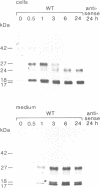
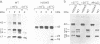Abstract
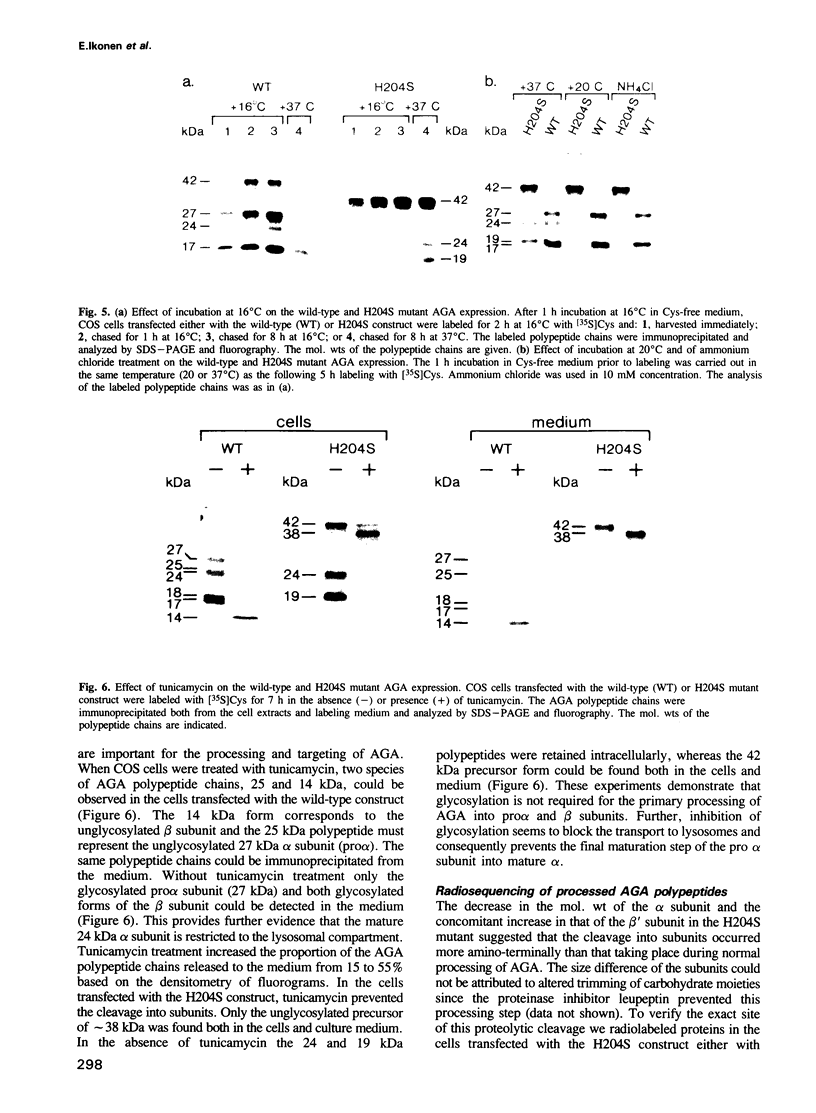
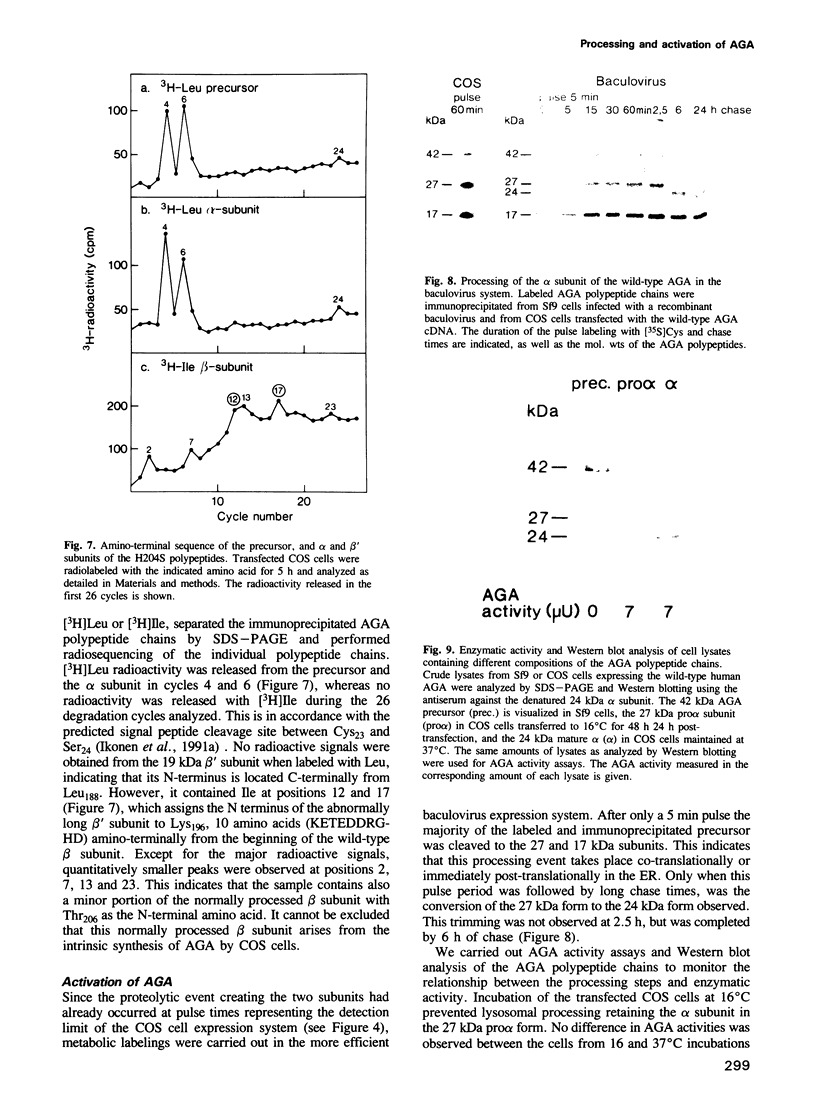
Aspartylglucosaminidase (AGA) is a lysosomal enzyme, the deficiency of which leads to a human storage disease, aspartylglucosaminuria (AGU). Although numerous mutations have been identified in AGU patients, elucidation of the molecular pathogenesis of the disease has been hampered by the missing information on the cellular events resulting in the maturation and activation of the enzyme. Here we used the expression of in vitro mutagenized constructs of the AGA cDNA to define three specific proteolytic trimming steps resulting in mature AGA. Removal of the signal peptide is immediately followed by proteolytic cleavage of the precursor into two subunits and results in biologically active enzyme already in the endoplasmic reticulum. This early activation has not previously been described for lysosomal enzymes. The subsequent lysosomal trimming does not influence the enzymatic activity of AGA. It consists only of a single proteolytic cleavage which removes 10 amino acids from the C-terminal end of the larger subunit, in contrast to the multistep lysosomal processing observed in several other hydrolases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Kuranda M. J. Lysosomal degradation of Asn-linked glycoproteins. FASEB J. 1989 Dec;3(14):2615–2622. doi: 10.1096/fasebj.3.14.2531691. [DOI] [PubMed] [Google Scholar]
- Baumann M., Peltonen L., Aula P., Kalkkinen N. Isolation of a human hepatic 60 kDa aspartylglucosaminidase consisting of three non-identical polypeptides. Biochem J. 1989 Aug 15;262(1):189–194. doi: 10.1042/bj2620189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. J., Aronson N. N., Jr Characterization of the mutation responsible for aspartylglucosaminuria in three Finnish patients. Amino acid substitution Cys163----Ser abolishes the activity of lysosomal glycosylasparaginase and its conversion into subunits. J Biol Chem. 1991 Jun 25;266(18):12105–12113. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., von Figura K., Conzelmann E., Nehrkorn H., Sandhoff K. Lysosomal enzyme precursors in human fibroblasts. Activation of cathepsin D precursor in vitro and activity of beta-hexosaminidase A precursor towards ganglioside GM2. Eur J Biochem. 1982 Jul;125(2):317–321. doi: 10.1111/j.1432-1033.1982.tb06685.x. [DOI] [PubMed] [Google Scholar]
- Hubbes M., Callahan J., Gravel R., Mahuran D. The amino-terminal sequences in the pro-alpha and -beta polypeptides of human lysosomal beta-hexosaminidase A and B are retained in the mature isozymes. FEBS Lett. 1989 Jun 5;249(2):316–320. doi: 10.1016/0014-5793(89)80649-0. [DOI] [PubMed] [Google Scholar]
- Ikonen E., Aula P., Grön K., Tollersrud O., Halila R., Manninen T., Syvänen A. C., Peltonen L. Spectrum of mutations in aspartylglucosaminuria. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11222–11226. doi: 10.1073/pnas.88.24.11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E., Baumann M., Grön K., Syvänen A. C., Enomaa N., Halila R., Aula P., Peltonen L. Aspartylglucosaminuria: cDNA encoding human aspartylglucosaminidase and the missense mutation causing the disease. EMBO J. 1991 Jan;10(1):51–58. doi: 10.1002/j.1460-2075.1991.tb07920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E., Enomaa N., Ulmanen I., Peltonen L. In vitro mutagenesis helps to unravel the biological consequences of aspartylglucosaminuria mutation. Genomics. 1991 Sep;11(1):206–211. doi: 10.1016/0888-7543(91)90120-4. [DOI] [PubMed] [Google Scholar]
- Ikonen E., Ulmanen I., Peltonen L. Deletion of the 3'-untranslated region of aspartylglucosaminidase mRNA results in a lysosomal accumulation disease. J Biol Chem. 1992 May 5;267(13):8715–8718. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makino M., Kojima T., Ohgushi T., Yamashina I. Studies on enzymes acting on glycopeptides. J Biochem. 1968 Feb;63(2):186–192. doi: 10.1093/oxfordjournals.jbchem.a128760. [DOI] [PubMed] [Google Scholar]
- Makino M., Kojima T., Yamashina I. Enzymatic cleavage of glycopeptides. Biochem Biophys Res Commun. 1966 Sep 22;24(6):961–966. doi: 10.1016/0006-291x(66)90344-5. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt R. J., Jenner F. A., Merskey H. Aspartylglycosaminuria. An inborn error of metabolism associated with mental defect. Lancet. 1968 Aug 3;2(7562):253–255. doi: 10.1016/s0140-6736(68)92355-6. [DOI] [PubMed] [Google Scholar]
- Quon D. V., Proia R. L., Fowler A. V., Bleibaum J., Neufeld E. F. Proteolytic processing of the beta-subunit of the lysosomal enzyme, beta-hexosaminidase, in normal human fibroblasts. J Biol Chem. 1989 Feb 25;264(6):3380–3384. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984 Sep;38(2):535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Steckel F., Hasilik A., von Figura K. Biosynthesis and maturation of arylsulfatase B in normal and mutant cultured human fibroblasts. J Biol Chem. 1983 Dec 10;258(23):14322–14326. [PubMed] [Google Scholar]
- Sussman D. J., Milman G. Short-term, high-efficiency expression of transfected DNA. Mol Cell Biol. 1984 Aug;4(8):1641–1643. doi: 10.1128/mcb.4.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen A. C., Ikonen E., Manninen T., Bengtström M., Söderlund H., Aula P., Peltonen L. Convenient and quantitative determination of the frequency of a mutant allele using solid-phase minisequencing: application to aspartylglucosaminuria in Finland. Genomics. 1992 Mar;12(3):590–595. doi: 10.1016/0888-7543(92)90452-x. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The isolation and structure of the core oligosaccharide sequences of IgM. Biochemistry. 1975 Dec 16;14(25):5516–5523. doi: 10.1021/bi00696a021. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollersrud O. K., Aronson N. N., Jr Comparison of liver glycosylasparaginases from six vertebrates. Biochem J. 1992 Mar 15;282(Pt 3):891–897. doi: 10.1042/bj2820891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]