CASE REPORTS
Case 1
In September 2012, a 74-year-old nonsmoker man was admitted to our hospital with dyspnea and general health degradation. A tumor in the upper lobe of the right lung, bilateral mediastinal lymphadenopathies, and a right pleural effusion were evident on abdominal computed tomography (CT) scan (Fig. 1B). Both pleural effusion and lung biopsies contained tumor cells presenting the p.L858R EGFR (exon 21) mutation. This alteration was also detected in the plasma sample collected before treatment (Fig. 1A).
FIGURE 1.
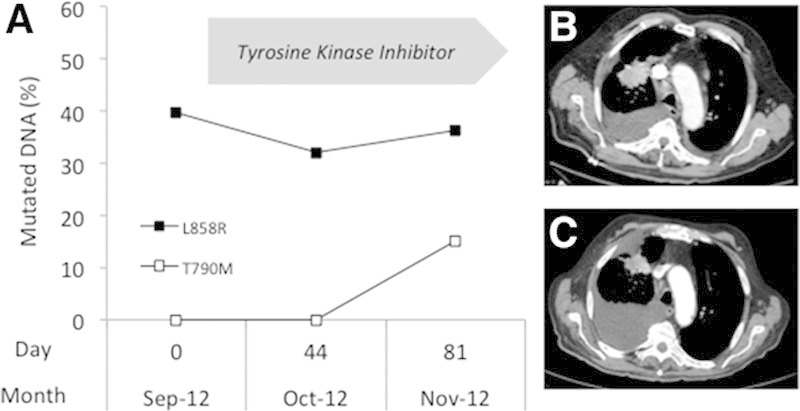
A, Detection of EGFR alterations in the plasma of patient 1. DNA was extracted from plasma, and EGFR alterations (p.L858R▪; p.T790M □) were detected by allele-specific amplification using the Therascreen RGQ EGFR kit as previously described.4 The fraction of mutated copies of the EGFR gene was determined using a standard curve generated using serial dilutions of mutated DNA extracted from control slides of formalin-fixed paraffin-embedded cell lines block with defined ratios of mutant alleles (Horizon Discovery, Cambridge, United Kingdom) into wild-type DNA. Chest computed tomography images at baseline (B) and after 3 months of tyrosine kinase inhibitor treatment (C).
Two months after tyrosine kinase inhibitor (TKI) treatment initiation, the patient presented a significant general health degradation and weight loss (7 kg). No significant tumor reduction was seen on the CT scan, and a pleural effusion was still present (Fig. 1C). The activating EGFR mutation was present at a stable level in plasma. In addition, the p.T790M mutation (not detected in the primary tumor and in the previous plasma samples) was detected in the last sample (Fig. 1A). The patient experienced progressive pleural effusion and important clinical deterioration and died in December 2012.
Case 2
A 63-year-old never-smoker woman was presented with cough and weight loss in August 2012. The initial CT scan showed a large tumor in the upper lobe of the right lung (Fig. 2B) and a single bone metastasis. Lung biopsies were found to contain thyroid transcription factor-1 positive adenocarcinoma cells, harboring an exon 19 deletion of the EGFR gene. This alteration was also detected in the plasma (Fig. 2A).
FIGURE 2.
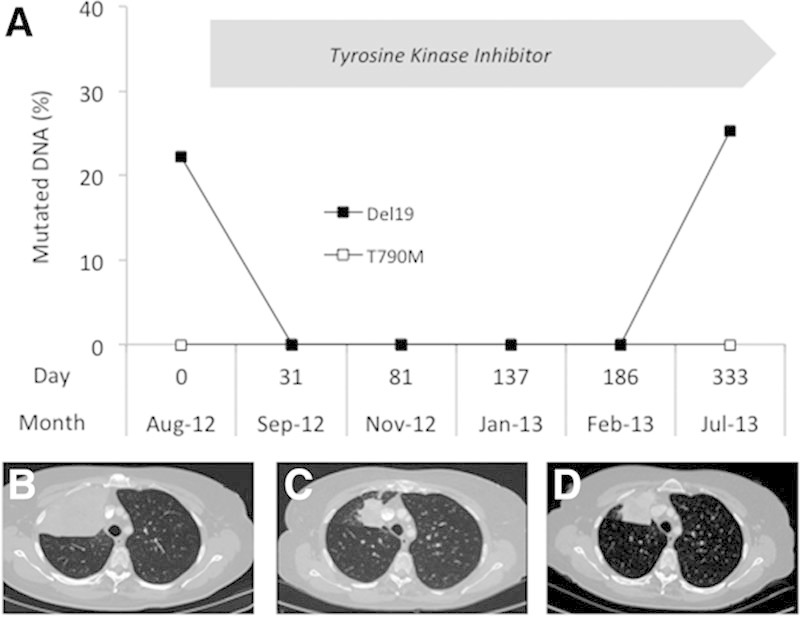
A, Detection of EGFR alterations in the plasma of patient 2. DNA was extracted from plasma, and EGFR alterations (Del19 ; p.T790M □) were detected and quantified as described in Figure 1. Chest computed tomography images at baseline (B) and after 6 months (C) and 11 months (D) of tyrosine kinase inhibitor treatment.
This patient was treated with an EGFR TKI. After 1 month, the patient reported improvement in both cough and weight. Two months after starting treatment, CT demonstrated a significant shrinkage of the lung tumor (50% objective response; Fig. 2C), and the EGFR mutation remained undetectable in plasma during 6 months (Fig. 2A).
A progression of the tumor was seen on the control CT scan performed 11 months following treatment initiation (Fig. 2D). The EGFR-activating mutation simultaneously reappeared in the plasma.
DISCUSSION
Liquid biopsies have recently emerged as an important source of biomarkers in clinical oncology. For instance, tumor cells circulating in blood can be used to determine the ALK (Anaplastic Lymphoma Kinase) status of patients with lung cancer,1 and EGFR alterations can be detected in cell-free circulating tumor DNA of patients before TKI treatment.2–4 Bai et al.5 recently demonstrated an effect of neoadjuvant chemotherapy on change in EGFR mutation in plasma samples. We present here the results obtained during follow-up of two patients during TKI treatment. Although in patient 1, who did not respond to TKI treatment, the EGFR mutation was detected at similar levels in all plasma samples, in patient 2 the EGFR mutation disappeared from plasma DNA during treatment response and reappeared at progression.
Our data suggest that the disappearance of circulating EGFR-mutated DNA may be a marker of TKI response. Few studies have attempted to detect EGFR mutations in plasma samples from non–small-cell lung cancer patients under targeted therapy or during follow-up period. But the techniques used (microfluidic digital polymerase chain reaction6; allele-specific arrayed primer extension),7 which are time consuming and require expensive hardware, are not suitable for use in a routine clinical biochemistry or DNA diagnosis laboratory. In a recent report, whole exome sequencing of plasma DNA was used to assess tumor dynamics of patient with lung tumor.8 But this very powerful technique is not yet compatible with routine clinical practice. In our study, DNA extraction and EGFR mutation detection using the approved and efficient9 Therascreen EGFR RGQ kit (Qiagen, Hilden, Germany) can be performed within 3 hours. We previously described that this procedure allowed us to detect activating EGFR mutations in plasma of advanced non–small-cell lung cancer patients before TKI treatment with a sensitivity of 94.7% and a specificity of 100%.4
Although promising, our data do not allow any reduction or change in the use of radiological examinations or even in the rebiopsy interest at the present time. But following confirmation of our results on a larger cohort, analysis of plasma DNA could turn out to be a useful biomarker for real-time monitoring of patients receiving EGFR TKI in routine clinical practice.
Acknowledgment
This work was supported by a grant from Astra-Zeneca.
Footnotes
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Ilie M, Long E, Butori C, et al. ALK-gene rearrangement: a comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann Oncol. 2012;23:2907–2913. doi: 10.1093/annonc/mds137. [DOI] [PubMed] [Google Scholar]
- 2.Goto K, Ichinose Y, Ohe Y, et al. Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol. 2012;7:115–121. doi: 10.1097/JTO.0b013e3182307f98. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Carcereny E, Gervais R, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 4.Vallée A, Marcq M, Bizieux A, et al. Plasma is a better source of tumor-derived circulating cell-free DNA than serum for the detection of EGFR alterations in lung tumor patients. Lung Cancer. 2013;82:373–374. doi: 10.1016/j.lungcan.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Bai H, Wang Z, Chen K, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:3077–3083. doi: 10.1200/JCO.2011.39.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yung TK, Chan KC, Mok TS, Tong J, To KF, Lo YM. Single-molecule detection of epidermal growth factor receptor mutations in plasma by microfluidics digital PCR in non-small cell lung cancer patients. Clin Cancer Res. 2009;15:2076–2084. doi: 10.1158/1078-0432.CCR-08-2622. [DOI] [PubMed] [Google Scholar]
- 7.Yam I, Lam DC, Chan K, et al. EGFR array: uses in the detection of plasma EGFR mutations in non-small cell lung cancer patients. J Thorac Oncol. 2012;7:1131–1140. doi: 10.1097/JTO.0b013e3182558198. [DOI] [PubMed] [Google Scholar]
- 8.Murtaza M, Dawson SJ, Tsui DW, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 9.Vallée A, Le Loupp AG, Denis MG. Efficiency of the Therascreen® RGQ PCR kit for the detection of EGFR mutations in non-small cell lung carcinomas. Clin Chim Acta. 2014;429:8–11. doi: 10.1016/j.cca.2013.11.014. [DOI] [PubMed] [Google Scholar]


