an asymptomatic, tan colored, scaly plaque over the left popliteal fossa (Figure 1). She had no systemic complaints and her past medical history was unremarkable. At the time of the visit, she had been taking oral doxycycline for 1 month to treat folliculitis of the legs. Potassium hydroxide (KOH) preparation of the scaly plaque demonstrated spores and short hyphae (Figure 2). The patient was treated with ketoconazole 2% cream once to twice daily for three weeks.
Figure 1.
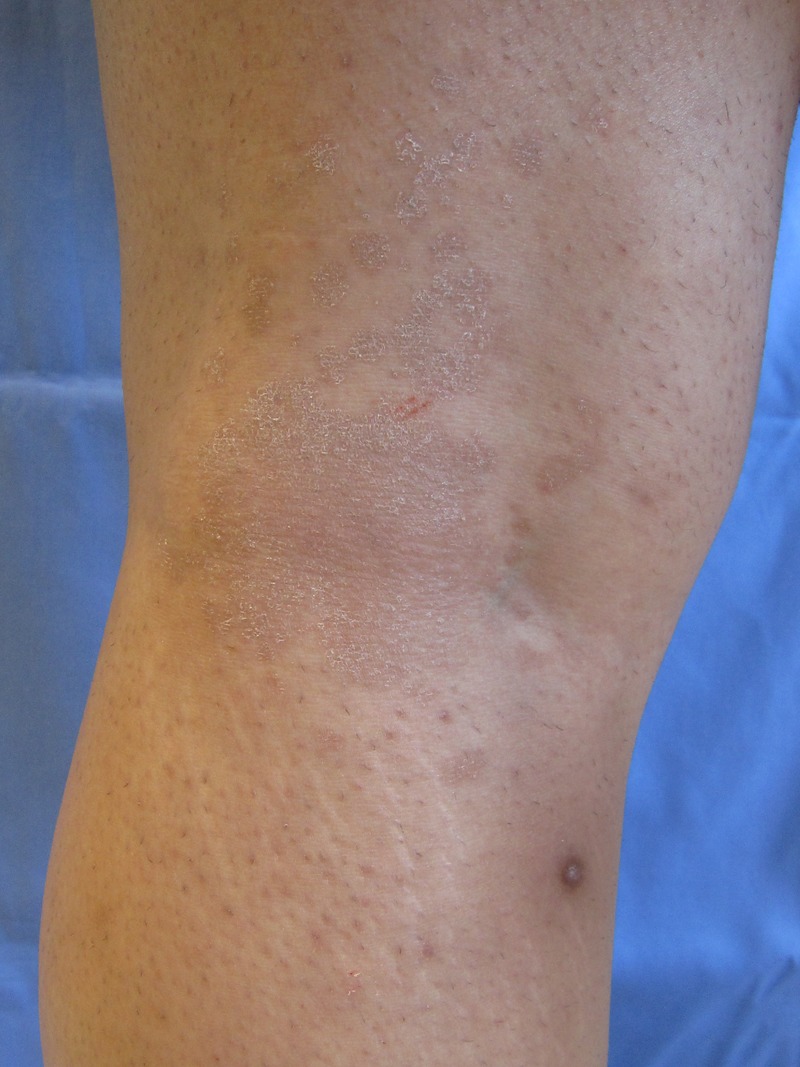
Multiple tan plaques with fine scale over the posterior left popliteal fossa. [Copyright: ©2014 Varada et al.]
Figure 2.
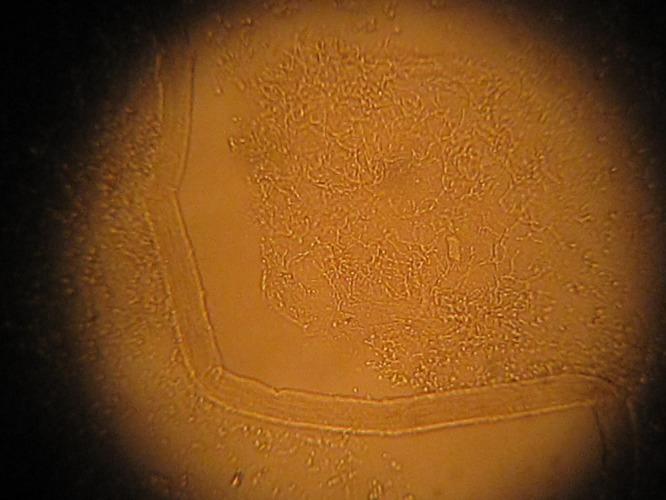
Potassium hydroxide (KOH) preparation demonstrating spores and short hyphae. [Copyright: ©2014 Varada et al.]
Case 2
A 16-year-old healthy Caucasian boy presented with a 1-year history of a mildly pruritic truncal rash that, over the past 3 months, had spread over the arms, legs, and groin. The lesions consisted of 1–2 cm salmon colored, round to oval, scaly papules coalescing into plaques (Figure 3). In the past, he was treated with an unknown prescription cream and selenium sulfide shampoo, which did not resolve the infection. KOH preparation of a lesion demonstrated yeast forms and short, septate, hyphal forms. He was subsequently treated with oral ketoconazole 400 mg once daily for 3 days, followed by ketoconazole 2% shampoo once a week for prophylaxis.
Figure 3.
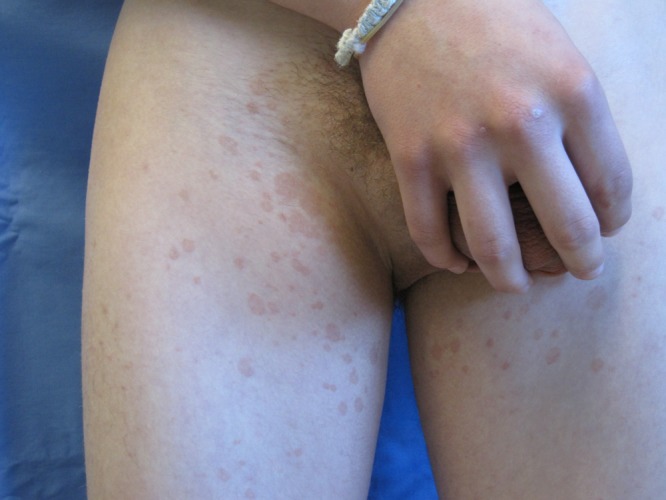
Multiple 1–2 cm salmon colored, round to oval, thin scaly plaques over the groin and medial thighs. [Copyright: ©2014 Varada et al.]
Discussion
The incidence of tinea versicolor varies by season and geographic location and is likely underreported, but it is one of the most common superficial mycoses worldwide. Prevalence ranges from as low as 1% in dry and temperate climates to 50% in the tropics [4]. The implicated pathogens are dimorphic saprophytes of the genus Malassezia (formerly Pityrosporum) currently comprising twelve known species, of which M. globosa, M. sympodialis, and M. furfur are the most commonly cultured [7]. The yeast forms, which exist commensally in the stratum corneum as part of human skin flora [4], are obligatorily lipophilic and therefore prefer regions of the body with the highest concentrations of sebaceous fatty acids such as the face, scalp, chest, and back [8]. Development of the clinical disease requires conversion of the yeast into the pathogenic mycelial form, an event that is likely provoked by many factors including malnutrition, hyperhidrosis, oral contraceptive use, and an altered immune response (such as corticosteroid use, AIDS, and solid organ transplant).4 Other risk factors for acquiring TV are: (1) age—the infection is most common in teens and young adults when sex hormones increase sebaceous secretions [9], (2) heat and humidity—it is more prevalent in tropical than temperate and dry regions, in the summer rather than winter, and in areas of the body covered by clothing [8], (3) use of topical oils which provide additional lipid substrate for yeast development [7], and (4) pregnancy. The disease is not contagious and factors such as gender, race, socioeconomic status, and skin hygiene are not implicated [4,7].
The largest single institution studies analyzing the anatomical distributions of TV show that the vast majority of infections involve the same locations. Of 25 patients from the United Kingdom, Roberts et al. [10] found the chest, upper back, shoulders, upper arms, and abdomen to be the most commonly affected. In this study, the two most frequently involved nonclassical locations were the scalp and groin, affecting 25% and 19% of patients respectively [11]. In a similar analysis of 47 patients studied by Bumgarner et al. [11], only two did not exhibit the classic distribution of trunk or upper arm involvement. It is unclear which factors predispose to the development of tinea versicolor in nonclassical distributions. Case series and reports have further described these associations.
Tinea versicolor of the flexures, as seen in one of our patients, is not entirely uncommon. Aljabre et al. reported an 11.8% (13 of 110 patients) incidence of flexural involvement at their institution but no statistically significant correlation with age, sex, immune status, infection duration, or infection relapse rate. They also noted that of flexures, the axillary vault and inguinal crease were the least frequently involved and were often spared even in cases of widespread infection [5,12] However, another report describes two cases solely affecting the axillae and inguinal regions respectively [2].
Several case reports have described a total of ten instances of tinea versicolor involving the penis including nine involving the penile shaft and one involving the glans [13–17]. Two of these occurred in renal transplant recipients receiving systemic immunosuppression [13,14], but the rest have involved healthy males lacking any of the above-mentioned risk factors other than age and geographic location. All of the reported cases were accompanied by extensive involvement of the chest, back, or other regions with no instances involving the penis alone.
Other case reports have described TV affecting the head and neck [15,18–21]. Several cases of TV of the face have been found more commonly in children than adults, with children exhibiting more extensive facial involvement and less involvement of other body regions [1,5]. Also, it has been found to occur more frequently in the tropics [10], although Boralevi et al. [19] report a more severe presentation occurring in two Caucasian patients in a temperate climate. Most of the reported cases have been in otherwise healthy patients without systemic illness and immune compromise—only one patient studied by Hughes et al [20] was immunocompromised from chemotherapeutic treatment of acute lymphoblastic leukemia.
Although uncommon, eruptions confined to the areola and periareolar areas have also been noted. Two patients had bilateral involvement of the areolae—one case occurred in a pubescent male with mild gynecomastia and one in an adult female, suggesting that localized seborrhea (perhaps hormone mediated) or increased heat and humidity from occlusion against clothing may play a role [22,23]. Another unilateral case was reported in a healthy male and was isolated to the left areola with no other skin involvement [24].
Conclusion
While the factors that predispose to development of TV are generally known, it remains unclear if there are specific etiological factors responsible for unusual variations in location. While these distributions occur more often in conjunction with the typical areas than alone, this is not necessarily so, as demonstrated in our first case. The clinician must be aware of this variability and consider performing a KOH preparation if the lesions have characteristic morphology despite an unusual location. As both topical and oral medications are highly effective in treating this mycosis, neglecting the full scope and distributions of tinea versicolor may result in suboptimal management; the patient may be prescribed topicals when oral medications may have been more appropriate, or may be unaware of all areas requiring topical application, resulting in incomplete resolution of the infection.
Footnotes
Funding: None.
References
- 1.Terragni L, Lasagni A, Oriani A. Pityriasis versicolor of the face. Mycoses. 1991;34(7–8):345–347. doi: 10.1111/j.1439-0507.1991.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph RI, Holzwanger JM. Letter: Inverse tinea versicolor. Arch Dermatol. 1975;111(9):1213. [PubMed] [Google Scholar]
- 3.Kaur I, Handa S, Kumar B. Tinea versicolor: involvement of unusual sites. Int J Dermatol. 1996;35(8):604–605. doi: 10.1111/j.1365-4362.1996.tb03674.x. [DOI] [PubMed] [Google Scholar]
- 4.Gupta AK, Bluhm R, Summerbell R. Pityriasis versicolor. J Eur Acad Dermatol Venereol. 2002;16(1):19–33. doi: 10.1046/j.1468-3083.2002.00378.x. [DOI] [PubMed] [Google Scholar]
- 5.Aljabre SH. Intertriginous lesions in pityriasis versicolor. J Eur Acad Dermatol Venereol. 2003;17(6):659–662. doi: 10.1046/j.1468-3083.2003.00727.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang WW, Tharp MD. A case of tinea versicolor of the eyelids. Pediatr Dermatol. 2013;30(6):e242–3. doi: 10.1111/j.1525-1470.2012.01753.x. Epub Apr 17 2012. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz RA. Superficial fungal infections. Lancet. 2004;364(9440):1173–1182. doi: 10.1016/S0140-6736(04)17107-9. [DOI] [PubMed] [Google Scholar]
- 8.Gupta AK, Batra R, Bluhm R, Faergemann J. Pityriasis versicolor. Dermatol Clin. 2003;21(3):413–429. v–vi. doi: 10.1016/s0733-8635(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 9.Mendez-Tovar LJ. Pathogenesis of dermatophytosis and tinea versicolor. Clin Dermatol. 2010;28(2):185–189. doi: 10.1016/j.clindermatol.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Roberts SO. Pityriasis versicolor: a clinical and mycological investigation. Br J Vener Dis. 1969;81(5):315–326. doi: 10.1111/j.1365-2133.1969.tb13990.x. [DOI] [PubMed] [Google Scholar]
- 11.Bumgarner FE, Burke RC. Pityriasis versicolor; atypical clinical and mycologic variations. Arch Dermatol Syphilol. 1949;59(2):192–195. doi: 10.1001/archderm.1949.01520270068007. [DOI] [PubMed] [Google Scholar]
- 12.Aljabre SH. Sparing of the upper axillary area in pityriasis versi-color. Rev Iberoam Micol. 2005;22(3):167–168. doi: 10.1016/s1130-1406(05)70033-4. [DOI] [PubMed] [Google Scholar]
- 13.Ryu HW, Cho JW, Lee KS. Pityriasis versicolor on penile shaft in a renal transplant recipient. Anna Dermatol. 2012;24(3):345–347. doi: 10.5021/ad.2012.24.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneshvar SA, Hashimoto K. An unusual presentation of tinea versicolor in an immunosuppressed patient. J Am Acad Dermatol. 1987;17(2 Pt 1):304–305. doi: 10.1016/s0190-9622(87)80325-0. [DOI] [PubMed] [Google Scholar]
- 15.Khaddar RK, Cherif F, Ben Hadid R, Mokni M, Ben Osman A. Penile shaft involvement in pityriasis versicolor. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17(2):86–89. [PubMed] [Google Scholar]
- 16.Nia AK, Smith EL. Pityriasis versicolor of the glans penis. Br J Vener Dis. 1979;55(3):230. doi: 10.1136/sti.55.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith EL. Pityriasis versicolor of the penis. Br J Vener Dis. 1978;54(6):441. doi: 10.1136/sti.54.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandhu K, Kanwar AJ. Extensive pityriasis versicolor of the face. J Dermatol. 2004;31(3):258–259. doi: 10.1111/j.1346-8138.2004.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 19.Boralevi F, Marco-Bonnet J, Lepreux S, Buzenet C, Couprie B, Taieb A. Hyperkeratotic head and neck Malassezia dermatosis. Dermatology. 2006;212(1):36–40. doi: 10.1159/000089020. [DOI] [PubMed] [Google Scholar]
- 20.Hughes BR. Tinea versicolor in immunosuppressed patients. J Am Acad Dermatol. 1988;19(2 Pt 1):357–358. doi: 10.1016/s0190-9622(88)80253-6. [DOI] [PubMed] [Google Scholar]
- 21.el-Gothamy Z, Ghozzi M. Tinea versicolor of the scalp. Int J Dermatol. 1995;34(8):533–534. doi: 10.1111/j.1365-4362.1995.tb02946.x. [DOI] [PubMed] [Google Scholar]
- 22.Sardy M, Korting HC, Ruzicka T, Wolff H. Bilateral areolar and periareolar pityriasis versicolor. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2010;8(8):617–618. doi: 10.1111/j.1610-0387.2010.07348.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith BL, Koestenblatt EK, Weinberg JM. Areolar and periareolar pityriasis versicolor. J Eur Acad Dermatol Venereol. 2004;18(6):740–741. doi: 10.1111/j.1468-3083.2004.01040.x. [DOI] [PubMed] [Google Scholar]
- 24.Anthony JL, Schosser RH, Gross DJ. Unilateral areolar and periareolar tinea versicolor. Int J Dermatol. 1991;30(8):600. doi: 10.1111/j.1365-4362.1991.tb02654.x. [DOI] [PubMed] [Google Scholar]


