Abstract
Introduction:
The PARAMOUNT phase III trial demonstrated that pemetrexed continuation maintenance significantly reduced the risk of disease progression (hazard ratio = 0.62) and death (hazard ratio = 0.78) versus placebo in patients with advanced nonsquamous non–small-cell lung cancer. To further understand the survival data, descriptive subgroup analyses were undertaken.
Methods:
Nine hundred thirty-nine patients received induction therapy (four 21-day cycles pemetrexed 500 mg/m2 and cisplatin 75 mg/m2), after which 539 nonprogressing patients with an Eastern Cooperative Oncology Group performance status (PS) of 0/1 were randomized (2:1) to maintenance pemetrexed (500 mg/m2) cycles or placebo until disease progression.
Results:
Baseline characteristics of patients surviving for longer periods were comparable to patients surviving shorter periods, suggesting overall survival (OS) benefit for all subgroups of patients on maintenance therapy. An examination of type and severity of induction adverse events also found no association with survival duration. Response to induction (tumor response versus stable disease) was not determinate of pemetrexed maintenance OS outcome as assessed by waterfall plot and scattergrams and by the distribution of patients among various OS intervals. The length of the interval before beginning maintenance therapy (<7 days versus ≥7/≤30 days) also did not impact the survival results. PS, a known prognostic factor, was the only baseline characteristic associated with improved OS; however, both PS 0 and PS 1 patients exhibited a survival benefit from pemetrexed maintenance.
Conclusions:
In PARAMOUNT, the OS benefit was seen across all subgroups. Other than PS, no baseline or clinical parameter clearly identified a subgroup more likely to benefit. Maintenance treatment decisions should be made on an individual basis.
Keywords: Nonsquamous non–small-cell lung cancer, Pemetrexed, Cisplatin, Induction, Maintenance, Phase III, Survival
Platinum-containing first-line chemotherapy has improved overall survival (OS) of patients with advanced non–small-cell lung cancer (NSCLC), but no additional benefit is realized after four to six cycles, and toxicities associated with platinum administration often prevent more than this number of cycles.1–3 As summarized in a recent review article, results of recent phase III trials have demonstrated that once platinum is discontinued, nonprogressing patients can derive further benefit through administration of maintenance therapy until disease progression, patient–physician decision, or unacceptable toxicity.4
Different concepts of maintenance therapy have been developed. “Continuation maintenance therapy” continues the administration of the nonplatinum component of the chemotherapeutic regimen used during the initial, first-line, “induction” therapy, and “switch maintenance therapy” introduces a different nonplatinum compound for maintenance therapy than that used for induction. For the treatment of advanced NSCLC, erlotinib has yielded a progression-free survival (PFS) and OS benefit in a switch maintenance format.5 The strong impact of the epidermal growth factor receptor (EGFR) mutation on the PFS outcome suggests that switch maintenance with erlotinib might be a valid option for patients with an EGFR mutation.6 Pemetrexed has shown PFS and OS benefit in both the switch maintenance and continuation maintenance settings.7–9 The accumulation of evidence detailing maintenance therapy efficacy has led to various clinical oncology guidelines that outline maintenance therapy as an option for some patients.1–3
In the PARAMOUNT continuation maintenance trial, PFS and OS were significantly improved when patients with advanced nonsquamous NSCLC, who had not progressed after four cycles of pemetrexed–cisplatin induction, were treated with pemetrexed continuation maintenance therapy compared with placebo.8,9 Pemetrexed continuation maintenance resulted in a statistically significant reduction in the risk of disease progression over placebo (hazard ratio [HR] = 0.62; 95% confidence interval [CI], 0.49–0.79; p < 0.0001) and improved OS (HR = 0.78; 95% CI, 0.64–0.96; p = 0.0195), with median OS, measured from randomization, 13.9 months pemetrexed versus 11.0 months placebo.
Additional prespecified analyses of the OS and PFS PARAMOUNT data yielded evidence that the relative treatment effect of pemetrexed was internally consistent across subgroups and similar to that observed in the primary PFS and OS analyses.8,9 Nevertheless, the ongoing need to identify those patients who will benefit most from maintenance therapy and those who will not benefit suggests a more detailed look of the PARAMOUNT data is needed. Some have proposed that switch maintenance may be preferred for patients who exhibit stable disease (SD) after induction, as with the erlotinib results in the Sequential Tarceva in Unresectable NSCLCC (SATURN) trial,10 and that continuation maintenance may be favored for patients who exhibit a partial response (PR) or complete response (CR) during induction.11 A more detailed examination of PARAMOUNT data with this proposed paradigm in mind is in order. Likewise, awareness that the SD designation is given to a fairly heterogeneous group, including patients whose response to induction therapy varies from minimal response to minimal progression, also supports a more detailed examination of the PARAMOUNT data set. Therefore, additional descriptive subgroup analyses of the PFS and OS data of the PARAMOUNT phase III clinical trial were undertaken to determine if the efficacy of pemetrexed continuation maintenance therapy is more pronounced in certain subgroups.
METHODS
Study Design and Patients
Other articles have described study methodology in depth.8,12 To summarize, this phase III trial had two treatment phases: an induction phase of four cycles of pemetrexed (intravenously [IV], 500 mg/m2) and cisplatin (IV, 75 mg/m2) administered on day 1 of a 21-day cycle and a maintenance phase in which eligible patients were randomized (2:1) to continuation pemetrexed (IV, 500 mg/m2) or placebo (IV, 0.9% sodium chloride), both on day 1 of 21-day cycles. Criteria for patients to be eligible for induction included advanced nonsquamous NSCLC (stage IIIB/IV) (Lung Cancer Staging Guidelines, Version 5),13 at least one measureable lesion per Response Evaluation Criteria in Solid Tumors (RECIST 1.0),14 Eastern Cooperative Oncology Group performance status (PS) of 0/1,15 and no previous systemic chemotherapy for lung cancer. Patient eligibility for maintenance included PR or CR or SD following four cycles of pemetrexed–cisplatin induction therapy and PS of 0/1.
Patients were randomized to a maintenance arm and began treatment within 7 days, 21 to 42 days from day 1 of induction cycle 4. Randomization to treatment was stratified by the following prognostic factors: disease stage before induction (IIIB versus IV), tumor response to induction (CR–PR versus SD), and PS before randomization (0 versus 1). Maintenance treatment continued until disease progression, unacceptable toxicity, or patient–physician decision. Patients were followed until death or study closure. During both phases of the study, patients were administered folic acid, vitamin B12 supplementation, and prophylactic dexamethasone as recommended on the pemetrexed label. Dose adjustments and cycle delays (≤42 days) were permitted to resolve toxicities. Tumor measurement proceeded as previously reported.8 Toxicity was assessed before each cycle using the Common Terminology Criteria for Adverse Events, version 3.0.16
The protocol was approved at each site by an ethics review board. Principles of good clinical practice and the Declaration of Helsinki ethical principles were used to guide study conduct. Each patient provided written informed consent before treatment initiation.
Statistical Analyses
All patients randomized to maintenance were included in the efficacy analyses (intent-to-treat). The unadjusted Cox proportional hazards regression model was used to estimate PFS and OS HRs and 95% CI.17 Kaplan–Meier analyses were used to estimate median PFS and OS.18 Waterfall plots were also used to illustrate treatment efficacy for individual patients. Differences in survival estimates between pemetrexed and placebo arms were assessed using a two-sided log-rank test. Using known prognostic variables, post hoc descriptive analyses were undertaken to expand on OS analyses in the Statistical Analysis Plan. Statistical Analysis Software (version 9.1.3; SAS Institute, Cary, NC) was used for all statistical analyses.
RESULTS
As previously reported, 939 patients were enrolled in the induction phase of the study at 83 primarily European sites; 700 of these patients (75%) achieved disease control (tumor response or SD) and 637 patients (68%) completed four cycles of pemetrexed–cisplatin.8 Subsequently, 539 patients who completed four cycles of induction and who exhibited both disease control and PS 0 to 1 were randomized 2:1 to maintenance treatment (359 pemetrexed, 180 placebo).
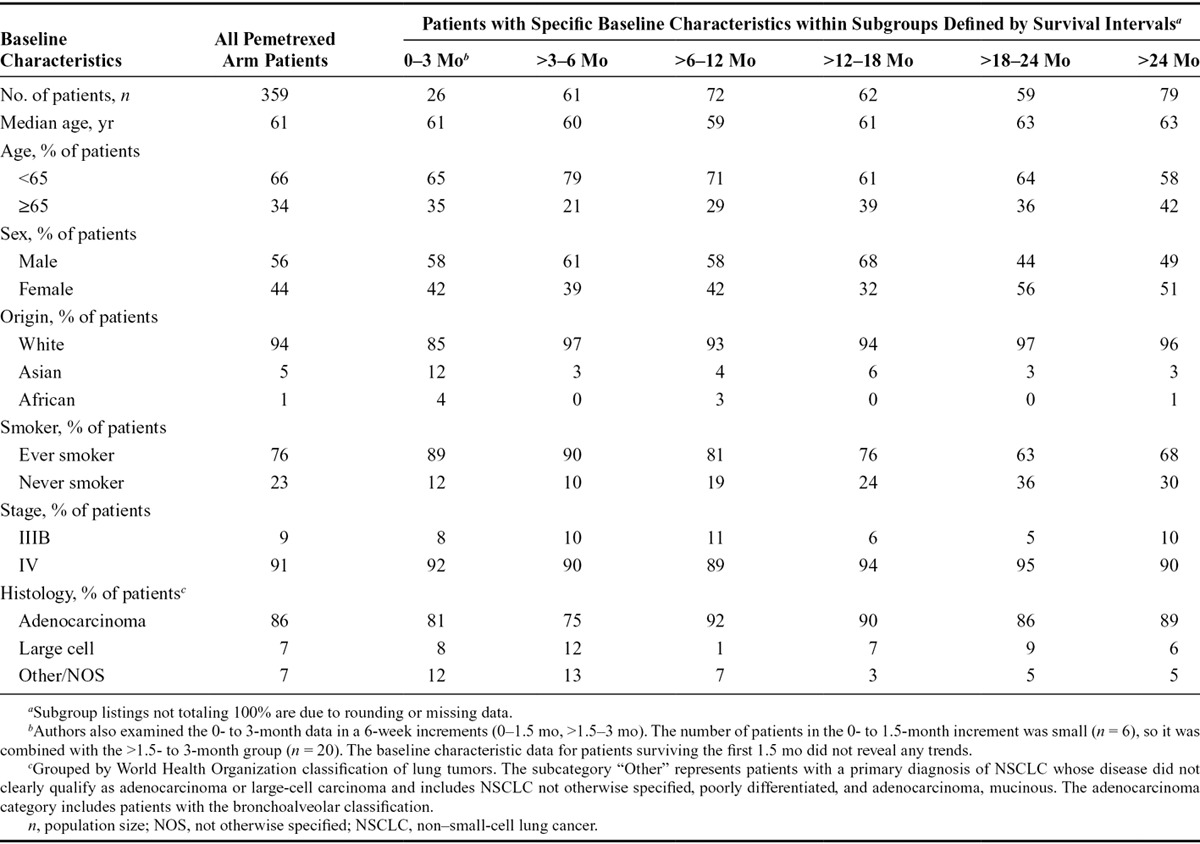
To explore whether any patient or disease characteristic was indicative of longer or shorter survival among patients receiving pemetrexed maintenance therapy, patients were divided into groups based on survival time (Table 1). Although the small patient numbers in some subgroups preclude definitive conclusions, in general, the indicated baseline patient and disease characteristics of patients surviving longer periods were comparable to those surviving shorter periods. This includes patient characteristics of age, sex, ethnic origin, prior smoking status, and tumor histology and stage. These data suggest that there is no baseline characteristic that identifies those patients who receive a long-term survival benefit from pemetrexed maintenance therapy from those patients who stop maintenance treatment early due to disease progression.
TABLE 1.
Comparison of Baseline Characteristics of Pemetrexed Arm Patients Surviving Longer vs. Shorter Periods
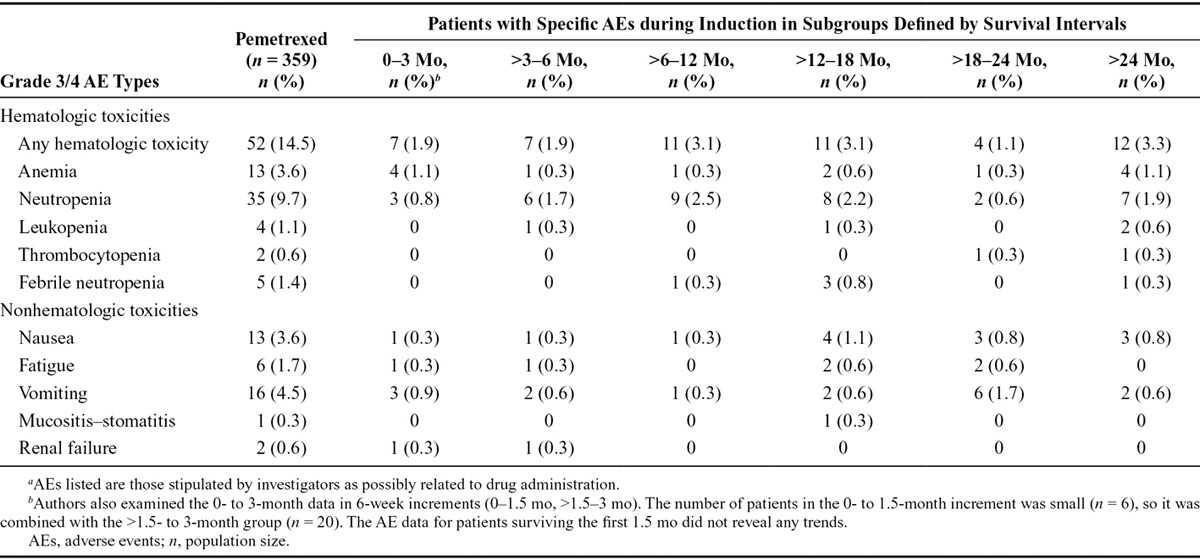
Since adverse events (AEs) during induction therapy can impact physician decision whether to continue with maintenance therapy, we examined if induction AEs were indicative of survival duration among patients receiving pemetrexed maintenance therapy (Table 2). No grade 3 and 4 induction toxicities were experienced disproportionately by patients with OS of shorter duration. Likewise, low grade toxicities (grade 1–2) were not experienced more frequently by patients with shorter survival times (data not shown).
TABLE 2.
Select AEs as Indicators of Length of Overall Survival among Patients Receiving Pemetrexed Continuation Maintenance Therapya
Patient response to induction therapy has also been proposed as an indicator of the utility of maintenance therapy. A prior report noted that both CR–PR and SD subgroups of the pemetrexed maintenance arm showed numerically positive survival results, although the study was not powered for these subgroups and the differences were not statistically significant.8 Further analysis also showed that the response by treatment interaction term was not significant as assessed using the Cox model of response, treatment, and response by treatment interaction (CR–PR versus SD; p = 0.731).8 A compilation of maintenance cycle data for the induction response subgroups revealed that on the pemetrexed arm, patients with an induction response of CR–PR received a similar mean number of cycles as those with an induction response of SD (8.5 versus 7.5; range, 0–44 and 0–37, respectively) but a greater median number of cycles (six versus four, respectively). On the placebo arm, both induction response groups received similar mean and median number of cycles (CR–PR: median, 4.7 cycles; mean, four cycles; range, 0–38 cycles; SD: median, 5.1 cycles; mean four cycles; range, 0–36 cycles). In addition, patients on pemetrexed maintenance therapy were found to have a greater mean number of cycles than patients on placebo maintenance therapy.
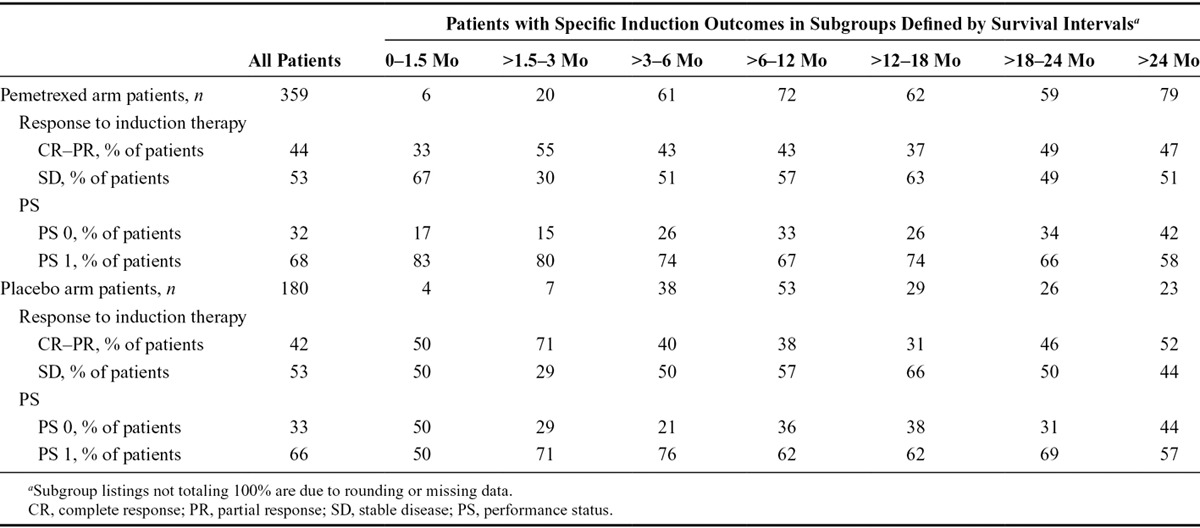
To further analyze induction response as a potential indicator of survival outcome, patients on both arms were separated into induction response subgroups (CR–PR versus SD) then segregated further into groups based on length of survival (Table 3). Similar to the data displayed in Tables 1 and 2, among patients who received maintenance pemetrexed, the proportion of patients with an induction response of CR–PR versus SD was largely consistent over time. Patients who experienced limited benefit from maintenance treatment (OS < 3 mo) are few in number, hindering analysis, but this population contains both patients with a tumor response and those with SD. In the two induction response groups (CR–PR and SD), similar proportions of patients lived greater than 18 months (as measured from randomization to maintenance therapy). Interestingly, OS also did not seem to be impacted by type of induction response among patients who received placebo for maintenance therapy. In general, these results suggest that tumor response to induction as assessed by RECIST designations of tumor response and SD are not indicative of OS outcome for pemetrexed continuation maintenance treatment.
TABLE 3.
Induction Response and PS as Indicators of Overall Survival among Patients Receiving Continuation Maintenance Pemetrexed or Placebo
Because RECIST tumor efficacy designations collapse smaller positive tumor responses (<30% decrease in tumor size) and smaller negative response (<20% increase in tumor size) into one category (SD), and, likewise, group all tumor responses less than complete tumor disappearance into a PR grouping, it is of value to look at tumor size change in individual patient records to further examine its relationship with survival. For each randomized patient, the percent change in the sum of the longest diameter of target lesions diameters (i.e., a normalized index of tumor growth or shrinkage) from baseline to the fourth induction cycle was graphed in a waterfall plot (left y axis), along with an indication of each patient’s PFS or OS time (right y axis) (Fig. 1A and B). The plot illustrates the expected variation within the SD designation (green bars), with a few patients showing a slight increase (up to 23%) in tumor size, with the majority of patients showing either no change in tumor size or modest decrease in tumor size (<30% decrease in the sum of target tumor lesion diameters). OS for patients with SD is variable, with the associated points ranging from approximately 0 to 37 months, as reported above. Likewise, patients with the designation of PR or CR also show a variability in the percent change of the sum of target lesion measurements ranging from 30% to 100%. OS for this group of patients ranged from 0 to 44 months, a range similar to the patients with SD. In general, these results suggest that the degree of tumor shrinkage after induction is not an indicator of OS outcome. Analysis of the data in Figure 1B (through scattergram, plotting individual patient induction response tumor data versus OS time [months]) confirmed this finding (data not shown). Computation of Spearman’s rank correlation coefficient revealed no correlation between the percentage of tumor reduction and final OS (Spearman’s rho = –0.019; p = 0.674).
FIGURE 1.
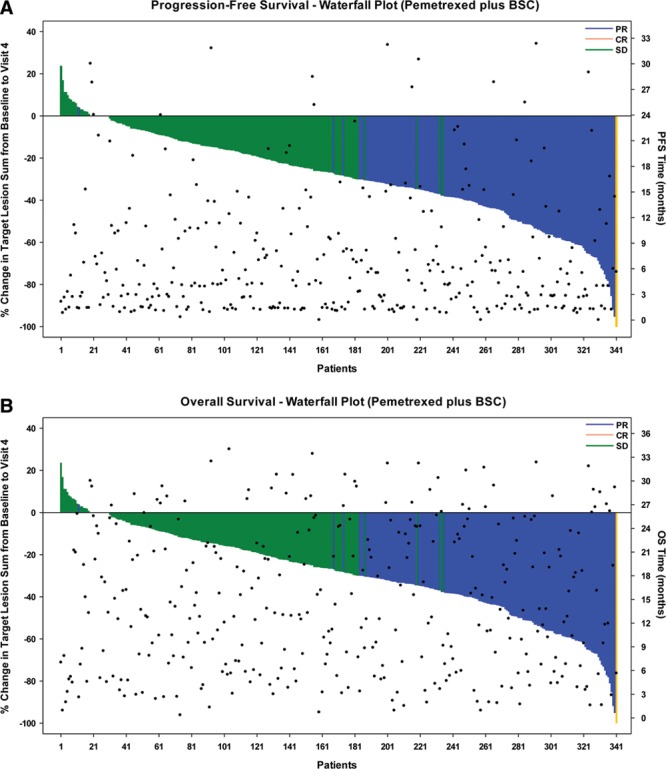
Waterfall plot of induction response of patients treated with pemetrexed. The waterfall plots display individual patient induction response data expressed as percent change in the sum of the longest diameter of target lesions as measured at baseline and visit 4 of the induction period. Best induction tumor response for each patient (SD, PR, or CR) is indicated by the color of the data bar. A, PFS time (months) (right y axis) for each patient as indicated by points. B, OS time (months) (right y axis) for each patient, also indicated by points. SD, stable disease; PR, partial response; CR, complete response; PFS, progression-free survival; OS, overall survival; BSC, best supportive care.
Additional analyses focused on the impact of PS on efficacy outcomes among patients with advanced NSCLC receiving maintenance therapy. When patients on both arms were divided into subgroups based on PS (0 versus 1) and OS, a greater proportion of pemetrexed arm patients with PS 1 had shorter survival times than those with PS 0 (Table 3). Although the study was not powered for subgroup analyses, data were analyzed by Kaplan–Meier estimation for descriptive purposes (Fig. 2A and B). Among patients on the pemetrexed arm, PS 0 patients were found to survive longer than PS 1 patients: median OS PS 0 patients: 17.2 months (95% CI, 14.2–22.7) versus PS 1 patients: 12.9 months (95% CI, 11.8–15.3) (log rank p = 0.023). When median OS was compared between PS 0 and PS 1 patients who received placebo for maintenance therapy, there was no statistical difference (p = 0.423) (Table 4). Nevertheless, both PS 0 and PS 1 patients receiving pemetrexed maintenance therapy survived longer than patients with similar PS receiving placebo (pemetrexed PS 0: 17.2 months versus placebo PS 0: 12.9, p = 0.059; pemetrexed PS 1: 12.9 months versus placebo PS 1: 10.7, p = 0.121). These results were directional, but not significant, likely influenced by inadequate sample size as the study was not powered for this analysis.
FIGURE 2.
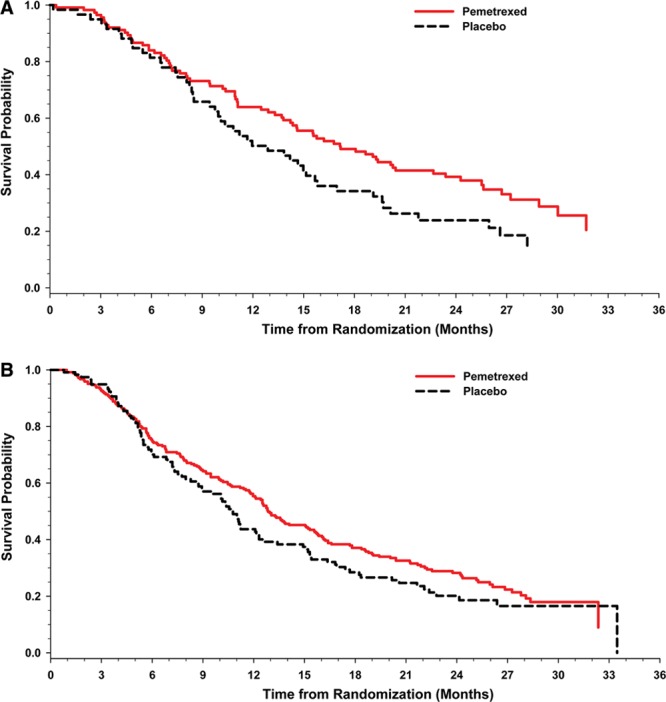
Kaplan–Meier plots of overall survival in performance status subgroups. The Kaplan–Meier plots depict overall survival by performance status 0 (A) and overall survival by performance status 1 (B).
TABLE 4.
OS of Maintenance Therapy Subgroups
A further parameter that might influence the efficacy of pemetrexed continuation maintenance therapy is the length of the interval between completion of induction therapy and the start of maintenance therapy. By protocol specification, maintenance therapy was to start within 21 to 42 days after the start of cycle 4. Median time from start of induction therapy to the start of maintenance treatment was 3 months on both arms. Time from end of induction to the start of maintenance assuming a 21-day cycle 4: median 3 days, range –2 to 30 days, with the majority of patients (68%) initiated maintenance therapy immediately (<7 days) after the completion of induction. The difference in survival in patients who started maintenance therapy less than 7 days from the completion of induction treatment and patients starting greater than or equal to 7 days after the completion of induction treatment was determined. As shown in Table 4, median OS is not significantly different among patients who begin maintenance therapy in less than 7 days versus greater than or equal to 7 days (but ≤30 days).
DISCUSSION
Phase III clinical trials have identified switch and continuation maintenance chemotherapy strategies that result in improved PFS and OS, while maintaining quality of life in advanced NSCLC patients.7–9,19 Examination of the Kaplan–Meier PFS and OS plots in the PARAMOUNT trial reveals that approximately 25% of patients progressed or died at the first 6-week evaluation of pemetrexed continuation maintenance therapy, whereas 32% of PARAMOUNT patients had survived 2 years after randomization.8,9 Similarly, other maintenance trials have patients who survive substantially greater or lesser periods than others in the treatment arm. Interest has grown in predicting which patients may benefit most from maintenance therapy to balance any increased toxicities with quality-of-life concerns and for economic considerations. Previous studies have identified a number of factors that may have bearing on the success of maintenance therapy outcome. These have been summarized in the recent review article by Gerber and Schiller4 and include tumor histology, response to first-line therapy, PS, likelihood of receiving therapy at progression, and molecular characteristics, in particular for those agents with tumor targets with variable expression (e.g., activating EGFR and KRAS mutations).
Tumor histology (nonsquamous subgroups) and other baseline and disease characteristics including patient age, sex, ethnic origin, smoking status, and disease stage were previously examined by Forest Plot for their effect on the PFS and OS efficacy parameters.8,9 In each subgroup, the impact of pemetrexed continuation maintenance treatment on OS and PFS was consistently positive. Likewise, in this analysis summary, neither those patients with limited survival (OS <3 mo) nor those patients with extensive survival (OS >24 mo) were observed to cluster in any particular baseline patient or disease characteristic group. The median age and the proportion of patients greater than 65 years were nearly constant. Likewise, subgroups within smoking status, disease stage, ethnic origin, and sex categories had a relatively consistent percentage of patients in the various survival time categories. The survival interval of 0 to 3 months had too few patients to draw conclusions, but one could note that these patients who benefitted minimally from treatment were represented in all of the subgroups. Type and severity of AEs experienced by patients during induction were also examined to determine if they impacted length of survival; no clear association was observed.
Another parameter widely considered potentially impactful on maintenance results is the response to induction therapy. The initial pemetrexed switch maintenance trial7 and the current PARAMOUNT trial8,9 reported that tumor response during induction did not have bearing on survival parameters. In contrast, the SATURN trial found that erlotinib as a switch maintenance agent only yielded an OS benefit for patients with SD after induction (HR = 0.72 SD versus HR = 0.90 for patients with a tumor response),10 an outcome reflected in the maintenance indication for which erlotinib is approved for treatment in Europe. In a separate study by Fidias et al.,20 docetaxel was found to be effective as a switch maintenance agent only for the cohort of patients with a tumor response after induction (HR = 0.61 versus 1.02 for patients with SD).4 Results from the study by Pérol et al.11 led to the suggestion that induction response may affect survival results when using continuation maintenance. The post hoc analyses reported herein sought to further explore the initial PARAMOUNT finding. When patients were divided into induction response groups (CR–PR versus SD) and the percentage of pemetrexed arm patients was compared with OS time falling within specific intervals, the proportion of patients in the induction response groups was relatively consistent. When this descriptive analysis was expanded to look at individual patient data through a waterfall plot, both the PFS and OS outcomes were entirely random relative to the induction tumor response. This was true for patients who manifested SD as slight tumor growth and those who exhibited modest target tumor shrinkage. Likewise, patients who had been characterized as partial responders with target tumor shrinkage in the range of greater than 30% to 93% exhibited a wide variation in PFS and OS. Further analysis with a scattergram confirmed the lack of correlation, thus leading to the conclusion that degree of tumor reduction does not predict outcome of continuation maintenance in the PARAMOUNT study.
This finding differs from that of SATURN10 and other studies that indicate patients with SD following first-line chemotherapy have a poorer prognosis than patients with a tumor response. Comparing the SATURN study with PARAMOUNT, one notes that the induction regimen for SATURN consisted of four cycles of a mix of platinum-based doublet chemotherapy, whereas in PARAMOUNT, all patients received pemetrexed–cisplatin for induction. Whether this difference has bearing on the similar responsiveness of the induction response subgroups (SD and PR–CR) remains to be investigated.
The waterfall plots used to confirm the dissociation of induction tumor response and survival outcomes were also a useful tool to visualize the substantial variability in tumor response within a group of patients. Expressing tumor response data in terms of RECIST categories may be helpful for analytical purposes, but it yields an incomplete picture of the results. In particular, the SD category represents a highly heterogeneous population as evidenced by the waterfall plots and corresponding survival data. Indeed, these plots underscore the need to review how well a patient responds to treatment through tolerability, symptomatic, and radiological assessment, when determining whether to recommend maintenance treatment.
PS is a known prognostic factor for patient survival in advanced NSCLC.21 Descriptive analyses identified a significant improved survival outcome for pemetrexed continuation maintenance patients with PS 0 over those with PS 1. Interestingly, PS impacted results sufficiently that the PS 1 pemetrexed cohort had a median OS value comparable to the placebo PS 0 cohort. Nonetheless, both PS 0 and PS 1 pemetrexed arm subgroups had greater median OS than patient subgroups with the same PS who received placebo maintenance therapy. These results are comparable to recently published PS and symptom burden analyses of the phase III switch maintenance pemetrexed trial.22 Although both phase III trial analyses were retrospective and derived from studies not suitably powered for subgroup analysis, they both support the prognostic value of PS and underscore its importance as an indicator of suitability for maintenance. Furthermore, they substantiate the notion of not delaying maintenance treatment in favor of second-line treatment as the delay might entail PS decline. Interestingly, a recent retrospective analysis concluded that patients with poorer PS (≥2) should be considered candidates for well-tolerated maintenance therapy because as a cohort, they are less likely to receive second-line therapy.23 Nevertheless, a study by Brodowicz et al.24 observed that patients with poorer PS (Karnofsky PS ≤ 80) did not exhibit a survival benefit with continuation maintenance gemcitabine, and a second trial that included 60% PS 2 patients also failed to demonstrate improved survival with continuation maintenance gemcitabine.25 Hence, further studies will be necessary to determine if patients with PS ≥2 receive any benefit from continuation maintenance pemetrexed.
Further examination of the concept of delayed treatment led to the analysis whether longer or shorter intervals between the conclusion of induction and the initiation of maintenance treatment might have bearing on survival results. An analysis of survival among patients receiving pemetrexed who initiated maintenance within a week (<7 days) after the end of induction versus those who initiated maintenance after a longer period, but one still within protocol specifications (>7 and ≤30 days), showed that the brief delay in maintenance initiation had no measurable effect on survival results. Nevertheless, prospective studies are needed to fully examine the ongoing consideration of maintenance treatment versus second-line treatment.26 Such studies should include prespecified, fully powered subgroup analyses to examine the impact of potentially predictive factors and assess factors identified with a lower likelihood of receipt of second-line chemotherapy as possible predictors of patients who will benefit from maintenance therapy.4
In addition to the aforementioned limitations inherent in retrospective analyses of study data not powered for subgroup analysis, the PARAMOUNT study and associated analyses are limited by the lack of data on the molecular characteristics of patient tumors associated with longer or shorter survival outcomes. Arguably, although analysis of such tissue at this time would be an open-ended inquiry, molecular genomic studies are thought of as pivotal future determinants of individualized patient care; therefore, appropriate tissue samples should be acquired when possible.
In summary, the analyses summarized here show that other than the PS prognostic marker, no baseline or clinical parameter identifies a subgroup of patients more or less likely to benefit from pemetrexed maintenance therapy. Survival was consistent across subgroups, including tumor response to induction. Degree of tumor shrinkage was not correlated with survival; both long-term and short-term survivors were variable in tumor response, with some long-term survivors characterized as having SD. These data underscore the usefulness of pemetrexed continuation maintenance therapy following four cycles of cisplatin-containing induction therapy for nonprogressing patients with PS 0 to 1. Furthermore, these analyses emphasize the importance of making maintenance treatment decisions based on a full clinical assessment of each patient and with an awareness of each patient’s preferences and needs.
ACKNOWLEDGMENT
Supported by Eli Lilly and Company.
Footnotes
Disclosure: Dr. Reck has consulted for Hoffmann-LaRoche, BMS, Pfizer, AstraZeneca, Daiichi-Sankyo, and Eli Lilly and Company but not in the context of this study. Dr. Paz-Ares has consulted for Eli Lilly in conjunction with this study. Dr. Molinier has received an honorarium from Eli Lilly and Company. Dr. John, Ms. Zimmermann, and Dr. Visseren-Grul are employees of Eli Lilly and Company. Dr. Gridelli has consulted for Roche and for Eli Lilly and Company, both in conjunction with this study and separately; he has received travel support from Eli Lilly and Company. All other authors declare no conflict of interest.
Trial registration: ClinicalTrials.gov, NCT00789373
Presented, in part, as an abstract and poster presentation at the European Society for Medical Oncology 2012 Conference, in Vienna, Austria, 28 September–2 October, 2012. The study rationale and design were published in BMC Cancer 2010(March 8);10:85. Data associated with the primary efficacy end point (progression-free survival) were published in Lancet Oncology 2012;9:247–255; data associated with the overall survival end point were published in Journal of Clinical Oncology 2013;31:2895–2902.
REFERENCES
- 1.NCCN National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Non-Small Cell Lung Cancer Version 3.2012. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl. Accessed April 16, 2013. [Google Scholar]
- 2.Azzoli CG, Temin S, Aliff T, et al. American Society of Clinical Oncology. 2011 focused update of 2009 American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2011;29:3825–3831. doi: 10.1200/JCO.2010.34.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7 Suppl):vii56–vii64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 4.Gerber DE, Schiller JH. Maintenance chemotherapy for advanced non–small-cell lung cancer: new life for an old idea. J Clin Oncol. 2013;31:1009–1020. doi: 10.1200/JCO.2012.43.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. SATURN Investigators. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 6.Brugger W, Triller N, Blasinska-Morawiec M, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non–small-cell lung cancer. J Clin Oncol. 2011;29:4113–4120. doi: 10.1200/JCO.2010.31.8162. [DOI] [PubMed] [Google Scholar]
- 7.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 8.Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2010;13:247–255. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, De Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately following induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 10.Coudert B, Ciuleanu T, Park K, et al. Survival benefit with erlotinib maintenance therapy in patients with advanced non-small-cell lung cancer (NSCLC) according to response to first-line chemotherapy. Ann Oncol. 2012;23:388–394. doi: 10.1093/annonc/mdr125. [DOI] [PubMed] [Google Scholar]
- 11.Pérol M, Chouaid C, Pérol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2012;30:3516–3524. doi: 10.1200/JCO.2011.39.9782. [DOI] [PubMed] [Google Scholar]
- 12.Paz-Ares LG, Altug S, Vaury AT, et al. Treatment rationale and study design for a phase III, double-blind, placebo-controlled study of maintenance pemetrexed plus best supportive care versus best supportive care immediately following induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small cell lung cancer. BMC Cancer. 2010;10:85. doi: 10.1186/1471-2407-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming ID, Cooper JS, Henson DE. Lung. In: ID Fleming, JS Cooper, DE Henson, et al., editors. AJCC Cancer Staging Manual. 5th Ed. Philadelphia, PA: Lippincott-Raven; 1997. pp. 127–137. [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 16.Common Terminology Criteria for Adverse Events v3.0 (CTCAE), 8/2006 update. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30. Accessed June 12, 2013. [Google Scholar]
- 17.Cox DR, Snell EJ. Analysis of Binary Data, 2nd Ed. London, England:: Chapman & Hall; 1989. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Gridelli C, de Marinis F, Pujol JL, et al. Safety, resource use, and quality of life in PARAMOUNT: a phase III study of maintenance pemetrexed versus placebo after induction pemetrexed plus cisplatin for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2012;7:1713–1721. doi: 10.1097/JTO.0b013e318267cf84. [DOI] [PubMed] [Google Scholar]
- 20.Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:591–598. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 21.Socinski MA, Morris DE, Masters GA, Lilenbaum R American College of Chest Physicians. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest. 2003;123(1 Suppl):226S–243S. doi: 10.1378/chest.123.1_suppl.226s. [DOI] [PubMed] [Google Scholar]
- 22.Obasaju C, Bowman L, Wang P, et al. Identifying the target NSCLC patient for maintenance therapy: an analysis from a placebo-controlled, phase III trial of maintenance pemetrexed (H3E-MC-JMEN). Ann Oncol. 2013;24:1534–1542. doi: 10.1093/annonc/mdt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun JM, Park JO, Won YW, et al. Who are less likely to receive subsequent chemotherapy beyond first-line therapy for advanced non-small cell lung cancer? Implications for selection of patients for maintenance therapy. J Thorac Oncol. 2010;5:540–545. doi: 10.1097/JTO.0b013e3181d3504d. [DOI] [PubMed] [Google Scholar]
- 24.Brodowicz T, Krzakowski M, Zwitter M, et al. Central European Cooperative Oncology Group (CECOG) Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52:155–163. doi: 10.1016/j.lungcan.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Belani C. Phase III study of maintenance gemcitabine (G) and best supportive care (BSC) versus BSC, following standard combination therapy with gemcitabine-carboplatin (G-Cb) for patients with advanced non-small cell lung cancer (NSCLC).. Oral presentation presented at the American Society of Clinical Oncology (ASCO) Conference; June 4–8, 2010; Chicago, IL. p. abstract 7506: slide 17. [Google Scholar]
- 26.Edelman MJ, Le Chevalier T, Soria J-C. Maintenance therapy and advanced non-small-cell lung cancer: a skeptic’s view. J Thorac Oncol. 2012;7:1331–1336. doi: 10.1097/JTO.0b013e3182629e37. [DOI] [PubMed] [Google Scholar]


