Abstract
Background:
Bone metastasis (BM) is a frequent complication in patients with advanced lung cancer and it causes skeletal-related events (SREs). Our study aim is to prospectively investigate the incidence of BM, incidence and types of SRE, and predictive factors of BM and SREs.
Methods:
Newly diagnosed, advanced non–small-cell lung cancer (NSCLC) or small-cell lung cancer (SCLC) patients were enrolled into the study. Patients were followed up every 4 weeks to monitor the development of SREs. Treatment for lung cancer was performed at the discretion of the investigator.
Results:
Two hundred seventy-four patients were enrolled in this study between April 2007 and December 2009 from 12 institutions. Patients included 77 cases of SCLC and 197 of NSCLC (stage IIIB/IV = 73/124). Median follow-up time was 13.8 months. The incidence of BM at initial diagnosis was 48% in stage IV NSCLC and 40% in extensive stage (ED)-SCLC. Forty-five percent of patients who developed BM had SREs consisting of pathologic fracture (4.7%), radiation to bone (15.3%), spinal cord compression (1.1%), and hypercalcemia (2.2%). Multivariate analysis revealed that factors predicting BM are stage IV, performance status 1 or greater and higher bone alkaline phosphatase in NSCLC patients, higher lactate dehydrogenase, and lower parathyroid hormone–related peptide in SCLC patients. Factors predicting SREs were stage IV, age 64 or younger, and lower albumin in NSCLC patients. Multivariate analysis of SRE was not performed for SCLC because of the small number of events.
Conclusion:
Predictive factors should be taken into consideration in future randomized studies evaluating BM and SREs.
Keywords: Bone metastasis, Skeletal-related event, Lung cancer, Predictive factor, Prospective study
Although the overall incidence of bone metastasis (BM) is unknown, BMs are a frequent complication in patients with advanced cancer. The most common human cancers such as breast, prostate, and lung have a great avidity for bone, leading to painful skeletal symptoms. How long patients live with a tumor is likely to influence whether BMs will occur. For example, in patients who quickly die of cancer because of an aggressively growing primary tumor, BMs will be relatively uncommon, simply because they have not adequate time to develop. This in no way implies that the tumor cells lacked the potential to grow in bone.1,2 In the case of advanced non–small-cell lung cancer (NSCLC), the median survival time (MST) has increased from 8 to 12 months to 15 to 17 months, during the past 10 years.3–6 Furthermore, when stage IIIB or stage IV NSCLC patients harboring a sensitive epidermal growth factor receptor (EGFR) gene mutation were treated with gefitinib, MST lengthened to 21.6 to 38.8 months.7–9 On the other hand, for patients in limited stage (LD) small-cell lung cancer (SCLC), the MST is approximately 2 years.10,11 For patients with extensive stage (ED) SCLC, survival is much more limited, ranging from 9.3 to 12.8 months.12,13 Accordingly, the chance of developing BM has increased, both in advanced NSCLC and LD-SCLC.
BMs can be associated with skeletal-related events (SREs), which include pathologic fracture, the need for surgery or radiation to bone, spinal cord compression, and hypercalcemia of malignancy (HCM).14–16 Because patient quality of life (QOL) deteriorates tremendously once SREs develop, it is important for investigators to treat patients with BM with an appropriate treatment, as early as possible. To our knowledge, there have been no prospective studies investigating the incidence and predictive factors of BM and SREs in patients with advanced lung cancer. Accordingly, it is worthwhile to investigate how therapeutic interventions such as chemotherapy, radiotherapy, and bisphosphonate could affect the clinical course of lung cancer patients with BM and the development of SREs. The aim of this study is to prospectively investigate: (1) the incidence of BM at initial diagnosis in patients with SCLC and stage IV NSCLC, (2) the time interval for the development in BM in patients with SCLC and stage IIIB NSCLC who have no BM at initial diagnosis, (3) the time interval for the development of SRE from BM, and (4) the predictive factors of BM and SRE.
MATERIALS AND METHODS
Study Design
This study is a prospective multicenter cohort study. Adult patients (at least 20 years of age) with newly diagnosed SCLC in all stages and NSCLC in stage IIIB or stage IV were eligible.
All patients were required to have received no prior chemotherapy or bisphosphonate therapy, and were allowed to undergo palliative surgical or radiation treatment for skeletal complications before registration. Once patients were enrolled into the study, treatment for lung cancer and the use of zoledronate were at the discretion of the investigator. Zoledronate was administered only after the development of BMs. No patients received denosumab during the study period, because it had not been approved by the Japanese government. The study was approved by the institutional review boards of the respective institutions and was conducted in compliance with international guidelines regulating patient safety. All patients provided written informed consent. QOL assessment and other therapeutic factors affecting BM and SREs will be reported, separately.
Staging of Lung Cancer and Subsequent Follow-Up Schedule
Table 1 shows the study schedule. Before patient enrollment, a complete physical examination was performed and a medical history was taken, which included history of SREs, antineoplastic history, and Eastern Cooperative Oncology Group performance status (PS). Blood chemistry and bone turnover markers (parathyroid hormone–related peptide [PTHrP], bone alkaline phosphatase [BALP], and urine cross-linked N-telopeptide of type I collagen [NTx]) were obtained. Tumor assessment was undertaken using chest and abdominal computed tomography (CT), radionuclide bone scan, or integrated positron emission tomography (PET)-CT. Magnetic resonance imaging or enhanced CT of the brain was performed when patients were symptomatic. Bone survey was performed before enrollment, with thoracic and lumbar spine radiograph, pelvic radiograph, and bone scan or integrated PET-CT. When BM was suspected only with bone scan or PET-CT, its confirmation with bone magnetic resonance imaging, CT scan, or normal radiograph was mandatory.
TABLE 1.
Examination Schedule
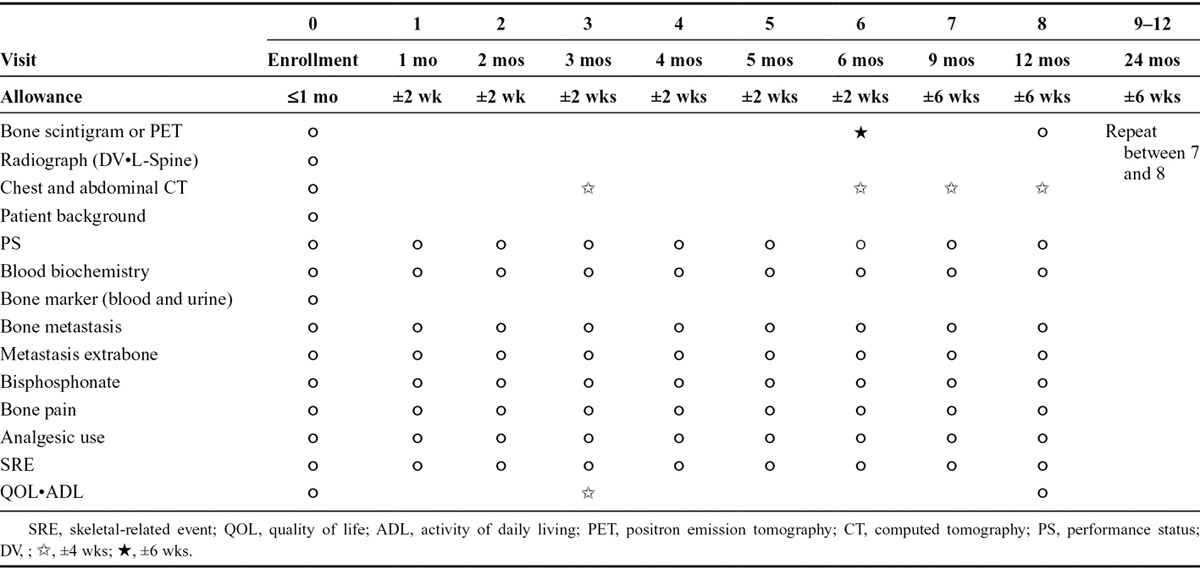
After enrollment, tumor assessment, Eastern Cooperative Oncology Group PS, and SREs were assessed every 4 weeks. Bisphosphonate use, pain, and analgesic scores were recorded at each visit, every 4 weeks. Blood chemistry was measured at each visit, every 4 weeks. Bone scan or integrated PET-CT and skeletal survey were assessed every 6 months. After 12 months of follow-up, the above-mentioned assessments were repeated every 3 months. Patients were closely monitored for 24 months after enrollment into the study.
Assessment of Outcome
SRE was defined as pathologic fracture, spinal cord compression, radiation or surgery to bone, or HCM. Fractures were identified by two expert radiologists at each institution. Spinal cord compression reported by investigators was confirmed by a neurologist and an orthopedic surgeon at each institution. Radiation to bone was given to control pain, treat or prevent pathologic fractures, or treat or prevent spinal cord compression. Surgery to bone included procedures to prevent imminent fractures or spinal cord compression or to set/stabilize fractures. Hypercalcemia was defined as a serum calcium concentration of greater than 11 mg/dl.
Statistical Analyses
The incidence provability rate was estimated for the time to the first occurrence of BM from diagnosis of lung cancer, and the time to the first occurrence of SREs from the diagnosis of lung cancer or from the first occurrence of BM, using the Kaplan–Meier method. Predictive factors for BMs and SREs were explored with Cox proportional hazard regression model for the following: Tumor type (stage IIIB NSCLC, stage IV NSCLC, LD-SCLC, and ED-SCLC), sex, age (65 or older/64 or younger), PS (PS1 or greater/PS0), and bone markers (lactate dehydrogenase [LDH], albumin, calcium, PTHrP, BALP, and urine NTx). The results were checked for consistency by conducting both a univariate Cox regression model, in which each factor was individually tested as an explanatory variable, and a multivariate Cox regression model, in which all variables were entered simultaneously into the model. To investigate whether the use of a chemotherapeutic agent and bisphosphonate inhibits the occurrence of SREs, Cox regression analysis was performed using these factors as time-dependent covariates. All analyses used the SAS statistical software package (version 9.1.3; SAS Institute, Cary NC).
The main purpose of this study is to survey lung cancer patients regarding the timing of elapsed events from onset to BM, to SRE, and is not intended as a comparison of treatment methods. Accordingly, the target number of cases was not designed for intergroup comparison, but to describe the time distribution until BM, search for predictors which affect metastasis to bone, describe what kind of SREs occur, and examine the effects on QOL, etc. The target number of cases was set at 50, as a number of cases for which descriptive statistics of some accuracy can be calculated, even for patients with stage IIIB NSCLC, which is anticipated to have comparatively small enrollment, for a total of approximately 400 cases from the percentage of each cancer type.
RESULTS
Patients
A total of 274 patients with lung cancer were enrolled in the study, between April 2008 and December 2009, from 12 institutions in Japan. Patient demographics and baseline characteristics are shown in Table 2. One hundred ninety-seven patients had NSCLC (stage IIIB, 73; stage IV, 124), and 77 patients had SCLC (LD, 30; ED, 47). The majority of patients were male (70.4%) and the median age was 68 years (range, 35–89). Ninety percent of patients had good PS (PS = 0, 27.7%; PS = 1, 62.4%). The number of patients with BM at initial diagnosis was 59 (47.6%) in stage IV NSCLC and 19 (40.4%) in ED-SCLC. Twenty patients of stage IV NSCLC (16.1%) and four patients in ED-SCLC (8.5%) already had SRE at the time of enrollment. The majority of patients (92.3%) were being treated with chemotherapy. The number of patients who received zoledronate was eight in stage IIIB NSCLC, 49 in stage IV NSCLC, two in LD-SCLC, and four in ED-SCLC after the development of BM during the study period. EGFR gene mutations were analyzed only in 92 (46.7%) of 197 NSCLC patients because it was not routinely tested during this period. Among these, 38 patients (19.3%) had EGFR gene mutations and 30 patients (15.2%) had active mutations (L858R or exon 19 deletion). Nine of 30 patients were treated with EGFR tyrosine kinase inhibitor (TKI) during the first regimen, 19 during the second regimen, and two during the third regimen. Eighteen patients (9.2%) were not treated with chemotherapy at all. One hundred fifty-two patients (77.2%) were treated with some kind of chemotherapy within 30 days of enrollment. The other patients (13.6%) were treated with chemotherapy after 31 days of enrollment.
TABLE 2.
Patient Characteristics
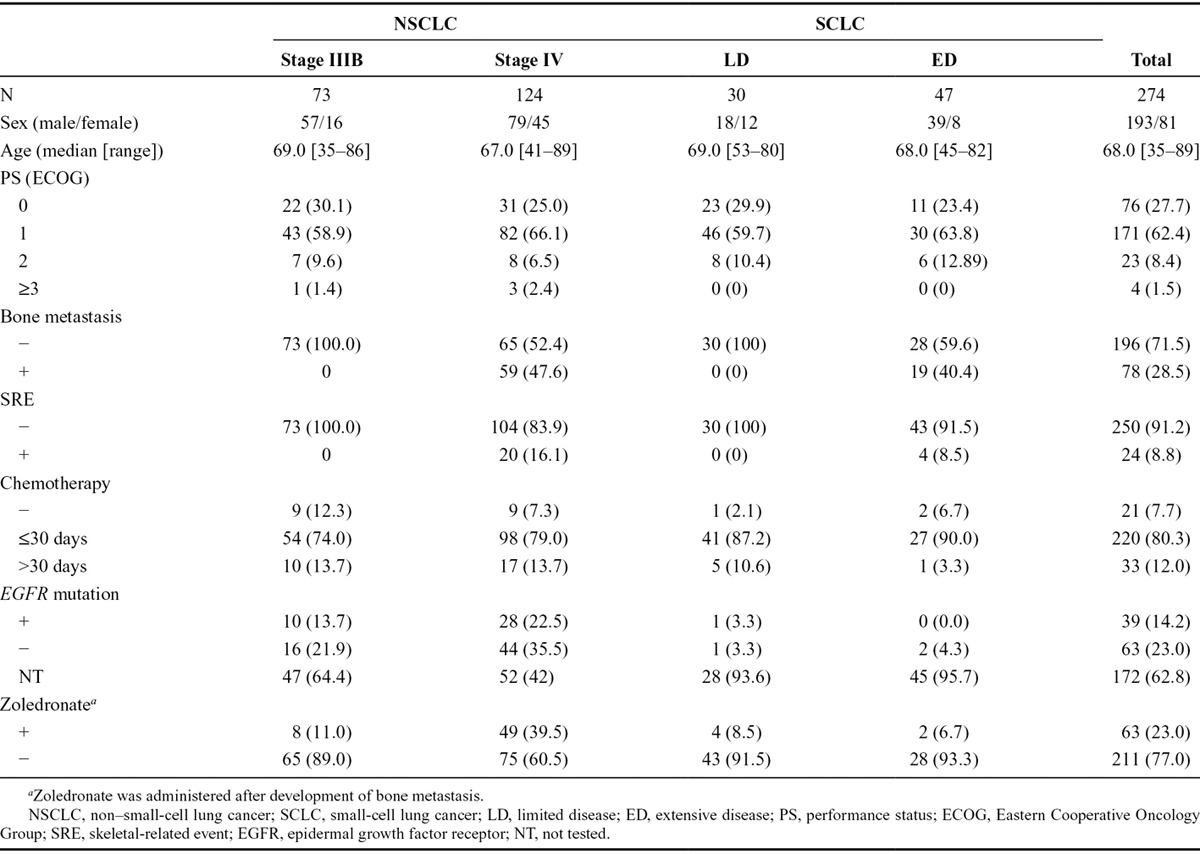
During the treatment of SCLC, three patients (3.9%) were not treated with any kind of chemotherapy, 68 (88.3%) patients were treated with some kind of chemotherapy within 30 days of enrollment, whereas six patients (7.8%) were treated with chemotherapy after 31 days of enrollment. The number of patients with platinum plus CPT-11 and platinum and etoposide in the first regimen were 19 (24.7%) and 40 (51.9%), respectively. The median follow-up duration was 13.8 months (range, 0–28.5 months).
Time Interval of Development of BM and SREs
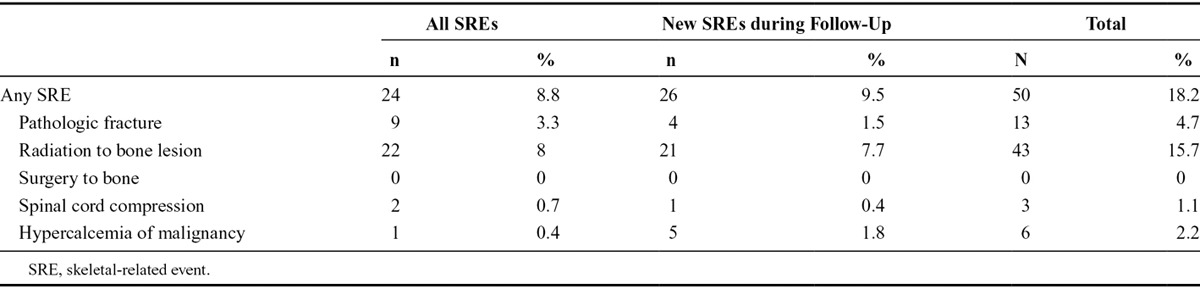
The incidence of BM and SREs at enrollment and during follow-up is presented in Figure 1 and Table 3. Among 274 lung cancer patients, BM was detected in 78 patients (28.5%) at enrollment and in another 34 patients (12.4%) during follow-up. SREs were reported in 24 patients (8.8%) at the time of enrollment and in another 26 patients (9.5%) during follow-up. Total number of patients who developed SREs was 52 (19.0%). The type of SRE was pathologic fracture in 14 (5.1%), radiation to bone in 45 (16.4%), spinal cord compression in three (1.1%), and HCM in six (2.2%). The median time to BM from diagnosis of lung cancer and to SRE from BM was 19.0 months (95% confidence interval [CI], 10.7 to not reached) and 9.5 months (95% CI, 5.1–16.9), respectively (Fig. 2A and C). However, the median time to SRE from diagnosis of lung cancer was not reached (Fig. 2B).
FIG. 1.

A, Time to bone metastasis from diagnosis of lung cancer. B, Time to SRE from diagnosis of lung cancer. C, Time to SRE from bone metastasis. SRE, skeletal-related event.
TABLE 3.
Incidence and Type of SREs (N = 274)
Predictive Factors of BM and SREs
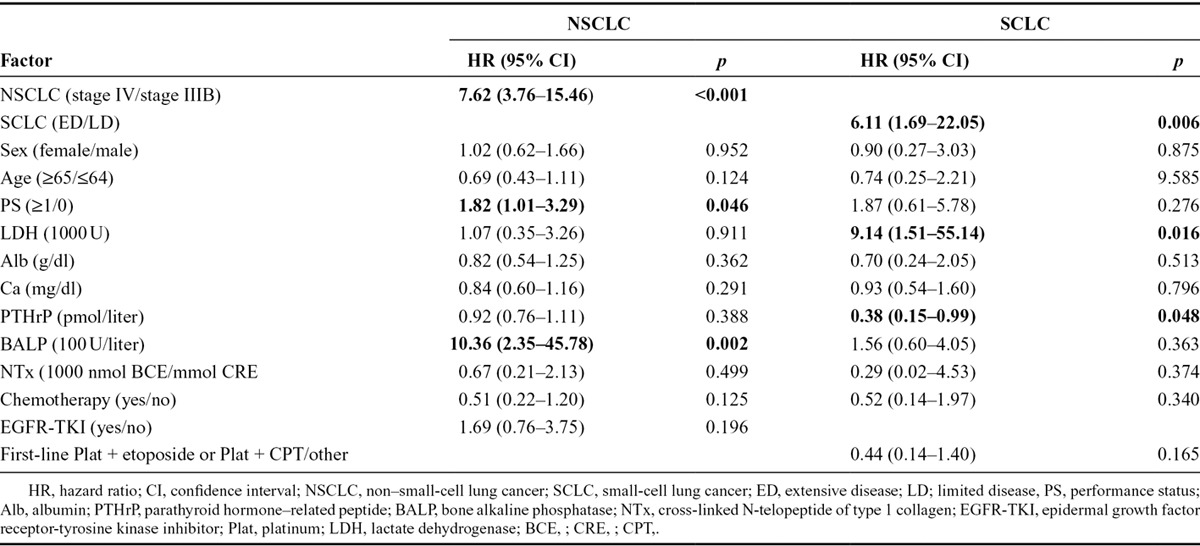
We performed subgroup analysis by separating NSCLC and SCLC because their biological characteristics and clinical behavior differ. To identify the predictive factors of BM and SREs, Cox proportional hazard regression analysis was performed using stage (stage IIIB and stage IV in case of NSCLC, and ED and LD in case of SCLC), sex, age, PS, LDH, albumin, calcium, PTHrP, BALP, NTx, and treatment (chemotherapy and EGFR-TKI in case of NSCLC, chemotherapy and first-line platinum + etoposide or first-line platinum and CPT in case of SCLC) (Table 4). The multivariate analysis of the NSCLC patients demonstrated that stage IV (hazard ratio [HR] = 7.62; 95% CI, 3.76–15.46; p < 0.001), PS 1 or greater (HR = 1.82; 95% CI, 1.01–3.29; p = 0.046), and higher serum BALP level (HR = 10.36; 95% CI, 2.35–45.78; p = 0.002) were significant predictive factors for BM (Table 4). However, neither chemotherapy (HR = 0.51; 95% CI, 0.22–1.20; p = 0.125) nor EGFR-TKI (HR = 1.69; 95% CI, 0.76–3.75; p = 0.196) were predictive factors. The multivariate analysis of the SCLC patients revealed that ED stage (HR = 6.11; 95% CI, 1.69–22.05; p = 0.006), higher serum LDH at baseline (HR = 9.14; 95% CI, 1.51–55.14; p = 0.016), and higher serum PTHrP at baseline (HR = 0.38; 95% CI, 0.15–0.99; p = 0.048) were significant predictive factors, but chemotherapy (HR = 0.52; 95% CI, 0.14–1.87; p = 0.340) and first-line use of platinum + etoposide or platinum + CPT (HR = 0.44; 95% CI, 0.14–1.40; p = 0.165) were not predictive factors.
TABLE 4.
Cox Regression Analysis for Bone Metastasis Incidence (Multivariable Analysis)
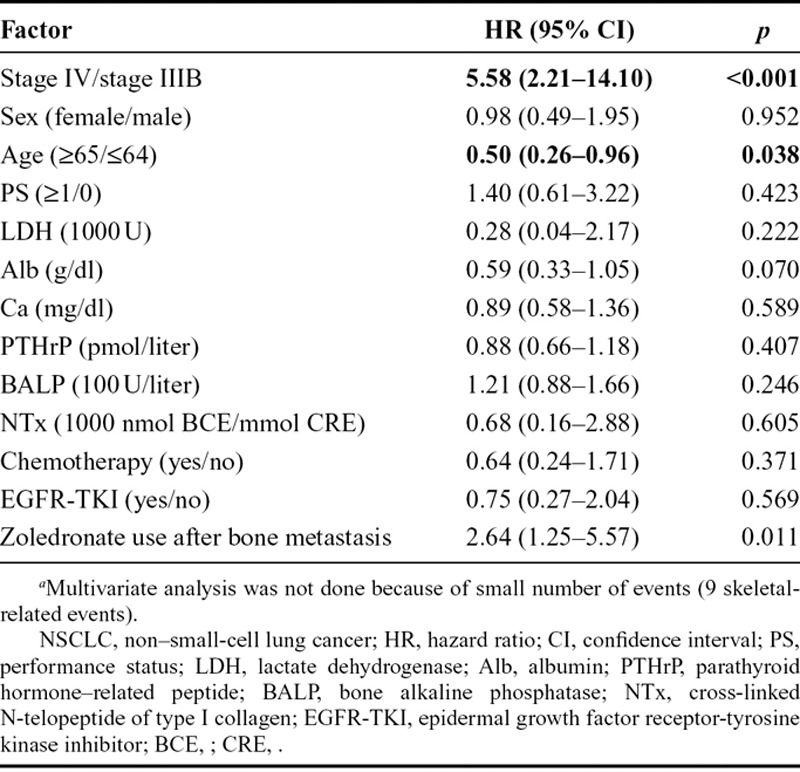
Regarding SREs, multivariate analysis of NSCLC patients demonstrated that stage IV (HR = 5.58; 95% CI, 2.21–14.10; p < 0.001), age 65 or older (HR = 0.50; 95% CI, 0.26–0.96; p = 0.038), and zoledronate use after BM (HR = 2.64; 95% CI, 1.25–5.57; p = 0.011) were predictive factors for SREs (Table 5). As for SCLC patients, multivariate analysis was not performed because of the small number of SRE events (only 9 events).
TABLE 5.
Cox Regression Analysis for Skeletal-Related Event Incidence in Patients with NSCLC (Multivariate Analysisa)
DISCUSSION
According to several studies published before 1991, the incidence of BM in NSCLC, as detected by bone scans, is between 8% and 34%, with a mean of 20%.17 More recent reviews have reported incidences of 24% and 30% in American and Japanese patient populations, respectively.18,19 Moreover, in retrospectively analyzed studies, the incidence of BM in patients with stage IV NSCLC was 41% to 54.8%.19,20 Our prospective study demonstrated that the incidence of BM in stage IV NSCLC and ED-SCLC was 46.7% and 40.4%, respectively. As newer imaging modalities such as PET and bone scan have been developed, the incidence of BM may have increased.21,22 However, it has been reported that the accuracies of PET and bone scan are 94% and 85% (p < 0.05), the sensitivity values were 91% and 75%, and the specificity values were 96% and 95%, respectively.22
The frequency of SREs among 274 patients in the current prospective study was in the order of frequency, radiation to bone (15.7%), pathologic fracture (4.7%), HCM (2.2%), and spinal cord compression (1.1%). Because spinal cord compression is considered an oncologic emergency, it requires urgent evaluation and treatment with corticosteroids and either radiotherapy or surgical decompression. Fortunately, we were able to reduce the incidence of spinal cord compression, as much as possible, because of close monitoring and appropriate treatment. A prospective study of 250 patients with a variety of solid tumors (120 of whom had NSCLC) complicated with BM found that the incidence of SRE was radiation to bone in 32%, pathologic fracture in 21%, surgery to bone in 4%, spinal cord compression in 4%, and HCM in 3% when patients were not treated prophylactically with zoledronate.15 In a retrospective review of Japanese patients with NSCLC, among 135 stage IV patients, the most common SREs were the need for radiotherapy in 34.3% and hypercalcemia in 20%.19
Another retrospective study of 196 NSCLC patients with BM revealed that 47 of 110 patients without initial SRE eventually experienced their first SREs while receiving chemotherapy, whereas the accompanying type of SRE was radiotherapy in 39, pathologic fracture in 11, surgery to bone in six, and spinal cord compression in four patients.23
The median time to BM from diagnosis of lung cancer and to SREs from BM in our study was 19.0 and 9.5 months, respectively. To our knowledge, there seems to be no single prospective study which reports the interval to BM from diagnosis of stage IIIB NSCLC or LD-SCLC. Our result may be useful in future trials to prevent BM from lung cancer with bisphosphonate or denosumab, regarding the initiation of treatment. The median interval to SRE from BM was 9.5 months, compared with 171 days for NSCLC patients treated with 4 mg of zoledronate.15 In a randomized phase III study comparing zoledronate with denosumab in the treatment of BMs in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma, the median time to first on-study SRE was 20.6 months for denosumab and 16.3 months for zoledronate. The effect of denosumab on time to first on-study SRE relative to zoledronate by tumor stratification factors resulted in an HR of 0.84 for NSCLC (95% CI, 0.64 to 1.10; p = 0.20).16 However, a direct comparison is not possible, as all of the patients in our study were not treated with zoledronate or denosumab, and were also treated with various types of anticancer drugs, including EGFR-TKIs, which were quite effective in EGFR mutation–positive patients.8,9
We evaluated the predictive factors of BM via multiple regression analysis separately in patients with SCLC and patients with NSCLC, because their biologic characteristics and clinical behavior differ. In case of NSCLC, stage IV (HR = 7.62; p < 0.001), PS 1 or greater at enrollment (HR = 1.82; p = 0.046), and high serum BALP at baseline (HR =10.36; p = 0.002) were found to be positive predictive factors. Two factors, excluding BALP, represent the tumor burden at the time of diagnosis of lung cancer. Therefore, the chance of developing BM increases with tumor progression. BALP has been investigated as a bone formation marker, but their association with clinical characteristics seems to vary depending on the tumor type, the nature of the BMs, and the effects of treatment. BALP measured in our study may represent active bone turnover as a result of subclinical metastasis to bone. Unexpectedly, EGFR-TKI treatment was not a negative predictive marker of BM. This may be related to the small number of active EGFR mutations (30 patients, 15.2%) and the fact that that most patients were treated with EGFR-TKI as a second- or third-line regimen (21 patients, 70%) instead of a first-line regimen. In SCLC, ED at enrollment (HR = 6.11; p = 0.006), higher serum LDH at baseline (HR = 9.14; p = 0.016), and higher serum PTHrP at baseline (HR = 0.38; p = 0.048) were found to be predictive factors. The former two factors represent the tumor burden at the time of diagnosis of lung cancer as in NSCLC while increasing the chance to develop BM. The reason why the higher serum PTHrP at baseline was a negative predictive factor of BM was not clear, but it may be acting as a nonfunctioning tumor marker. A first-line regimen with platinum + CPT-11 or platinum + etoposide tended to be a negative predictive factor of BM (HR = 0.44; p = 0.165) but did not reach a significant level, probably because of the small sample size.
Another multiple regression analysis evaluating the predictive factors of SREs from initial documentation of BM demonstrated that stage IV NSCLC (HR = 5.58; p < 0.001) and zoledronate use after BM were positive factors, whereas age 65 or older (HR = 0.50; p = 0.038) was a negative predictive factor for SRE in NSCLC. Multiple regression analysis was not performed for SCLC, because the number of patients with SREs was only nine and all SREs occurred in ED-SCLC. The reason why an age of more than 65 years in NSCLC was a negative predictive factor for SREs is not clear in our study. This might be related to the physical activity of younger patients, as they enjoyed moving, without pain or limitation, and needed to receive radiation therapy more frequently than elder patients. Otherwise, older patients might have undergone single-agent chemotherapy for longer periods, or received molecular-targeted agents more often than younger patients. These factors need to be elucidated in a future trial. Surprisingly, zoledronate use after BM was a positive predictive factor of SREs. This may be because of the fact that zoledronate was used at the discretion of the investigators, and that they tended not to administer zoledronate at an early stage, but at a progressive stage of BM (bias due to the treatment by indication).
Serum albumin was a marginally significant negative predictive factor of SRE (HR = 0.59; p = 0.070) by multivariate analysis. High serum albumin reflects that patients have a good appetite, and can maintain good general condition, such as body weight. These two factors are also known as a good prognostic factor.26–28 In contrast to other reports, NTx was not a predictive factor of SRE (p = 0.758) in our study.29,30 Brown et al.29 reported a statistically significant correlation between N-telopeptide levels at baseline and a range of skeletal complications in 121 bisphosphonate-treated patients with breast or prostate cancer with BM. Their definition of SRE was radiotherapy to bone, hypercalcemia, spinal cord compression, pathological fracture, surgery to bone, hospital admissions for control of bone pain, and/or death due to metastatic bone disease. The total number of SREs was 111 in 121 patients during 6 months. In another report, which focused on metastatic NSCLC without zoledronate therapy, high NTx level at baseline was associated with an increased rate of first SRE, and this increased rate was maintained throughout the study period. Overall, there were a total of 110 SREs in 115 patients during 21 months.30 However, there were only 50 SREs among 111 lung cancer patients with BM over a median follow-up duration of 13.8 months, and the number of SREs was relatively low in the current study, compared with these two reports. This may be the reason why NTx at baseline was not a predictive marker of SREs in our study.
CONCLUSIONS
The incidence of BM at initial diagnosis was 48% in patients with stage IV NSCLC and 40% in patients with ED-SCLC. Forty-six percent of the patients who developed BM had SREs at enrollment and during the follow-up period. Multivariate analysis revealed that the factors predicting BMs were stage IV, PS 1 or greater, higher serum BALP at baseline in case of NSCLC and ED, higher serum LDH at baseline, and higher serum PTHrP at baseline in case of SCLC, and that the factors predicting SRE in NSCLC were stage IV, age 64 or younger, and zoledronate use after BM. These factors should be taken into consideration in future randomized studies evaluating BM and SREs.
ACKNOWLEDGMENTS
The authors thank Mr. Hitoshi Inoue and other staff members of Comprehensive Support Project for Health Outcomes Research (CSP-HOR) data center for data management.
Footnotes
Disclosure: The authors declare no conflict of interest.
Previously presented at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, 3–7 June, 2011, and the 16th Congress of The European Multidisciplinary Cancer, Stockholm, Sweden, 23–27 September, 2011.
REFERENCES
- 1.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18:317–323. doi: 10.1093/annonc/mdl377. [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares L, Marinis FD, Dediu M, et al. PARAMOUNT: final overall survival (OS) results of the phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo (plb) plus BSC immediately following induction treatment with pem plus cisplatin (cis) for advanced nonsquamous (NS) non-small cell lung cancer (NSCLC). ASCO Meeting Abstracts. 2012;30:7507. [Google Scholar]
- 6.Katakami N, Gemma A, Sakai H, et al. Randomized phase III trial of S-1 plus cisplatin versus docetaxel plus cisplatin for advanced non-small-cell Lung cancer (TCOG0701)—CATS TRIAL (cisplatin and TS-1 TRIAL). ASCO Meeting Abstracts. 2012;30:7515. [Google Scholar]
- 7.Yang CH, Fukuoka M, Mok TS, et al. Final overall survival (OS) results from a phase III, randomised, open-label, first-line study of gefitinib (G) v carboplatin/paclitaxel (C/P) in clinically selected patients with advanced nonsmall cell lung cancer (NSCL) in Asia (IPASS). Ann Oncol. 2010;21(Suppl 8):LBA 2. [Google Scholar]
- 8.Inoue A, Kobayashi K, Maemondo M, et al. Final overall survival results of NEJ002, a phase III trial comparing gefitinib to carboplatin (CBDCA) plus paclitaxel (TXL) as the first-line treatment for advanced non-small cell lung cancer (NSCLC) with EGFR mutations. ASCO Meeting Abstracts. 2011;29:7519. [Google Scholar]
- 9.Mitsudomi T, Morita S, Yatabe Y, et al. Updated overall survival results of WJTOG 3405, a randomized phase III trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor (EGFR). ASCO Meeting Abstracts. 2012;30:7521. [Google Scholar]
- 10.Noda K, Nishiwaki Y, Kawahara M, et al. Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 11.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 12.Turrisi AT, 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 13.Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–3060. doi: 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 14.Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 15.Rosen LS, Gordon D, Tchekmedyian S, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 16.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 17.Kosteva J, Langer C. The changing landscape of the medical management of skeletal metastases in nonsmall cell lung cancer. Curr Opin Oncol. 2008;20:155–161. doi: 10.1097/CCO.0b013e3282f54cf2. [DOI] [PubMed] [Google Scholar]
- 18.Kosteva J, Langer CJ. Incidence and distribution of skeletal metastases in NSCLC in the era of PET [abstract]. Lung Cancer. 2004;46(Suppl 1):S45. [Google Scholar]
- 19.Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer. 2007;57:229–232. doi: 10.1016/j.lungcan.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Schumacher T, Brink I, Mix M, et al. FDG-PET imaging for the staging and follow-up of small cell lung cancer. Eur J Nucl Med. 2001;28:483–488. doi: 10.1007/s002590100474. [DOI] [PubMed] [Google Scholar]
- 22.Cheran SK, Herndon JE, 2nd, Patz EF., Jr Comparison of whole-body FDG-PET to bone scan for detection of bone metastases in patients with a new diagnosis of lung cancer. Lung Cancer. 2004;44:317–325. doi: 10.1016/j.lungcan.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Bae HM, Lee SH, Kim TM, et al. Prognostic factors for non-small cell lung cancer with bone metastasis at the time of diagnosis. Lung Cancer. 2012;77:572–577. doi: 10.1016/j.lungcan.2012.05.094. [DOI] [PubMed] [Google Scholar]
- 24.Coleman RE, Whitaker KB, Moss DW, Mashiter G, Fogelman I, Rubens RD. Biochemical prediction of response of bone metastases to treatment. Br J Cancer. 1988;58:205–210. doi: 10.1038/bjc.1988.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berruti A, Dogliotti L, Gorzegno G, et al. Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. Clin Chem. 1999;45(8 Pt 1):1240–1247. [PubMed] [Google Scholar]
- 26.Finkelstein DM, Ettinger DS, Ruckdeschel JC. Long-term survivors in metastatic non-small-cell lung cancer: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1986;4:702–709. doi: 10.1200/JCO.1986.4.5.702. [DOI] [PubMed] [Google Scholar]
- 27.Hoang T, Xu R, Schiller JH, Bonomi P, Johnson DH. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on eastern cooperative oncology group data. J Clin Oncol. 2005;23:175–183. doi: 10.1200/JCO.2005.04.177. [DOI] [PubMed] [Google Scholar]
- 28.Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P International Staging Committee and Participating Institutions. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol. 2008;3:457–466. doi: 10.1097/JTO.0b013e31816de2b8. [DOI] [PubMed] [Google Scholar]
- 29.Brown JE, Thomson CS, Ellis SP, Gutcher SA, Purohit OP, Coleman RE. Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer. 2003;89:2031–2037. doi: 10.1038/sj.bjc.6601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown JE, Cook RJ, Major P, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]


