Abstract
Background:
In this open-label phase I study, the maximum-tolerated dose of cetuximab with concurrent chemoradiotherapy (C-CRT) in stage III non–small-cell lung cancer together with individualized, isotoxic accelerated radiotherapy (RT) was investigated.
Methods:
Patients with stage III non–small-cell lung cancer, World Health Organization performance status 0–1, forced expiratory volume in 1 second more than 50%, carbon monoxide diffusing capacity more than 50%, weight loss less than 10%, and no severe comorbidity were enrolled. Patients without progression after one to two cycles of gemcitabine–carboplatin were included and treated with cetuximab 400 mg/kg d7 and 250 mg/kg weekly together with RT and cisplatin (50 mg/m2 d1, 8; 40 mg/m2 d22)–vinorelbine for 5 weeks. Vinorelbine was escalated in three steps; (1) 10 mg/m2 d1, 8 and 8 mg/m2 d22, 29; (2) 20 mg/m2 d1, 8 and 8 mg/m2 d22, 29; (3) 20 mg/m2 d1, 8; 15 mg/m2 d22, 29. An individualized prescribed RT dose based on normal tissue dose constraints was applied (e.g., mean lung dose 19 Gy). The primary endpoint was the maximum-tolerated dose 3 months after the end of C-CRT; secondary endpoints were toxicity and metabolic response as assessed by positron emission tomography.
Results:
Between September 2007 and October 2010, 25 patients (12 men, 13 women, mean age 59 years) were included. The mean RT dose was 62 ± 6.6 Gy. The vinorelbine dose could be escalated to dose level 3. Twelve of 25 patients experienced greater than or equal to grade 3 toxicity (esophagitis 3, rash 1, diarrhea 1, cough 1, dyspnea 1, vomiting 1, and pulmonary embolism 1). No dose-limiting toxicities were observed. One patient with a complete pathological response in dose level 3 developed a fatal hemoptysis 4 months after RT. Metabolic remissions were observed in 19 of 22 patients.
Conclusion:
C-CRT with cetuximab and cisplatin–vinorelbine is safe to deliver at full dose. The recommended phase II dose is therefore cetuximab 400 mg/m2 d7 and 250 mg/m2 weekly, cisplatin 50 mg/m2 d1, 8; 40 mg/m2 d22 and vinorelbine 20 mg/m2 d1, 8; 15 mg/m2 d22, 29 for 5 weeks together with RT.
Keywords: NSCLC, Concurrent chemoradiotherapy, Cetuximab, Phase I
Approximately 30% of patients with non–small-cell lung cancer (NSCLC) present with locally advanced disease (stage III), for which concurrent chemoradiotherapy (C-CTRT) is mostly the treatment of choice.1,2 Even recent series only show a 5-year survival of approximately 25%.3 Local progression occurs in more than 40% of patients and most develop distant metastases.4 Cisplatin has been the backbone in most series of C-CTRT, with an additional drug such as etoposide or vinorelbine, although cisplatin alone leads to similar outcomes as doublet chemotherapy.5,6
As there is much room for improvement, investigators have combined chemotherapy with cetuximab, a monoclonal antibody against the epidermal growth factor receptor.7 Preclinical studies indicated a strong radiosensitizing effect of cetuximab, which was supported by the improved survival of patients with locally advanced squamous cancer of the head and neck treated with cetuximab and radiation therapy compared with radiotherapy (RT) alone.8,9 Laboratory studies have also shown that the tumor uptake of cetuximab can vary significantly.10,11 Local tumor control of NSCLC shows a dose–response relationship, with higher doses of RT delivered in a short overall treatment time leading to higher overall survival (OS) rates.12 Isotoxic accelerated radiotherapy (INDAR) is a way to deliver to each individual patient the highest dose of RT in the shortest possible treatment time without increasing toxicity.13,14 As the variability of the radiation dose between patients to the lungs is lower with INDAR than with a standard approach where the dose to the tumor is kept constant but the radiation dose to the organs at risk differs widely, this strategy is particularly appealing for phase I trials in which the primary endpoint is typically toxicity. The derived estimations of the maximum-tolerated dose (MTD) with INDAR in combination with novel drugs will be more reliable than if the dose–volume parameters to the normal tissues would differ significantly between enrolled patients. Furthermore, phase I trials of drugs with radiation have specific features previously described.15
As lowering the cetuximab dose would not be logical for biological and toxicity reasons and lowering the dose of radiation would be methodologically incorrect,15 we decided to combine INDAR with the standard dose of cisplatin and cetuximab with escalating doses of vinorelbine. Here, we report the long-term data of a phase I trial aiming to determine the recommended phase 2 dose of concurrent cisplatin–vinorelbine–cetuximab and individualized INDAR in stage III NSCLC.
PATIENTS AND METHODS
The study was performed in two hospitals in The Netherlands. For RT, patients were referred to a single institute (MAASTRO Clinic). The study was designed as an open-label phase I study.
Inclusion criteria were as follows: pathologically proven NSCLC, stage III disease, World Health Organization (WHO) performance status 0–1, forced expiratory volume in 1 second more than 50%, carbon monoxide diffusing capacity more than 50%, weight loss less than 10%, no severe cardiac disease, creatinine clearance (Cockcroft) more than 60 ml/min. Patients with mixed histology, malignant pleural, or pericardial effusion were excluded.
The study was approved by the local medical ethics committees, and all patients signed informed consent before entering the study. The study was registered at clinicaltrials.gov (NCT00522886).
Treatment
Treatment scheme and dose escalation are displayed in Figure 1. Patients were treated with two cycles of gemcitabine (1250 mg/m2 d1, 8) and carboplatin (area under the curve 5 d1 every 3 weeks). In 2010, however, the study was amended after the publication of Cancer and Leukemia Group B (CALGB) 30105, which showed no benefit of induction chemotherapy.16 For practical reasons, a single cycle of induction carboplatin–gemcitabine was still administered before the start of C-CRT. Patients without progression after gemcitabine/carboplatin were included in the phase I part and treated with cetuximab 400 mg/m2 d7 (1 week before the start of RT) and 250 mg/m2 weekly thereafter together with RT and cisplatin (50 mg/m2 d1, 8; and 40 mg/m2 d22) plus vinorelbine for 5 weeks. Vinorelbine was escalated in three steps—step 1: 10 mg/m2 d1, 8 and 8 mg/m2 d22, 29; step 2: 20 mg/m2 d1, 8 and 8 mg/m2 d22, 29; step 3: 20 mg/m2 d1, 8 and 15 mg/m2 d22, 29. The radiation dose was 45 Gy, given in 30 twice daily fractions of 1.5 Gy, immediately followed by once-daily fractions of 2 Gy until a mean lung dose of 19 Gy or another normal tissue constraint, as previously described,13,14 was reached. The maximal allowed dose was 69 Gy in 6 weeks.
FIGURE 1.
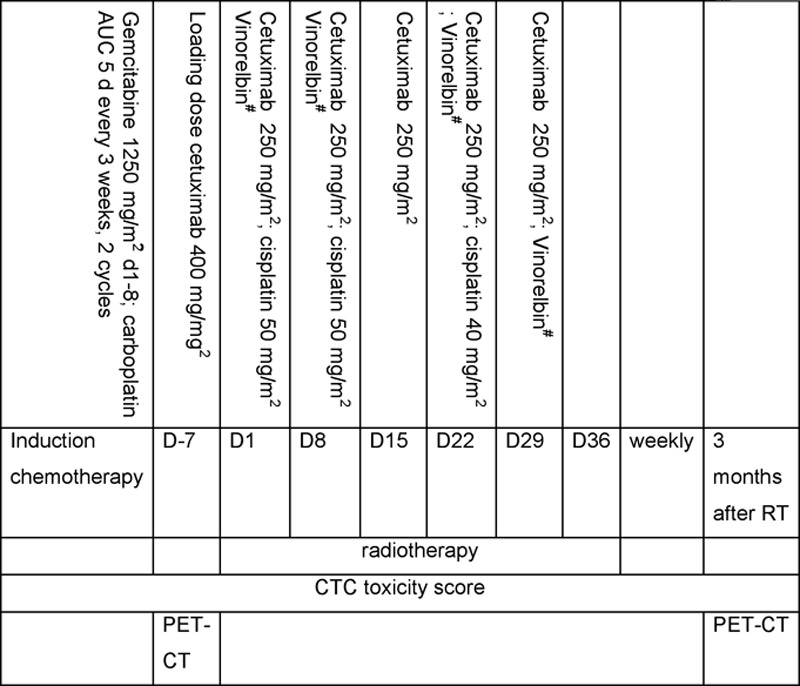
Treatment and dose escalation scheme. #Vinorelbine dose escalation. Step 1: 10 mg/m2 d1, 8 and 8 mg/m2 d22, 29; step 2: 20 mg/m2 d1, 8 and 8 mg/m2 d22, 29; step 3: 20 mg/m2 d1, 8 and 15 mg/m2 d22, 29.
RT planning was done as previously described.13,14 In short, a positron emission tomography (PET)-computed tomography (CT), with intravenous contrast for the CT, was used for RT planning. The gross tumor volume (GTV) of the primary tumor was delineated on the CT with lung window, with the lymph nodes on mediastinal settings. Only fluorodeoxyglucose (FDG)-avid nodes or those proven to be positive on endoscopic examination with biopsy were included in the nodal GTV. The clinical target volume was defined as the GTV with a margin of 5 mm, edited according to organs at risk (e.g., the bones). The planning target volume was made by expanding the clinical target volume with a margin of an average 10 mm, according to the movement of the tumor and the setup error. The organs at risk (lungs, esophagus, spinal cord, and heart) were contoured on each slide throughout the thorax.
Calculation of the dose distribution was done with a CMS (XiO) treatment planning system using a superposition–convolution algorithm, according to International Commission on Radiation Units (ICRU) 50 standards. Portal imaging and exit dosimetry were used for quality control during treatment.
Toxicity
Toxicity was scored from baseline until 3 months after the last day of RT, according to the Common Toxicity Criteria for Adverse Events version 3.0. As clinical dyspnea and pneumonitis overlap in Common Toxicity Criteria for Adverse Events version 3.0, it was decided to report dyspnea only.
Endpoints
The primary endpoint of the study was the MTD. Secondary endpoints were toxicity and metabolic response as assessed by FDG-PET 3 months after C-CTRT, according to the Positron Emission Tomography Response Criteria in Solid Tumors of the European Organization for Research and Treatment of Cancer.17,18
Study Design
Patients were followed for toxicity until 3 months after the last day of C-CTRT: weekly during C-CTRT and every 4 weeks thereafter till 3 months after C-CTRT. Every cohort consisted of six patients: when six patients were entered in a certain cohort, the study was “on hold” until all patients had been followed for 3 months after RT and no MTD-defining toxicity observed in the cohort. MTD was defined as: greater than or equal to two of six patients had greater than or equal to grade 3 pneumonitis, diarrhea, liver, or renal toxicity or greater than or equal to three of six patients had greater than or equal to grade 3 esophagitis. If there was no MTD in a cohort, then if one of six patients in the cohort had developed grade 4 skin or neurological toxicity or grade 5 hematological toxicity, the dose level was extended with six patients (Fig. 2). Patients were included in the next dose level when all patients in a given dose level had been followed for 3 months and MTD was not reached. Safety data were analyzed by an independent safety data monitoring board. OS was defined as the time from study inclusion till death. Progression-free survival (PFS) was defined as time from inclusion till progression or death. Patients were censored at date of last follow-up.
FIGURE 2.
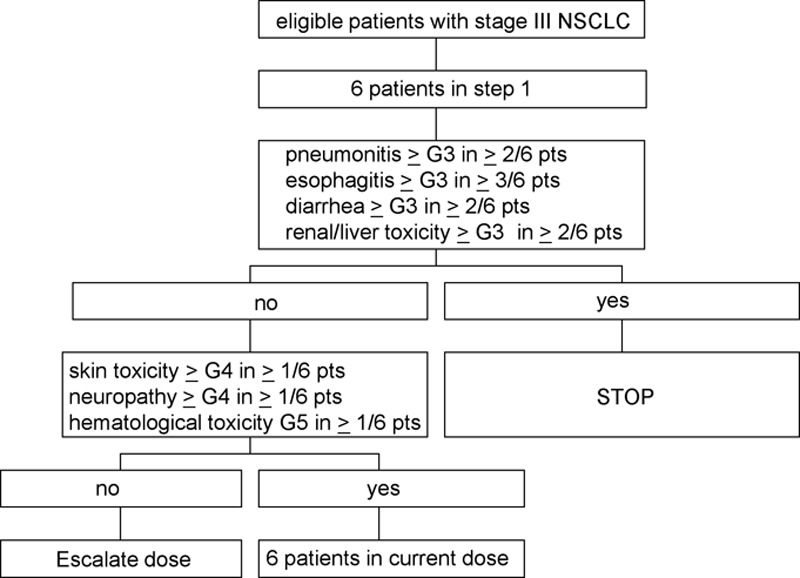
Dose escalation scheme.
RESULTS
Patient Characteristics
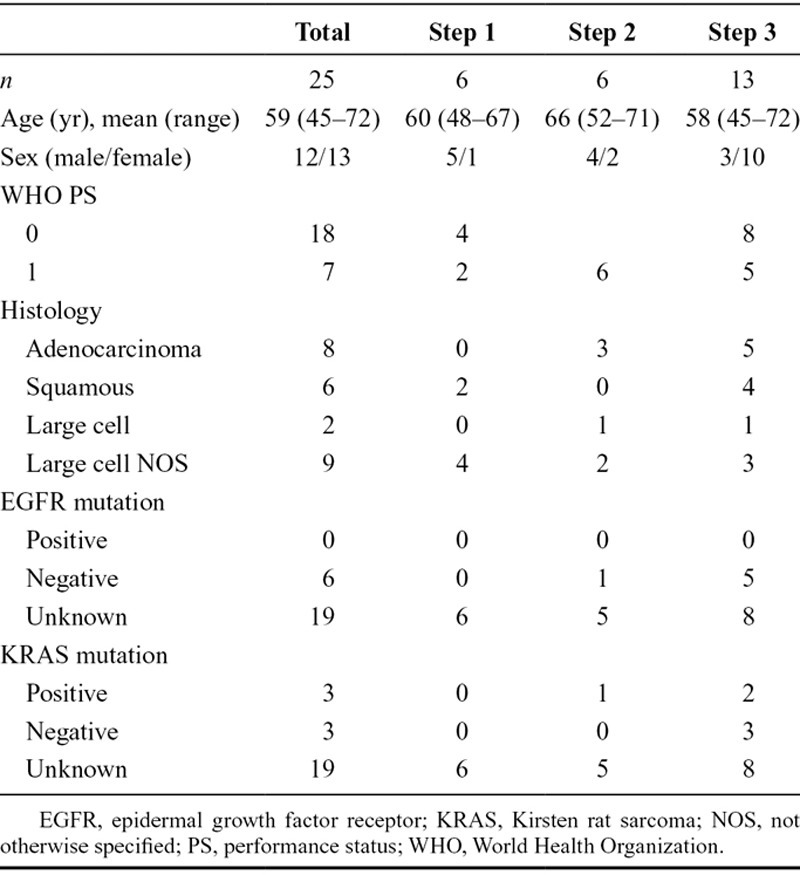
Between September 2007 and October 2010, 27 patients were included in the trial: six in dose level 1, six in dose level 2, and 15 in dose level 3. Six extra patients were enrolled in dose level 3, at the request of the independent safety monitoring board, of whom three were replaced (two patients did not enter the phase I part of the study because of toxicity of the induction treatment and disease progression after induction treatment; one patient withdrew consent and stopped treated after one cycle of C-CRT without an MTD-defining toxicity). Finally, 25 of 27 enrolled in the phase I study and were included in the safety analysis and 24 of 27 in the survival analysis. The patient characteristics of these 25 patients (12 men and 13 women, mean age 59 years) are described in Table 1. As the study was amended in January 2010, patients in cohort 3 (extension) received one course instead of two courses of induction treatment. This amendment was submitted because at the time of inclusion of the extra patients in the level 3 cohort the standard care of patients with stage III NSCLC had changed; it had been shown that induction chemotherapy is not additive.
TABLE 1.
Patients Characteristics
Dose Intensity and Toxicity
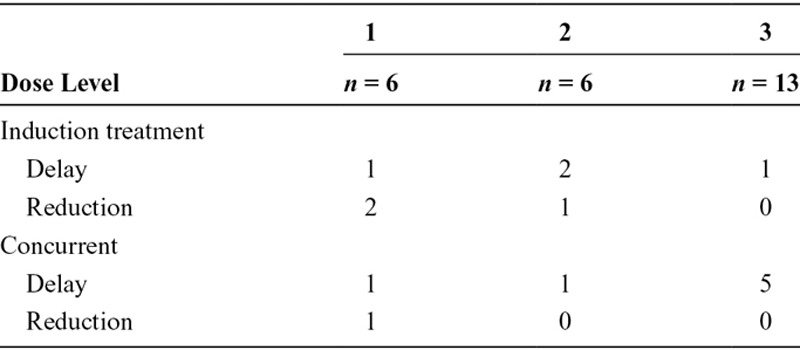
All assessable patients received the planned number of courses of chemotherapy. The first 18 patients, six in each cohort, received two courses of induction treatment with carboplatin and gemcitabine; dose reduction or delay of the second cycle was necessary in five of 18 patients (two dose level 1 and three in dose level 2), all because of hematological toxicity. In the concurrent phase of the treatment in dose level 1, a dose reduction and a delay of treatment were necessary in one patient because of renal toxicity; in dose level 2, in one patient the dose of chemotherapy was reduced because of hematological toxicity; in dose level 3, chemotherapy was delayed in five of 12 assessable patients: one because of logistic reasons, one because of hematological toxicity, one because of patient decision, one because of infection, and one unknown (Table 2). As stated before in this cohort, one patient stopped prematurely; she withdrew consent and stopped treatment because of psychological reasons.
TABLE 2.
Dose Reductions and Delays
Median overall treatment time, defined as first day of induction chemotherapy till last day of RT was 75 days (range 24–93 days); for the 18 patients with two cycles of induction treatment, the median overall treatment time was 76 days and for the six patients with one cycle of induction treatment, 57 days. Median total radiation dose was 65 Gy (range 9–69 Gy). Median lung dose was 17 Gy (range 5.0–20.2 Gy), and median esophagus dose was 25.6 Gy (range 14.4–37.8 Gy).
The dose could be escalated to dose level 3. No dose-limiting toxicities were observed. Twelve of 25 patients experienced greater than or equal to grade 3 toxicity (fatigue, esophagitis, skin toxicity, diarrhea, cough, dyspnea, vomiting, and pulmonary embolism; Table 3). Dose-limiting toxicity (DLT) was not reached. The most frequent toxicity was skin rash.
TABLE 3.
Treatment-Related Toxicity CTCAE 3.0 More Than Grade 2
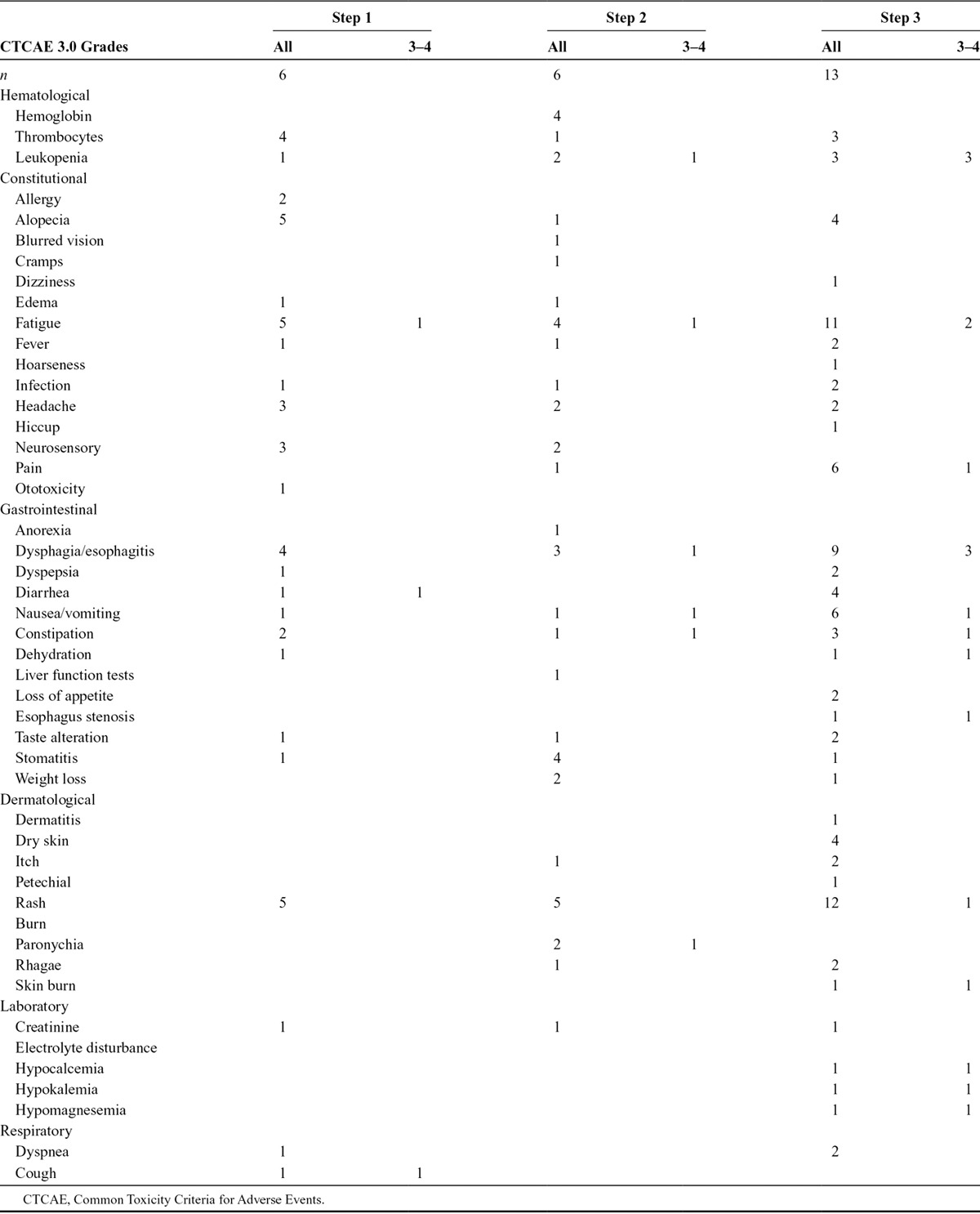
One patient in dose level 3 developed a fatal hemoptysis 4 months after RT. This was a patient with a complete metabolic response at 3 months. Necropsy was performed. No cancer was found. Although this event occurred after the defined MTD period (3 months after C-CRT), it was decided after discussion with the independent safety monitoring board to enroll six extra patients at dose level 3. In this additional cohort, two patients did not enter the phase I trial and one patient stopped treatment without a DLT before the MTD period of 3 months after C-CRT. This patient was however included in the analysis for toxicity and survival.
The recommended phase II dose is therefore cetuximab 400 mg/m2 d7 and 250 mg/m2 weekly, cisplatin 50 mg/m2 d1, 8; 40 mg/m2 d22 and vinorelbine 20 mg/m2 d1, 8; 15 mg/m2 d22, 29 for 5 weeks together with RT.
The database was closed in October 2012 when the last patient had been followed up for 2 years. Median OS was 21.0 months (95% confidence interval 19.0–22.8 months), and 2-year survival rate was 42%. Median PFS was 14.8 months, 95% confidence interval 5.5 to 24.0 months; 2-year PFS rate was 40% (Fig. 3).
FIGURE 3.
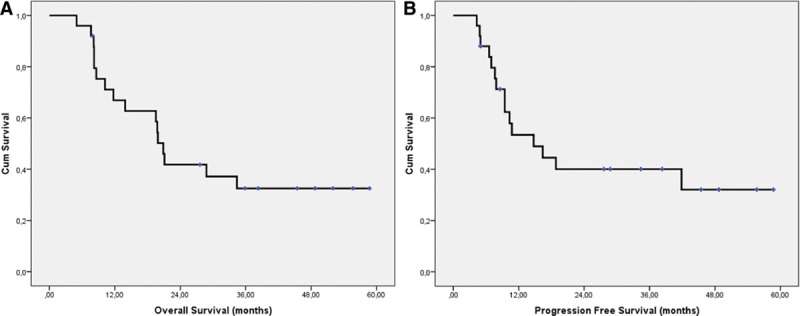
A, Overall survival; (B) progression-free survival.
FDG-PET scans 3 months after C-CRT were available for 22 patients. According to Positron Emission Tomography Response Criteria in Solid Tumors, eight patients had a complete metabolic response, 11 a partial response, and three patients a progressive disease.
DISCUSSION
Several attempts have been made to improve the outcome of stage III NSCLC patients. Strong preclinical data and other early clinical trials in NSCLC support the use of cetuximab delivered concurrently with chemotherapy and RT.14 At the time of developing this protocol, no data were available about the combination of cetuximab with cisplatin and vinorelbine. Moreover, we chose to use INDAR as a way to deliver to each individual patient the highest dose of RT in the shortest possible treatment time without increasing toxicity.13,14 As this is an “equitoxic” approach, the variability of the radiation dose between patients to the lungs is lower with INDAR than with a standard strategy where the dose to the tumor is kept constant but the radiation dose to the organs at risk differs widely. The derived estimations with INDAR of the MTD of INDAR in combination with novel drugs will be more reliable than if the dose–volume parameters to the normal tissues would differ significantly between enrolled patients.
Because cisplatin is the backbone of C-CRT schedules and lowering the cetuximab dose did not seem to be necessary in view of its low toxicity, we designed a trial in which vinorelbine was escalated. The aim of this study was to establish the MTD of C-CRT with cetuximab–vinorelbine–cisplatin.
The MTD was not reached, and the dose could be escalated to the full dose of vinorelbine. The MTD observation period was 3 months after C-CTRT, implying that between the different dose levels during a period of 6 months (treatment period of last patient enrolled and 3 months follow-up) no patients were enrolled in the study that is common in phase 1 trials involving RT.15 This, together with the decision to enroll six additional patients in dose level 3 because of a grade 4 hemoptysis 4 months after the end of treatment, prolonged the duration of this study considerably. At the time, the study was designed using gemcitabine–carboplatin as induction chemotherapy was common in our region. Evidence about induction treatment changed and resulted in an amendment to omit the two cycles of induction chemotherapy and to use for practical reasons only a single cycle of induction chemotherapy. We believe, however, that the amendment has not influenced the outcome of the study, as in the later cohorts (with only one cycle of induction treatment) certainly no less toxicity was observed.
In two other studies, the addition of cetuximab to C-CRT in locally advanced NSCLC was investigated.19,20 In the CALGB trial 30407, carboplatin (AUC6)–pemetrexed 500 mg/m2 was studied with or without the addition of cetuximab.19 In this randomized phase II study, systemic doses of chemotherapy and cetuximab were used for four cycles and four additional cycles of pemetrexed monotherapy, starting RT on day 1 of the chemotherapy. The main toxicity in the CALBG study was grade 4 thrombocytopenia, requiring dose reduction of carboplatin to AUC5 after 19 patients had been enrolled. No difference in toxicity was observed between the arms with and without cetuximab. Although the primary endpoint (OS at 18 months) was reached in this study, the difference between the arms was not significant (58% versus 54%).The median failure-free survival was 12.6 versus 12.3 months.
The RTOG 0324 is a single-arm phase II study with the standard RTOG scheme of weekly carboplatin AUC2 and paclitaxel 45 mg/m2 for 6 weeks concurrent with the RT followed by two cycles of consolidation chemotherapy with carboplatin AUC6 and paclitaxel 200 mg/m2. Weekly cetuximab was added to the standard treatment. The primary endpoint was safety and compliance. In the 87 assessable patients, median OS was 22.7 months, which is longer than historical data from RTOG. Toxicity was mainly hematologic, with 20% grade 4 toxicities. Only 8% grade 3 esophagitis was reported. Esophagitis grade 3 or more in our study was 16% and comparable with data from other studies.
Toxicity in our series was in line with published trials and our own experience,21–24 despite the administration of cetuximab in addition to cisplatin and vinorelbine and the delivery of intensified, high-dose RT. Severe radiation dermatitis associated with concurrent cetuximab treatment, as was seen in patients with head and neck cancer,25 was not a major problem in our study with only one patient having grade 3 rash. Severe neutropenia seems more frequent in cetuximab-treated patients according to a systematic review,26 most frequently in colorectal cancer and NSCLC. In our study, four of 25 patients (16%) experienced a grade 3 neutropenia. In the CALBG 30407, no increase in febrile neutropenia was observed in the arm with cetuximab (8% versus 6%).19 In the RTOG 0324 study, with carboplatin and paclitaxel as backbone, 20% grade 4 hematological toxicities were observed.20 Surprisingly, we did not see patients with severe dyspnea indicating radiation-induced pneumonitis in our study. Only three patients had dyspnea, one in dose level 1 and two in dose level 3, all grade 1 to 2. This can be because of the short period of toxicity reporting in our phase I study (till 3 months after C-CRT) and the relatively small group of patients. Only one patient presented with hemoptysis, but in this case it was a very severe, fatal event in a patient with a complete pathological response. This patient had a very large (T4) adenocarcinoma in the right upper lobe with invasion of the mediastinum and congruent with lymph nodes 4R and 7, with a good response after C-CRT. On CT, no cavitation was visible; however, pathological postmortem examination showed necrosis in the right upper lung in close relation with the vena cava superior and showed aspergillus infection in the necrotic tissue. In none of the other patients, hemoptysis was reported in the follow-up.
Despite a high rate of metabolic responses on FDG-PET-CT scan 3 months after C-CRT, the OS was less than in previous trials and also inferior to our own series with concurrent INDAR and cisplatin–vinorelbine.10 Although we cannot rule out an interaction between cetuximab and other drugs, patient selection, the decreased vinorelbine dose in part of the patients, and the low number of patients may be reasons for the lower than expected OS in this phase I study that was not designed for OS assessment. Two recently published studies on the use cetuximab together with C-CRT did not show improvement in OS.27,28 Both these studies used a different chemotherapy and radiation schedule, being low-dose daily cisplatin with 66 Gy in 24 once-daily fractions and weekly carboplatin and paclitaxel with 60 Gy or 74 Gy in 2-Gy fractions (RTOG0167), respectively. The combination of concurrent chemotherapy and RT with cetuximab is thus not useful in unselected patients.
Recently, it has been shown that high EGFR expression could be used as a biomarker to predict outcome in NSCLC patients treated with cisplatin–vinorelbine and cetuximab in the FLEX trial.7 Unfortunately, in our study, histological biopsies were not mandatory and a retrospective survey showed that not enough tumor material was available to do this analysis. Furthermore, in a recently published study in the subgroup of patients with available material for EGFR testing, patients who had H-score more than 200 did not have better outcome when treated with cetuximab and C-CRT.27
There is still much room for improvement of treatment in stage III with C-CRT as the 5-year survival rates seems to stuck approximately 25%. Neither induction nor consolidation chemotherapy have shown better outcomes.29,30 Both improvement of local control by more sophisticated RT31 and prevention of metastasis by systemic therapy are important. As the addition of cetuximab to intensified, accelerated RT and cisplatin–vinorelbine did not show DLT, this strategy, possibly strengthened by EGFR imaging or specific molecular markers, may be further pursued. The concept of using an isotoxic RT scheme to decrease the variability in toxicity due to dose–volume effects in phase I trials was shown to be practically feasible.
ACKNOWLEDGMENTS
The authors acknowledge financial support from the Center for Translational Molecular Medicine (CTMM) framework (AIRFORCE project, grant 030-103), European Union (EU) 7th framework program (EURECA, ARTFORCE), euroCAT (IVA Interreg—www.eurocat.info), NGI Pre-Seed grant (no 93612005), Kankeronderzoekfonds Limburg from the Health Foundation Limburg and the Dutch Cancer Society (KWF UM 2008–4210, KWF UM 2011–5020).
Footnotes
Disclosure: Cetuximab was donated by Merck KGaA, Darmstadt, Germany. A-M.C.D. received honoraria from Roche, Eli Lilly, Pfizer, and Amgen and is on the speaker’s bureau for Roche. All the other authors declare no conflict of interest.
REFERENCES
- 1.De Ruysscher D, Botterweck A, Dirx M, et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol. 2009;20:98–102. doi: 10.1093/annonc/mdn559. [DOI] [PubMed] [Google Scholar]
- 2.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 4.Machtay M, Paulus R, Moughan J, et al. Defining local-regional control and its importance in locally advanced non-small cell lung carcinoma. J Thorac Oncol. 2012;7:716–722. doi: 10.1097/JTO.0b013e3182429682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pöttgen C, Eberhardt WE, Gauler T, et al. Intensified high-dose chemoradiotherapy with induction chemotherapy in patients with locally advanced non-small-cell lung cancer-safety and toxicity results within a prospective trial. Int J Radiat Oncol Biol Phys. 2010;76:809–815. doi: 10.1016/j.ijrobp.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Pirker R, Pereira JR, von Pawel J, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13:33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Morsbach F, Sander D, et al. EGF receptor inhibition radiosensitizes NSCLC cells by inducing senescence in cells sustaining DNA double-strand breaks. Cancer Res. 2011;71:6261–6269. doi: 10.1158/0008-5472.CAN-11-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 10.Aerts HJ, Dubois L, Hackeng TM, et al. Development and evaluation of a cetuximab-based imaging probe to target EGFR and EGFRvIII. Radiother Oncol. 2007;83:326–332. doi: 10.1016/j.radonc.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Aerts HJ, Dubois L, Perk L, et al. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J Nucl Med. 2009;50:123–131. doi: 10.2967/jnumed.108.054312. [DOI] [PubMed] [Google Scholar]
- 12.Mauguen A, Le Péchoux C, Saunders MI, et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol. 2012;30:2788–2797. doi: 10.1200/JCO.2012.41.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Baardwijk A, Reymen B, Wanders S, et al. Mature results of a phase II trial on individualised accelerated radiotherapy based on normal tissue constraints in concurrent chemo-radiation for stage III non-small cell lung cancer. Eur J Cancer. 2012;48:2339–2346. doi: 10.1016/j.ejca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 14.van Baardwijk A, Wanders S, Boersma L, et al. Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non-small-cell lung cancer. J Clin Oncol. 2010;28:1380–1386. doi: 10.1200/JCO.2009.24.7221. [DOI] [PubMed] [Google Scholar]
- 15.Pijls-Johannesma M, van Mastrigt G, Hahn SM, et al. A systematic methodology review of phase I radiation dose escalation trials. Radiother Oncol. 2010;95:135–141. doi: 10.1016/j.radonc.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 17.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 18.Aerts HJ, van Baardwijk AA, Petit SF, et al. Identification of residual metabolic-active areas within individual NSCLC tumours using a pre-radiotherapy (18)Fluorodeoxyglucose-PET-CT scan. Radiother Oncol. 2009;91:386–392. doi: 10.1016/j.radonc.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol. 2011;29:3120–3125. doi: 10.1200/JCO.2010.33.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumenschein GR, Jr, Paulus R, Curran WJ, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol. 2011;29:2312–2318. doi: 10.1200/JCO.2010.31.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehing-Oberije C, De Ruysscher D, Petit S, et al. Development, external validation and clinical usefulness of a practical prediction model for radiation-induced dysphagia in lung cancer patients. Radiother Oncol. 2010;97:455–461. doi: 10.1016/j.radonc.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Nalbantov G, Kietselaer B, Vandecasteele K, et al. Cardiac comorbidity is an independent risk factor for radiation-induced lung toxicity in lung cancer patients. Radiother Oncol. 2013;109:100–106. doi: 10.1016/j.radonc.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Petit SF, van Elmpt WJ, Oberije CJ, et al. [18F]fluorodeoxyglucose uptake patterns in lung before radiotherapy identify areas more susceptible to radiation-induced lung toxicity in non-small-cell lung cancer patients. Int J Radiat Oncol Biol Phys. 2011;81:698–705. doi: 10.1016/j.ijrobp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 24.De Ruysscher D, Dehing C, Yu S, et al. Dyspnea evolution after high-dose radiotherapy in patients with non-small cell lung cancer. Radiother Oncol. 2009;91:353–359. doi: 10.1016/j.radonc.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Giro C, Berger B, Bölke E, et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiother Oncol. 2009;90:166–171. doi: 10.1016/j.radonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Chen YZ, Shi D, Shi XY, Zou Z, Zhao JH. Incidence and risk of severe neutropenia in advanced cancer patients treated with cetuximab: a meta-analysis. Drugs R D. 2011;11:317–326. doi: 10.2165/11598190-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Heuvel MM, Uyterlinde W, Vincent AD, et al. Additional weekly cetuximab to concurrent chemoradiotherapy in locally advanced non-small cell lung carcinoma: efficacy and safety outcomes of a randomized, multi-center phase II study investigating. Radiother Oncol. 2014;110:126–131. doi: 10.1016/j.radonc.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Bradley J, Masters GA, Hu C, et al. An intergroup randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) chemoradiotherapy (CRT) +/- cetuximab (cetux) for stage III non-small cell lung cancer (NSCLC): results on cetux from RTOG 0617. J Thorac Oncol. 2013;8(suppl 2) [PubMed] [Google Scholar]
- 29.Hanna N, Neubauer M, Yiannoutsos C, et al. Hoosier Oncology Group; US Oncology. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 30.Vokes EE, Herndon JE, 2nd, Kelley MJ, et al. Cancer and Leukemia Group B. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698–1704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 31.Roelofs E, Engelsman M, Rasch C, et al. ROCOCO Consortium. Results of a multicentric in silico clinical trial (ROCOCO): comparing radiotherapy with photons and protons for non-small cell lung cancer. J Thorac Oncol. 2012;7:165–176. doi: 10.1097/JTO.0b013e31823529fc. [DOI] [PubMed] [Google Scholar]


