Abstract
Introduction:
Poor prognosis patients with bulky stage III locally advanced non–small-cell lung cancer may not be offered concurrent chemoradiotherapy (CRT). Following a phase III trial concerning the effect of palliative CRT in inoperable poor prognosis patients, this analysis was performed to explore how tumor size influenced survival and health-related quality of life (HRQOL).
Methods:
A total of 188 poor prognosis patients recruited in a randomized clinical trial received four courses intravenous carboplatin day 1 and oral vinorelbine day 1 and 8, at 3-week intervals. The experimental arm (N = 94) received radiotherapy with fractionation 42 Gy/15, starting at the second chemotherapy course. This subset study compares outcomes in patients with tumors larger than 7 cm (N = 108) versus tumors 7 cm or smaller (N = 76).
Results:
Among those with tumors larger than 7 cm, the median overall survival in the chemotherapy versus CRT arm was 9.7 and 13.4 months, respectively (p = 0.001). The 1-year survival was 33% and 56%, respectively (p = 0.01). Except for a temporary decline during treatment, HRQOL was maintained in the CRT arm, regardless of tumor size. Among those who did not receive CRT, patients with tumors larger than 7 cm experienced a gradual decline in the HRQOL. The CRT group had significantly more esophagitis and hospitalizations because of side effects regardless of tumor size.
Conclusion:
In patients with poor prognosis and inoperable locally advanced non–small-cell lung cancer, large tumor size should not be considered a negative predictive factor. Except for performance status 2, patients with tumors larger than 7 cm apparently benefit from CRT.
Keywords: Non–small-cell lung cancer, Locally advanced, Bulky tumors, Chemoradiotherapy
Approximately 2800 Norwegians are diagnosed with lung cancer each year.1 In a study of all lung cancer patients diagnosed in southern Norway between 2002 and 2005, Rolke et al.2 found that 72% of patients were in an advanced, nonoperable stage at the time of diagnosis. Twenty years ago, palliative radiotherapy was considered the main treatment for advanced non–small-cell lung cancer (NSCLC), yielding effective symptom relief for a limited time, regardless of dose/fractionation.3 Presently, the Norwegian national guidelines recommend combined radiotherapy and chemotherapy for patients with locally advanced stage III disease and good prognostic factors.
Large tumor volumes have been associated with a decreased overall survival (OS),4,5 especially when combined with N3 disease.6 After the development of three-dimensional radiation planning and therapy, researchers have found that large gross tumor volume as determined by computed tomography (CT) and three dimensional-chemoradiotherapy (CRT) to be a negative prognostic factor.7–9 On behalf of The International Association for the Study of Lung Cancer (IASLC) Staging Committee and Participating Institutions, Ball et al.10 recently reported data from approximately 900 NSCLC patients selected for radical radiotherapy or CRT. They did not observe any negative prognostic effect of tumor size larger than 3 cm.
Based on a comparison with treatment effect on smaller tumors, some authors have cautioned against the routine use of concurrent CRT to patients with larger volume tumors.3,11 Several trials have excluded patients with large tumors from combined modality treatment.4,5,12–14 However, a poor prognosis does not preclude possible positive treatment effect, and the role of CRT for patients with NSCLC stage III nonresectable large volume tumors remains unresolved.
We have recently reported the results of a phase III trial comparing palliative concurrent CRT with palliative chemotherapy alone in patients with poor prognosis nonresectable locally advanced NSCLC (Conrad study).15 Because the majority of included patients (54%) had tumors 8 cm or larger in diameter, a subgroup analysis according to tumor size seemed feasible and warranted.
The aim of this subgroup analysis was to explore how tumor size influenced treatment outcomes in nonresectable NSCLC stage III poor prognosis patients receiving palliative CRT or chemotherapy alone. Investigated outcomes were OS; time to progression (TTP), toxicity, and health-related quality of life (HRQOL).
PATIENTS AND METHODS
Patients
This subset analysis is based on the Conrad study, which was designed to compare palliative CRT versus palliative chemotherapy alone to poor-prognosis patients with unresectable locally advanced NSCLC stage III.15 The primary endpoint was OS, and the secondary endpoints were HRQOL, TTP, and toxicity. The patients were stratified by age and performance status (PS).
Included patients were to have unresectable, locally advanced NSCLC stage III disease with one or more negative prognostic factors (tumor size ≥8 cm, PS ≥2, and/or weight loss of >10% during the past 6 months). There was no upper age limit. The patients were staged by CT examination alone because positron emission tomography was not universally available in the years of inclusion. The patients should not have malignant pleural effusion, other active malignant disease, should not have received previous chemotherapy, or be candidates for radical radiotherapy. Patients were eligible provided they had World Health Organization PS16 0 to 2 and adequate hematological, liver, and kidney function.
Eligible patients were to receive four courses of chemotherapy in 3-week intervals: intravenous carboplatin (Calvert area under the curve 5) day 1 and oral vinorelbin 60 mg/m2 days 1 and 8.17 The experimental arm received in addition radiation with 42 Gy/15 fractions during 3 weeks between chemotherapy course two and three. If patients in the chemotherapy group later (after the study treatment was completed) required palliative thoracic radiation, they were administered hypofractionated RT (17 Gy/two fractions).
From November 2006 to November 2011, 191 eligible patients were included from 25 hospitals in Norway. The Regional Ethics Committee of North Norway approved the study.
HRQOL
European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30) questionnaire version 3.0 and the supplementary questionnaire module LC13 were used to assess HRQOL. The patients were to complete questionnaires at each chemotherapy course, as well as every 8th week after termination of treatment. Among domains covered by the QLQ-C30/LC13, we have chosen to focus on mean change from baseline to 52 weeks for global function, social function, physical functions, and dysphagia, comparing the two groups of different tumor size.18 The complete numbers of figures are found in a supplemental file.
Tumor Size Assessment
The largest tumor diameter (centimeter) was registered at randomization, according to the baseline CT examination. In the original Conrad protocol, tumor size 8 cm or larger was considered a negative prognostic factor. For inclusion, patients were to have tumors that could be included in a pragmatic radiation field.
Analyses and Statistical Considerations
To stay in accordance with the IASLC tumor, node, metastasis (TNM) classification, we chose tumor size 7 cm as cutoff for the present analysis.19 The Common Terminology Criteria of Adverse Events version 3.0 was used to grade toxicity.
Baseline characteristics were compared using chi-squared tests and Fisher’s exact test. PS and possible progression were registered during follow-up visits (weeks 12, 20, 28, 36, 44, and 52). Progressions were based on CT examination. Biopsies were not performed. OS and TTP were analyzed using the Kaplan–Meier method and compared using the long-rank test. Cox’s proportional hazard model was used for multivariate analysis to estimate the independent impact of clinical factors on survival.
The EORTC scoring manuals were used to analyze the HRQOL questionnaires.20,21 Mean scores were calculated from the reported scores only. The mean changes were calculated by subtracting baseline score from the score at each designated time point during and after the treatment. The scores were compared using analysis of variance (ANOVA) and the nonparametric Mann–Whitney U test. A mean change of 10 points or greater was considered clinically relevant.22,23 A higher score for symptom domains indicates more pronounced symptoms, whereas higher score for the functional domains indicates better function.
RESULTS
Patients and Study Treatment
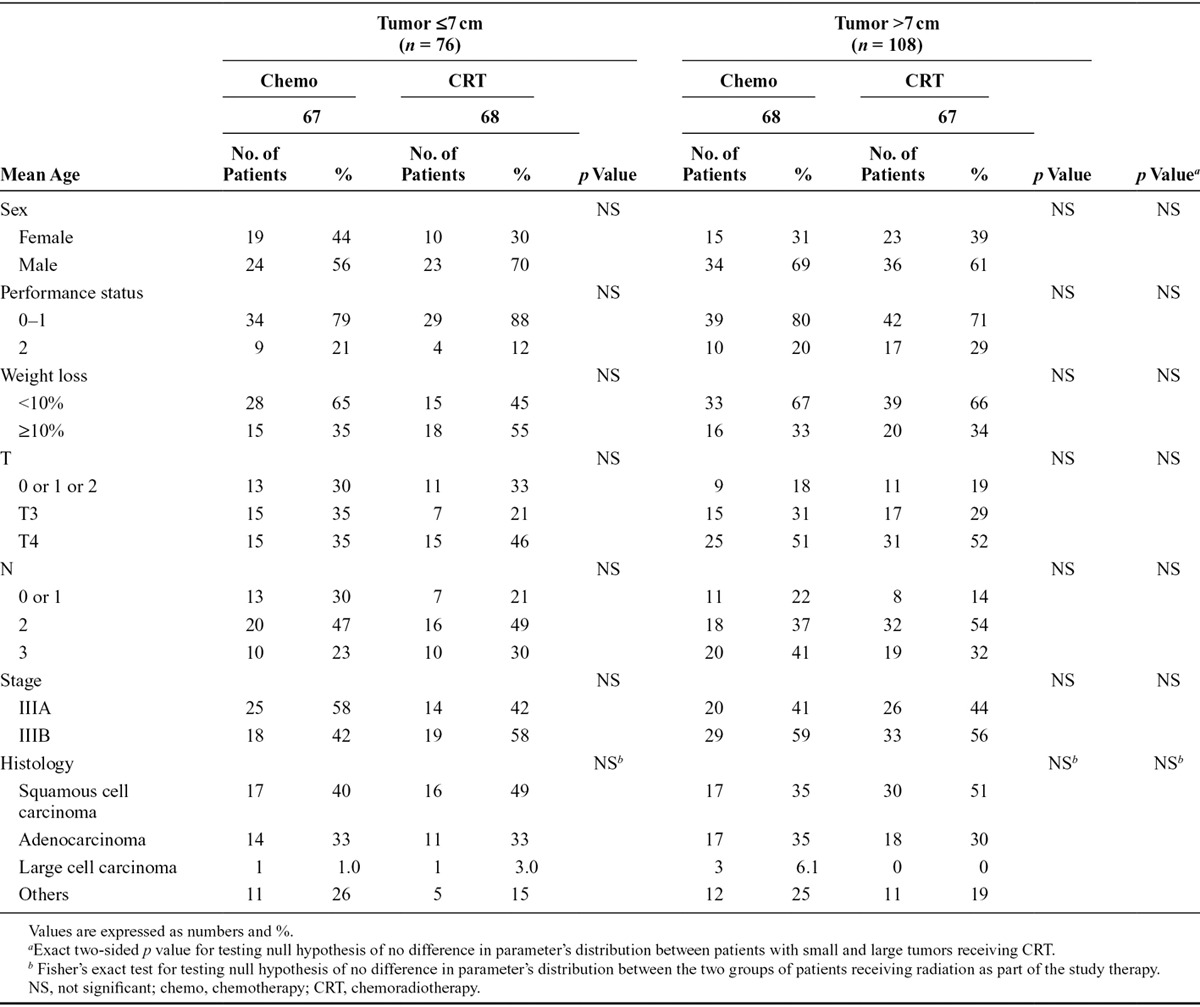
The demographic data for subgroup comparisons are shown in Table 1. Of the 188 eligible patients in the Conrad study, four were excluded because of missing information on tumor diameter. Seventy-eight patients had tumors 7 cm or smaller, and 108 patients had tumors larger than 7 cm. There were no significant differences between the groups with respect to demographic or clinical variables. There was a tendency toward more CRT (55% versus 43%) and more PS 2 patients (25% versus 17%) in the larger than 7 cm group when compared with the 7 cm or smaller group. Among patients receiving CRT, there was a relative predominance of women in the group with tumors larger than 7 cm compared with the group with tumors 7 cm or smaller (39% versus 30%, respectively). In the same group, we found a relative deficit of patients with weight loss larger than 10% (34% versus 55%, respectively).
TABLE 1.
Baseline Characteristics
Treatment Received
Treatment according to group is shown in Table 2. In the 7 cm or smaller group, the mean number of chemotherapy cycles was 3.5 and 3.7 (chemo versus CRT) versus 3.6 and 3.6 (chemo versus CRT) in the larger than 7 cm group.
TABLE 2.
Treatment According to Tumor Size

Among patients with tumors larger than 7 cm randomized to CRT, three patients did not receive radiotherapy: One patient because of death from complications after a femoral neck fracture (n = 1) and two patients because of significantly reduced PS after initial chemotherapy (n = 1) and myocardial infarction (n = 1). One patient died of arrhythmia during radiation treatment. Less than 10% of patients with tumors larger than 7 cm randomized to CRT discontinued treatment because of disease progression or unacceptable toxicity. The mean number of fractions was 13.6 of the planned 15 in this group. In the group of smaller tumors, all patients randomized to CRT completed radiotherapy.
Local Control and Poststudy Treatment
Data on lung progression/recurrence and poststudy treatment are presented in Table 3. CRT yielded a significantly better local control when compared with chemotherapy alone in the tumor larger than 7 cm group (p = 0.01). In 69% and 68% of patients receiving chemotherapy alone, the lungs were reported to be the first site of recurrence/progression. In comparison, this was seen in only 41% to 45% of patients treated with CRT. Consistently, the need for additional therapy seemed to be more pronounced among those treated with chemotherapy alone, irrespective of tumor size.
TABLE 3.
Lung Recurrence and Poststudy Treatment
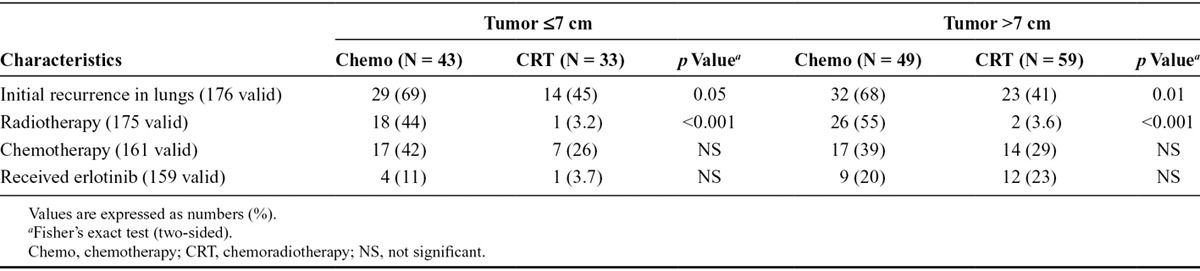
Although the information on the use of erlotinib is incomplete, it seems to be more pronounced for patients with tumors larger than 7 cm.
Outcomes
This subgroup analysis showed that the improved survival seen after CRT when compared with chemotherapy alone was primarily mediated by the group of patients with tumors larger than 7 cm (Fig. 1). Although the 2-year OS in patients with tumors 7 cm or smaller increased from 9.3% to 24% (p = 0.11) with the addition of concurrent radiotherapy, an increase from 6.1% to 32% (p = 0.001) was observed among those with tumors larger than 7 cm (Fig. 1). Consistently, median TTP seemed, regardless of tumor size, significantly longer after CRT (≤7 cm: 6.7 versus 4.5 months, p < 0.05; >7 cm: 7.2 versus 4.2 months, p < 0.001).
FIGURE 1.
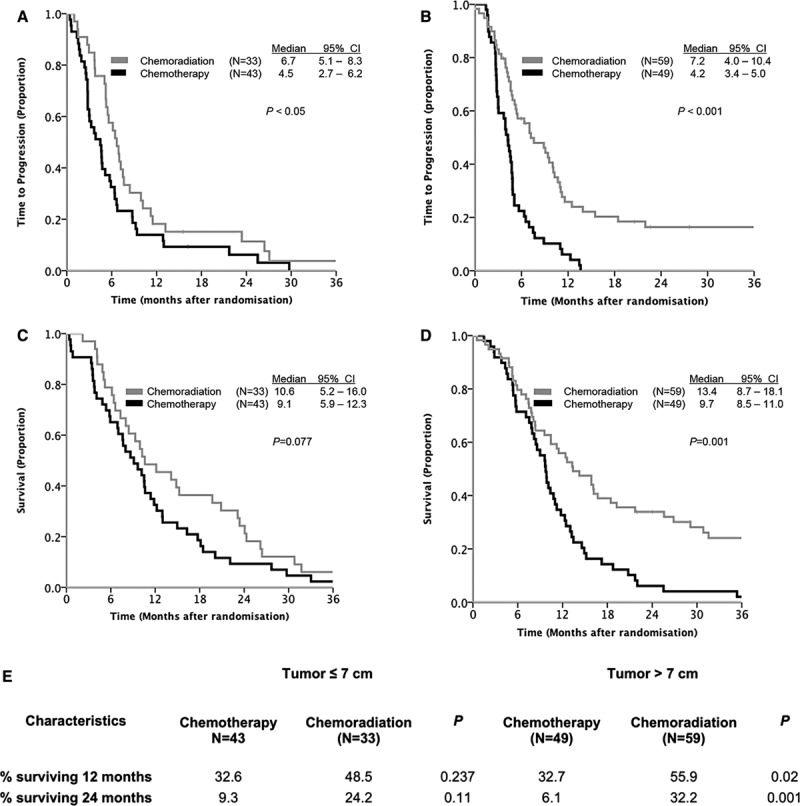
Kaplan–Meier plots for (A) TTP for patients with tumors 7 cm or smaller; (B) TTP for patients with tumors larger than 7 cm; (C) overall survival for patients with tumors 7 cm or smaller; (D) overall survival for tumors larger than 7 cm; (E) 1- and 2-year survival in both groups. CI, confidence interval; TTP, time to progression.
FIGURE 2.
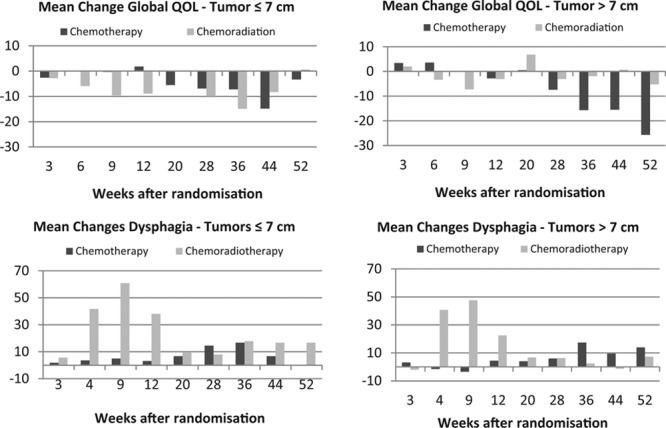
Mean changes in the selected domains of HRQOL measurements. A high score for dysphagia indicates more pronounced symptoms, whereas higher score for the change in Global Quality of Life indicates better function. HRQOL, health-related quality of life.
In a multivariate analysis adjusting for weight loss, PS, and sex, only PS and tumor size were found to have significant impact on survival: hazard ratio = 1.835 for PS 0 to 1 versus 2 (confidence interval, 1.26–2.67; p = 0.002) and hazard ratio = 0.937 (0.881–0.996, p = 0.037), respectively. Tumor size was not dichotomized in this analysis so that no information is lost.
Toxicity and side effects are summarized in Table 4. Except for anemia in the tumor 7 cm or smaller group, there were no significant differences in hematological toxicity related to tumor size. The number of patients with esophagitis and hospital admissions attributable to side effects was higher in both groups receiving CRT.
TABLE 4.
Toxicity According to Tumor Size and Treatment

Of 184 eligible patients, 182 (99%) completed the HRQOL questionnaire at randomization. The median percentage of completed questionnaires during the first 6 months after randomization was 91% in the 7 cm or smaller group and 86% in the larger than 7 cm group. The percentage of responders declined the past 6 months of the observation period (median, 72% versus 73%). Regardless of tumor size, patients receiving CRT recorded a temporal worsening in physical and social functioning before returning to baseline levels again. All groups experienced a certain decline in physical and social functions at the end of the observational period, but the decline was significantly more pronounced among the patients with tumors larger than 7 cm who did not receive CRT.
DISCUSSION
This subset analysis of a randomized phase III trial demonstrates that patients with large locally advanced NSLCL stage III tumors and negative prognostic factors may possibly be better served by concurrent CRT than by chemotherapy alone, with patients with PS 2 being the exception.
The preferred treatment for locally advanced (LA)-NSCLC is concurrent chemoradiation with high-dose chemotherapy.24 However, some authors question the benefit of CRT to patients with LA-NSCLC and large tumors.3,11 The aim of the Conrad trial was to study how poor prognosis NSCLC stage III patients responded to a palliative combination regimen of radiation and chemotherapy. Because we considered PS 2; weight loss larger than 10% and tumor size 8 cm or larger to be negative prognostic factors, the high percentage of patients with large tumors in this material is caused by the aim of the original study.
The original phase III trial (the Conrad study), on which this substudy is based, confirmed a significant benefit from palliative CRT compared with palliative chemotherapy alone, with a median survival of 12.6 months versus 9.7 months, respectively.15 Palliative CRT increased OS time regardless of tumor size, and the survival gain was most prominent for patients with tumors larger than 7 cm (1-year survival 56% versus 33%; 2-year survival 32% versus 6.1%). The median survival time for patients with tumors larger than 7 cm was comparable with the findings in other studies,5,7 where better prognosis patients received definitive CRT or radiation alone. In a smaller retrospective material, Wiersma et al.6 found that approximately one of five patients with large tumors survived 3 years when given treatment with definitive CRT containing a cisplatin doublet. In our material, we observed a slightly better 3-year survival (20%) in the larger than 7 cm tumor group receiving palliative CRT.
Several authors have reported a strong sex-dependent difference in survival among patients with NSCLC.25–27 Weight loss at time of diagnosis is also a factor known to have impact on survival.28–30 Sex and weight loss were not significant predictive factors in our study. However, there were somewhat less weight loss and more women in the tumor larger than 7 cm group, and especially for those treated with CRT. This may possibly have contributed to the increased survival in the group of tumors larger than 7 cm. In contrast, poor PS 2, being one of the strongest prognostic factors in NSCLC,30 was overrepresented in the tumor larger than 7 cm group and especially for the CRT treated.
The CRT patients experienced a significantly increased incidence of esophagitis and had significantly more hospitalizations attributable to side effects regardless of tumor size. Beyond this, the hematological toxicities were modest, and no serious pulmonary toxicity was observed. This may be explained by chemotherapy doses and radiation being adjusted to a palliative intent. Besides, the relatively large percentage of CRT patients who developed esophagitis may be diminished by modern radiation techniques in the future.31,32
A large tumor is expected to give more symptoms than a smaller one. CRT may relieve these symptoms. However, radiation and chemotherapy are related to certain side effects that will influence symptoms and quality of life during and shortly after the treatment period.33,34 In this study, profiles of the HRQOL recordings demonstrate an increase in dysphagia and a decline in functional values during treatment. As expected, this was most pronounced for those receiving CRT. However, the post-treatment beneficial HRQOL changes for those treated with CRT were most evident for patients with larger tumors (>7 cm).
The lack of available positron emission tomography-CT scanning in Norway at the time of inclusion may imply that the study group does not reflect the current stage III NSCLC population. By using CT alone, one may underestimate nodal involvement and/or overestimate tumor size by unintentionally including atelectasis. Considering that approximately 80% of our patients had N3 disease, this will not weaken the argument for CRT to patients with tumors larger than 7 cm.
Several authors have found the TNM classification system inaccurate or insufficient in predicting the treatment effect on survival in nonoperable NSCLC patients, especially with respect to the impact of tumor size.5,35,36 An unfortunate lack of distinction between predictive and prognostic factors may be one reason.37 It should be noted that a prognostic factor provides information on the likely outcome of a cancer disease in an untreated individual, whereas a predictive factor provides information about the likely effect of a treatment.38
Morgensztern et al.,4 who examined 12,315 patients with locally advanced stage III NSCLC N2-3 disease from the Surveillance, Epidemiology and End Results (SEER) registry, included patients strictly on the basis of TNM staging, regardless of the treatment. They found tumor size to be an independent prognostic factor. Ball et al. recently published the results data on 868 patients of all TNM stages included in The IASLC Staging Project. The tumor diameters were known, and the cancers were subjected to radical radiotherapy or combined chemotherapy and radiotherapy.10 The authors found that tumor size smaller than 3 cm was associated with a longer survival than larger tumors, but evidence on the prognostic effect by increasing size above the 3-cm cutoff was weak. The assessments have been made on comparisons with treatment effect on smaller tumors. Accordingly, they do not tell us much about how treatment on larger tumors compares with no treatment or best supportive care.
Werner-Wasik et al.39 specifically addressed treatment effect on different tumor volumes in a small study published in 2008. They found that larger tumor volumes were associated with larger risk of local failure, and smaller tumors were associated with improved OS. But these findings were not compared with a control group. Several other authors have addressed tumor size and volume as a prognostic factor for survival.5,7,9,36 All these studies have dealt with patients who received definitive treatment. But while these authors, to a varying degree, demonstrate that a large tumor may be a negative prognostic factor, the treatment effect was not specifically addressed. A poor prognosis does not preclude an excellent treatment effect. These studies cannot be used as an argument against treating bulky tumors.
Aupérin et al.40 found that CRT has a significant effect on locoregional control. This is consistent with the findings in our study: Regardless of tumor size, patients receiving CRT had a significant reduction in lung recurrence and progression. However, the percentages of recurrence and progression in the lungs after CRT in our study (approximately 40%) were considerably larger than those in the limited material of Alexander et al.5 (11%). This may reflect that the radiation dose in our study was too low.41 Further research should be performed on bulky NSCLC tumors, employing modern, more advanced, radiation techniques.
To our knowledge this is the first study to explore the effect of CRT compared with chemotherapy alone in poor-risk NSCLC patients with bulky tumor masses. Our results demonstrate that, although a large tumor may be considered a negative prognostic factor, it is not a negative predictive factor. Patients with large NSCLC tumors seem to benefit notably from concomitant chemoradiation.
ACKNOWLEDGMENTS
This research was supported by unrestricted grants from the North Norway Regional Health Authority and Pierre Fabre.
Footnotes
Disclosure: Dr. Strøm has received payment for lectures from Glaxo Wellcome and support for travel to meetings from Perre Fabre and Glaxo Wellcome. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Cancer Registry N. Cancer in Norway 2009. Cancer Registry of Norway. 2011. pp. Pp. 1–169.
- 2.Rolke HB, Bakke PS, Gallefoss F. Delays in the diagnostic pathways for primary pulmonary carcinoma in Southern Norway. Respir Med. 2007;101:1251–1257. doi: 10.1016/j.rmed.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Lester JF, Macbeth FR, Toy E, Coles B. Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev. 2006;(4):CD002143. doi: 10.1002/14651858.CD002143.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Morgensztern D, Waqar S, Subramanian J, Gao F, Trinkaus K, Govindan R. Prognostic significance of tumor size in patients with stage III non-small-cell lung cancer: a surveillance, epidemiology, and end results (SEER) survey from 1998 to 2003. J Thorac Oncol. 2012;7:1479–1484. doi: 10.1097/JTO.0b013e318267d032. [DOI] [PubMed] [Google Scholar]
- 5.Alexander BM, Othus M, Caglar HB, Allen AM. Tumor volume is a prognostic factor in non-small-cell lung cancer treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1381–1387. doi: 10.1016/j.ijrobp.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 6.Wiersma TG, Dahele M, Verbakel WF, et al. Concurrent chemoradiotherapy for large-volume locally-advanced non-small cell lung cancer. Lung Cancer. 2013;80:62–67. doi: 10.1016/j.lungcan.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Basaki K, Abe Y, Aoki M, Kondo H, Hatayama Y, Nakaji S. Prognostic factors for survival in stage III non-small-cell lung cancer treated with definitive radiation therapy: impact of tumor volume. Int J Radiat Oncol Biol Phys. 2006;64:449–454. doi: 10.1016/j.ijrobp.2005.07.967. [DOI] [PubMed] [Google Scholar]
- 8.De Petris L, Lax I, Sirzén F, Friesland S. Role of gross tumor volume on outcome and of dose parameters on toxicity of patients undergoing chemoradiotherapy for locally advanced non-small cell lung cancer. Med Oncol. 2005;22:375–381. doi: 10.1385/MO:22:4:375. [DOI] [PubMed] [Google Scholar]
- 9.Bradley JD, Ieumwananonthachai N, Purdy JA, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:49–57. doi: 10.1016/s0360-3016(01)01772-2. [DOI] [PubMed] [Google Scholar]
- 10.Ball D, Mitchell A, Giroux D, Rami-Porta R IASLC Staging Committee and Participating Institutions. Effect of tumor size on prognosis in patients treated with radical radiotherapy or chemoradiotherapy for non-small cell lung cancer. An analysis of the staging project database of the International Association for the Study of Lung Cancer. J Thorac Oncol. 2013;8:315–321. doi: 10.1097/JTO.0b013e31827dc74d. [DOI] [PubMed] [Google Scholar]
- 11.O’Rourke N, Roqué I Figuls M, Farré Bernadó N, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010;(6):CD002140. doi: 10.1002/14651858.CD002140.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Uitterhoeve ALJ, Koolen MGJ, van Os RM, et al. Accelerated high-dose radiotherapy alone or combined with either concomitant or sequential chemotherapy; treatments of choice in patients with non-small cell lung cancer. Radiat Oncol. 2007;2:27. doi: 10.1186/1748-717X-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belderbos J, Uitterhoeve L, van Zandwijk N, et al. EORTC LCG and RT Group. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973). Eur J Cancer. 2007;43:114–121. doi: 10.1016/j.ejca.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Bradley JD, Hope A, El Naqa I, et al. RTOG. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69:985–992. doi: 10.1016/j.ijrobp.2007.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109:1467–1475. doi: 10.1038/bjc.2013.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. 1979. p. 1. [Google Scholar]
- 17.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 18.Claassens L, van Meerbeeck J, Coens C, et al. Health-related quality of life in non-small-cell lung cancer: an update of a systematic review on methodologic issues in randomized controlled trials. J Clin Oncol. 2011;29:2104–2120. doi: 10.1200/JCO.2010.32.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groome PA, Bolejack V, Crowley JJ, et al. IASLC International Staging Committee; Cancer Research and Biostatistics; Observers to the Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 20.Fayers P, Bottomley A EORTC Quality of Life Group; Quality of Life Unit. Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2002;38(Suppl 4):S125–S133. doi: 10.1016/s0959-8049(01)00448-8. [DOI] [PubMed] [Google Scholar]
- 21.Fayers P, Aaronson N, Bjordal K, et al. EORTC QLQ-C30 Scoring Manual. 3rd Ed. Brussels, Belgium: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 22.Osoba D, Bezjak A, Brundage M, Zee B, Tu D, Pater J Quality of Life Committee of the NCIC CTG. Analysis and interpretation of health-related quality-of-life data from clinical trials: basic approach of The National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer. 2005;41:280–287. doi: 10.1016/j.ejca.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Brundage M, Osoba D, Bezjak A, Tu D, Palmer M, Pater J National Cancer Institute of Canada Clinical Trials Group. Lessons learned in the assessment of health-related quality of life: selected examples from the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:5078–5081. doi: 10.1200/JCO.2007.11.4645. [DOI] [PubMed] [Google Scholar]
- 24.Crinò L, Weder W, van Meerbeeck J, Felip E ESMO Guidelines Working Group. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v103–v115. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 25.Moore R, Doherty D, Chamberlain R. Sex differences in survival in non-small cell lung cancer patients 1974–1998. Acta Oncol. 2004;43:57–64. [PubMed] [Google Scholar]
- 26.Cerfolio RJ, Bryant AS, Scott E, et al. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006;130:1796–1802. doi: 10.1378/chest.130.6.1796. [DOI] [PubMed] [Google Scholar]
- 27.Visbal AL, Williams BA, Nichols FC, et al. Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004;78:209–215. doi: 10.1016/j.athoracsur.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Jeremić B, Miličić B, Milisavljevic S. Clinical prognostic factors in patients with locally advanced (stage III) nonsmall cell lung cancer treated with hyperfractionated radiation therapy with and without concurrent chemotherapy: single-Institution Experience in 600 Patients. Cancer. 2011;117:2995–3003. doi: 10.1002/cncr.25910. [DOI] [PubMed] [Google Scholar]
- 29.Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest. 2002;122:1037–1057. doi: 10.1378/chest.122.3.1037. [DOI] [PubMed] [Google Scholar]
- 30.Buccheri G, Ferrigno D. Importance of weight loss definition in the prognostic evaluation of non-small-cell lung cancer. Lung Cancer. 2001;34:433–440. doi: 10.1016/s0169-5002(01)00273-2. [DOI] [PubMed] [Google Scholar]
- 31.Werner-Wasik M, Paulus R, Curran WJ, Jr, Byhardt R. Acute esophagitis and late lung toxicity in concurrent chemoradiotherapy trials in patients with locally advanced non-small-cell lung cancer: analysis of the radiation therapy oncology group (RTOG) database. Clin Lung Cancer. 2011;12:245–251. doi: 10.1016/j.cllc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Phernambucq ECJ, Spoelstra FOB, Verbakel WFAR, et al. Outcomes of concurrent chemoradiotherapy in patients with stage III non-small-cell lung cancer and significant comorbidity. Ann Oncol. 2011;22:132–138. doi: 10.1093/annonc/mdq316. [DOI] [PubMed] [Google Scholar]
- 33.Wang XS, Fairclough DL, Liao Z, et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. J Clin Oncol. 2006;24:4485–4491. doi: 10.1200/JCO.2006.07.1126. [DOI] [PubMed] [Google Scholar]
- 34.Pijls-Johannesma M, Houben R, Boersma L, et al. High-dose radiotherapy or concurrent chemo-radiation in lung cancer patients only induces a temporary, reversible decline in QoL. Radiother Oncol. 2009;91:443–448. doi: 10.1016/j.radonc.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Ball D, Smith J, Wirth A, Mac Manus M. Failure of T stage to predict survival in patients with non-small-cell lung cancer treated by radiotherapy with or without concomitant chemotherapy. Int J Radiat Oncol Biol Phys. 2002;54:1007–1013. doi: 10.1016/s0360-3016(02)03046-8. [DOI] [PubMed] [Google Scholar]
- 36.Dehing-Oberije C, De Ruysscher D, van der Weide H, et al. Tumor volume combined with number of positive lymph node stations is a more important prognostic factor than TNM stage for survival of non-small-cell lung cancer patients treated with (chemo)radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1039–1044. doi: 10.1016/j.ijrobp.2007.07.2323. [DOI] [PubMed] [Google Scholar]
- 37.Oldenhuis CN, Oosting SF, Gietema JA, de Vries EG. Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer. 2008;44:946–953. doi: 10.1016/j.ejca.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Italiano A. Prognostic or predictive? It’s time to get back to definitions! J Clin Oncol. 2011;29:4718; author reply 4718–4718; author reply 4719. doi: 10.1200/JCO.2011.38.3729. [DOI] [PubMed] [Google Scholar]
- 39.Werner-Wasik M, Swann RS, Bradley J, et al. Increasing tumor volume is predictive of poor overall and progression-free survival: secondary analysis of the Radiation Therapy Oncology Group 93-11 phase I-II radiation dose-escalation study in patients with inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:385–390. doi: 10.1016/j.ijrobp.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 40.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L, West BT, Hayman JA, Lyons S, Cease K, Kong FM. High radiation dose may reduce the negative effect of large gross tumor volume in patients with medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;68:103–110. doi: 10.1016/j.ijrobp.2006.11.051. [DOI] [PubMed] [Google Scholar]


