Abstract
Metabolic syndrome is reaching epidemic proportions, particularly in developing countries. In this review, we explore the concept—based on the developmental-origin-of-health-and-disease hypothesis—that reprogramming during critical times of fetal life can lead to metabolic syndrome in adulthood. Specifically, we summarize the epidemiological evidence linking prenatal stress, manifested by low birth weight, to metabolic syndrome and its individual components. We also review animal studies that suggest potential mechanisms for the long-term effects of fetal reprogramming, including the cellular response to stress and both organ- and hormone-specific alterations induced by stress. Although metabolic syndrome in adulthood is undoubtedly caused by multiple factors, including modifiable behavior, fetal life may provide a critical window in which individuals are predisposed to metabolic syndrome later in life.
Keywords: nutrition, developmental-origin-of-health-and-disease hypothesis, in utero stress
1. INTRODUCTION
Compelling evidence indicates that stressful environmental conditions during sensitive periods of early development cause a predisposition to chronic disease later in life (1). The premise of this concept, known as the developmental-origin-of-health-and-disease hypothesis, is that the fetus adapts to its environment. Specifically, the developing fetus senses the environment during specific windows of sensitivity and optimizes future metabolic responses by reprogramming its genome. This reprogramming favors early survival and reproductive success but potentially causes a predisposition to disease in later stages of life (2).
Here we summarize the epidemiological evidence linking prenatal stress to the development of metabolic syndrome (MS) and review animal studies that reveal potential mechanisms for the long-term effects of fetal stress. Excellent reviews on the topic exist (3–5). Our focus is on the primary mechanisms and potential molecular pathways by which organ dysfunction occurs and leads to components of MS.
2. FETAL REPROGRAMMING
A quarter of a century ago, Barker & Osmond (1) sat the University of Southampton, England, crystallized the concept of fetal programming and early origin of adult disease by suggesting that stress in utero, as manifested by low birth weight (LBW), increases the risk of cardiovascular disease and stroke in specific areas of England and Wales. Prior researchers had formulated similar hypotheses on the basis of findings in humans (6, 7) and animals (8). Hales & Barker (9), however, provided a mechanistic explanation by proposing the thrifty phenotype hypothesis to complement the already existing thrifty genotype hypothesis (10). According to Hales & Barker’s hypothesis, fetuses exposed to suboptimal conditions during uterine life program developmental processes in anticipation of similar suboptimal conditions in postnatal life. If postnatal conditions are instead optimal and resources are abundant, the organism is ill-prepared to cope with the different environment and hence is more susceptible to developing diseases (11).
Integral to the concept of reprogramming is the existence of critical windows of sensitivity, during which the organism is particularly sensitive to the environment. The Barker hypothesis identified the uterine period as a key period of developmental plasticity; however, it is now clear that there are additional windows of sensitivity, including the preconception period and early postnatal life (Figure 1) (12).
Figure 1.
Critical windows of sensitivity during human pregnancy for the development of components of metabolic syndrome later in life. The different effects of stress during pregnancy can be explained by the cellular events that occur during particular periods of pregnancy. Data were obtained from the Dutch Famine Cohort (26). Glucose intolerance is a common consequence of in utero stress and is independent of the time of occurrence. Stress in early gestation leads to a greater risk of an abnormal atherogenic lipid profile, obesity in women, and coronary artery disease. Stress in the second trimester of pregnancy, during which the number of nephrons increases rapidly, is associated with a 3.2-fold increase in risk for microalbuminuria. Finally, stress during the third trimester of pregnancy, when fat deposition occurs, has a relatively higher effect in reducing birth weight. Additional windows of sensitivity include the preconception and the early postnatal periods. Surprisingly, animal studies show that even stress limited to the preimplantation period (5 days) can lead to impaired glucose intolerance (96, 97).
Indeed, some critics of the Barker hypothesis emphasize that early postnatal stress is at least as important as in utero stress in determining long-term effects and introduced an alternative hypothesis—that exposures or insults gradually accumulating through episodes of illness, adverse environmental conditions, and behaviors increase the risk of chronic disease and mortality (13). In this so-called life course hypothesis, lifestyle factors in adulthood make a much greater contribution than in utero stress. According to this view, in utero stress causes a predisposition to disease that is manifested only if an additional stress occurs later in postnatal life (e.g., obesity or high-fat diet). This is exemplified by the increasing incidence of diabetes in low- and middle-income countries, where the combination of poor nutrition in utero and overnutrition in later life is common (14).
Another important concept is that the effects of in utero stress differ according to the timing of its occurrence. In rats and sheep, maternal undernourishment during the period of conception and implantation, for example, leads to hypertension and cardiovascular dysfunction in offspring (15–19). Embryos cultured in vitro during the preimplantation period have long-term health problems (19).
Other than evidence from the Dutch famine, when caloric intake was severely reduced for a short, well-defined short period, it is often difficult to identify a specific time frame in which in utero stress can be analyzed. The only additional evidence of a temporally limited stress derives from follow-up of children conceived by assisted reproductive technology (ART). More than 3 million children have been born worldwide as a result of ART. Data exist to support the hypothesis that stress in the form of embryo culture during the preimplantation period may be associated with early differences in metabolic profile. Epidemiological data are limited by the relatively short history of ART. Because the first child conceived by in vitro fertilization (IVF) was born in 1978, IVF children are at most in their early thirties and are rather young to manifest components of MS. However, there is compelling evidence that IVF offspring have a different metabolic profile starting at birth. Among term singleton infants, those conceived through ART have a 2.6-fold-greater risk of LBW than do those conceived spontaneously (20).
Recently, a cohort study investigated metabolic and pubertal measures in 233 IVF children ages 8–18 and a similar number of age- and gender-matched spontaneously conceived controls born to subfertile parents (21). IVF children had significant increases in peripheral body mass and percentage of peripheral fat compared with controls, as well as a trend toward a higher percentage of total body fat (21). Waist circumference did not differ between the two groups. Among pubertal girls, those conceived by IVF also appear to have greater bone age than did controls, as evidenced by radiographs of the left hand (22). Even more interesting, systolic and diastolic blood pressure levels were higher in IVF children than in controls, and fasting glucose levels were higher in pubertal IVF children (23). These differences persisted despite adjustments for current body size, birth weight, and parental characteristics. A difference of 4 mm Hg in systolic blood pressure is clinically significant, as evidenced by the increase in hypertension with age (24).
On the contrary, one study described an improved metabolic profile at 6 years of age in 69 IVF children compared with 71 controls (25). IVF children were taller than controls after correction for parental height and had a more favorable lipid profile, with higher high-density lipoprotein and lower triglyceride levels. The different findings of these studies are likely secondary to the different age of the test subjects and to the low sample size of the two studies and underline the need for more research in the field.
Studies of the Dutch famine are particularly illuminating for evaluating in utero stress. During the 4-month duration of the famine (December 1944 to April 1945), the official daily ration was 400–800 calories (26). Fetuses undernourished during early gestation had an atherogenic lipid profile, increased risk for coronary heart disease in adulthood, and a decline in cognitive function (26, 27). Undernourishment during mid-gestation increased the incidence of microalbuminuria and obstructive airway disease. Finally, glucose tolerance was altered in all fetuses exposed to famine but was particularly evident after undernourishment in late gestation (Figure 1) (26, 28).
Another important point is the concept of sexually dimorphic effects after in utero stress (29). Women with less than average birth weight and higher weight at 1 year showed the highest incidence of cardiovascular death rates; this pattern was not present in men (30). Only in women with a birth weight greater than 4 kg (normal birth weight at term is between 2.5 and 4 kg) did systolic blood pressure increase in parallel with birth weight (31). In animal models, only female offspring of pregnant dams exposed to a high-fat diet exhibit hypertension in later life (32); in contrast, only adult male rats show alterations in triglycerides and expression of hepatic fatty acid enzymes after uterine artery ligation (33).
These sexually dimorphic effects are caused by multiple mechanisms that are not clearly understood. Apart from differences in sex steroids, these effects may reflect variations in activation of the hypothalamic-pituitary-adrenal (HPA) axis (32), different responses to oxidative stress (34), and the faster postnatal growth rate of male mice compared with female mice (29).
3. DEFINITION OF METABOLIC SYNDROME
MS is a constellation of metabolic risk factors that increase an individual’s predisposition to atherosclerotic cardiovascular disease, hypertension, and type 2 diabetes. Estimates suggest that the population-attributable fraction for MS is approximately 6–7% for all-cause mortality, 12–17% for cardiovascular disease, and 30–52% for diabetes (35). Among adults in the United States, the age-adjusted prevalence of MS is 23.7% or 47 million people (36). Importantly, the prevalence increases from 6.7% among individuals aged 20–29 years to more than 40% in those 60 years or older (36).
In both adults and children, the definition of MS is controversial (37–40). The most widely used definitions in adults are from the National Cholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III) (37) and the International Diabetes Federation (IDF) (38). Diagnostic signs include elevated blood pressure (>130/85 mm Hg), central obesity (>102 cm in men and >88 cm in women), dyslipidemia (serum triglycerides ≥ 150 mg dl−1 and high-density lipoprotein cholesterol <40 mg dl−1 in men and <50 mg dl−1 in women), and glucose intolerance (serum glucose ≥ 100 mg dl−1). The main difference between the two definitions is that abdominal obesity, defined by ethnicity-specific waist circumference measurements, is a required component of the IDF definition. As a result, MS is more prevalent when diagnosed with the IDF definition than with the NCEP/ATP III definition (39% versus 34.5%) (36).
The definition of MS in children and adolescent is more controversial, and to date, no unified criteria exist (41). Attempts have been made to characterize MS in the pediatric population using modified criteria from NCEP/ATP III, the World Health Organization, and the European Group for the Study of Insulin Resistance (42). In a study that applied eight different criteria for MS to 1,289 children aged 4–16 years, the prevalence of the syndrome varied significantly, with figures between 6% and 39% (42). Application of age-modified NCEP/ATP III criteria to participants 12–19 years from the National Health and Nutrition Examination Survey suggested a 9.2% prevalence among adolescents (43). In overweight children and adolescents, the prevalence was much higher: 38.7% in moderately obese individuals and 49.7% in severely obese individuals (44).
The utility of diagnosing MS as a whole versus individual risk factor components for the prediction of cardiovascular disease remains to be shown. The literature provides conflicting data on this issue (40, 45–47). MS did not predict cardiovascular mortality independently of its individual components in a large cohort of U.S. men aged 50 years at baseline (47), but it did in another study that included men and women ≥ 65 years followed for 4 years (46).
Regardless of the criteria used to define MS, the prevalence of the diagnostic signs that constitute MS is increasing worldwide, affecting individuals at a progressively younger age. This phenomenon is particularly evident in developing countries, where an accelerated economic and cultural transition and the metabolically obese phenotype (i.e., normal body weight with increased abdominal adiposity) is common. In these countries, the combination of poor nutrition in utero and overnutrition in later life is likely responsible for the observed epidemic of MS and diabetes (14). The incidence of the syndrome is increasing; MS could affect 40% of the population by 2025.
4. EVIDENCE OF FETAL REPROGRAMMING OF METABOLIC SYNDROME FROM HUMAN STUDIES
An important methodological challenge in analyzing human data is to identify in utero stress. LBW has been used to classify fetuses below the expected standard of growth. Two definitions of LBW are often used: (a) <2,500 g regardless of gestational age and (b) small for gestational age, defined as below the tenth percentile of the population-specific growth curve. These definitions are prone to error. In particular, false negatives may occur. For example, a fetus below the tenth percentile, and therefore defined as small for gestation age, may be constitutionally small for genetic reasons (both parents were of small size) but indeed have had a perfectly healthy gestation (a false positive). This fetus, although small, will not be predisposed to long-term consequences. Conversely, newborns above the tenth percentile and thus considered to be of “normal” weight may have been stressed in utero but may not have reached their ideal weight because of intrauterine growth restriction (IUGR) (a false negative). Here we use IUGR to indicate that fetuses were stressed in utero, regardless of their weight. Currently there are no clinical measures to differentiate between babies of the same birth weight who were or were not exposed to in utero stress.
Overall, the link between LBW and adult disease is broadly continuous. Heavier babies at birth (excluding overweight newborns of diabetic mothers) have a lower incidence of MS in adulthood than do those of normal birth weight. Epidemiological data support the use of birth weight as a continuous measure rather than as a dichotomous measure associated with long-term disease risk.
Epidemiological studies have convincingly linked a suboptimal gestational environment to an increased risk for components of adult-onset MS (e.g., hypertension, glucose intolerance, dyslipidemia, obesity). Fewer studies have specifically studied the association between in utero stress and MS as a whole (Table 1).
Table 1.
Selected human studies linking either a well-identified pregnancy stress or a marker of stress identified at birth (like LBW) to MS or its individual componentsa
| Type of stress | Stress marker | MS component | Details | Reference |
|---|---|---|---|---|
| IVF | RR 2.6 for low birth weight | 20 | ||
| IVF | HTN | Higher SBP: 109 ± 11 versus 105 ± 10 mm Hg | 23 | |
| IGT | Higher fasting glucose: 5.0 ± 0.4 versus 4.8 ± 0.4 mmol liter−1 | 23 | ||
| IVF | Obesity | Higher peripheral fat mass: 7.6 ± 4.2 versus 6.7 ± 3.2 kg | 21 | |
| IVF | Obesity | Prevalence of BMI > 25: 44.9% | 147 | |
| HTN, dyslipedemia, insulin resistance | Prevalence of 6.9% (12/173) | 147 | ||
| IVF | Dyslipidemia | Higher HDL: 1.67 ± 0.04 versus 1.53 ± 0.04 mmol liter−1 | 25 | |
| Dyslipidemia | Lower triglycerides: 0.65 ± 0.04 versus 0.78 ± 0.04 mmol liter−1 | 25 | ||
| Birth weight | HTN | Inverse correlation with SBP by 2 mm Hg kg−1 | 49 | |
| Birth weight | HTN | Inverse correlation with SBP by 1.52 mm Hg kg−1 (men), 2.80 mm Hg kg−1 (women) | 31 | |
| Biafran famine | HTN | OR 2.87 for systolic HTN in fetal-infant exposure | 66 | |
| PIH | HTN | Higher childhood SBP by 2 mm Hg in exposed group | 148 | |
| PIH | HTN | OR 1.88 for HTN in exposed group | 79 | |
| Weight at 1 year | IGT | Higher prevalence of IGT: 23% (<18 lb) versus 13% (>27 lb) | 53 | |
| IGT | Higher prevalence of DM: 17% (<18 lb) versus 0% (>27 lb) | 53 | ||
| Dutch famine | IGT | Higher mean 2-h glucose by 0.05 mmol liter−1 in exposed group | 28 | |
| Ponderal index | IGT | Inverse correlation with insulin resistance | 54 | |
| IUGR | IGT | Lower glucose-stimulated insulin uptake: 6.7 ± 2.9 versus 8.0 ± 1.9 mg kg−1 fat-free mass × min | 55 | |
| Biafran famine | IGT | OR 1.65 for IGT in fetal-infant exposure | 66 | |
| Abdominal circumference at birth | Dyslipidemia | Inverse correlation with total cholesterol by 0.25 mmol liter−1 per 1 inch | 56 | |
| Dutch famine | Dyslipidemia | OR 2.1 for high-fat diet in exposed group | 57 | |
| BMI at birth | Dyslipidemia | Inverse correlation with non-HDL cholesterol by 0.051 mmol liter−1 per 1 kg m−2 | 58 | |
| Birth weight | Dyslipidemia | No consistent association between birth weight and lipids | 59 | |
| Dutch famine | Obesity | Exposure during third trimester decreased obesity rates (0.82% versus 1.32%) | 60 | |
| Dutch famine | Obesity | Exposure during first and second trimester increased obesity rates (2.77% versus 1.45%) | 60 | |
| Birth weight | Obesity | Higher percentage body fat in low birth weight (29.3% versus 25.3%) | 61 | |
| Birth weight | Obesity | No association between birth weight and BMI after accounting for maternal weight | 62 | |
| Biafran famine | Obesity | OR 1.41 for overweight in fetal-infant exposure | 66 | |
| Birth weight | Obesity | Significant association between birth weight and childhood obesity | 149 | |
| Gestational DM | Obesity | OR 1.4 for adolescent overweight in exposed group | 75 | |
| Diet-controlled gestational DM | Obesity | No difference in prevalence of childhood obesity | 76 | |
| Dutch famine | MS | Exposure not associated with MS | 63 | |
| Birth weight | MS | RR 2.41 for MS associated with lowest birth weight tertile | 64 | |
| Birth weight | MS | OR 1.8 for MS associated with lowest birth weight tertile | 65 | |
| Maternal diabetes | MS | Higher prevalence of childhood MS in exposed LGA (50% versus 21%) | 73 | |
| Maternal obesity | MS | OR 1.81 for childhood MS in exposed group | 73 | |
| Birth weight | CAD | Decrease in standardized mortality ratios with increase in birth weight | 67 | |
| Birth weight | CAD | Decrease in standardized mortality ratios with increase in birth weight | 30 | |
| Birth weight | CAD | RR 1.49 for nonfatal CAD with birth weight <5 lb | 68 | |
| Birth weight | CAD | Higher prevalence of CAD in low birth weight (11% versus 3%) | 69 | |
| Birth weight | CAD | Age-adjusted HR 0.62 for CAD for every 1-kg increase | 70 | |
| Birth weight | CAD | Age-adjusted HR 0.67 for CAD for every 1-kg increase | 71 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; HDL, high-density lipoprotein; HR, hazard ratio; HTN, hypertension; IGT, impaired glucose tolerance; IUGR, intrauterine growth restriction; IVF, in vitro fertilization; LGA, large for gestational age; MS, metabolic syndrome; OR, odds ratio; PIH, pregnancy-induced hypertension; RR, relative risk; SBP, systolic blood pressure.
The evidence linking LBW and hypertension later in life is well documented, although few studies have questioned the strength of the association (48). In a few studies, for example, low birth weight was not associated with increased blood pressure (49) but was associated with other markers of IUGR, such as the ratio between placental weight and either newborn weight (49) or birth length (50). Yet an extensive, systematic review of 444,000 participants concluded that birth weight is inversely related to systolic blood pressure; the size of the effect is approximately 2 mm Hg kg−1 (51). Furthermore, the Bogalusa Heart Study suggests that higher blood pressure of black adolescents compared with white adolescents reflects a lower birth weight (52). A meta-analysis of 197,954 adults from 20 Nordic cohorts (birth years 1910–1987) confirmed the inverse association between birth weight and systolic blood pressure, even after adjusting for current body mass index (BMI) (31).
The literature also provides convincing data on the link between LBW and future risk for impaired glucose tolerance. The first evidence of this link came from a cohort of 468 men born in Hertfordshire, England, between 1920 and 1930 (53). Men with impaired glucose tolerance or undiagnosed diabetes on the basis of a 75-g glucose challenge had a lower mean birth weight and a lower weight at 1 year of age (53). Likewise, individuals who were in utero during the Dutch famine of 1944–1945 had both a lower birth weight and lower glucose tolerance at the age of 50 years than did individuals born the year before or after the famine. Those who were exposed during mid- to late gestation and who had an elevated BMI had the highest 2-h glucose levels (28). Smaller case-control studies have also concluded that thinness at birth as measured by ponderal index [mass (kg)/height (m3)] and IUGR is associated with insulin resistance later in life (54, 55).
In terms of hypercholesterolemia, the evidence for the association of in utero stress and an abnormal lipid profile is mixed. In 219 middle-aged men and women, Barker et al. (56) showed that abdominal circumference at birth, but not birth weight, correlated inversely with serum levels of total cholesterol and apolipoprotein B. Studies from the Dutch Famine Birth Cohort showed that, although lipid profiles did not differ between exposed and unexposed individuals at age 58, those exposed in utero were more likely to be on lipid-lowering medications (21% versus 15%) (57). In a similar analysis of the Helsinki Birth Cohort, excluding those who used lipid-lowering medications, researchers found that a decrease of 1 kg m−2 in BMI at birth was associated with a slight increase in non-high-density-lipoprotein cholesterol and apolipoprotein B concentrations (58). A review of the literature involving 38 studies and 28,578 individuals did not find strong evidence for a link between birth weight and lipid profiles later in life (59).
Data on the link between birth weight and waist circumference are limited; however, several studies have examined obesity and body composition. The first study to observe the association between intrauterine malnutrition and later obesity was based on the Dutch Famine Birth Cohort of 300,000 19-year-old men (60). Participants who were exposed to in utero malnutrition in the third trimester of pregnancy had lower obesity rates, defined as weight for height ≥ 120% of standard, whereas those who were exposed in the first and/or second trimesters had significantly higher obesity rates. This study accounted for socioeconomic status, but not for other known confounders such as maternal BMI. In a much smaller case-control study of 32 white men 64–72 years of age, those with LBW (mean 2.76 kg) had a higher percentage body fat and fat mass by dual-energy X-ray absorptiometry than did those with a higher birth weight (mean 4.23 kg) (61). Other studies have concluded that maternal or parental BMI as a confounder largely explains the association between birth weight and later obesity (62). The important role of maternal BMI also supports the importance of the in utero environment on future health.
The association between in utero stress and MS has also been explored. In an analysis of a subset of 783 men and women aged 57–59 years from the Dutch Famine Birth Cohort, the prevalence of MS, defined with NCEP/ATP III criteria, was not significantly greater among those exposed to malnutrition (63). In British cohorts, by contrast, in utero stress was associated with a higher prevalence of MS in both men and women in the seventh decade of life. This finding was confirmed in multiple study populations, including postmenopausal Caucasian women living in the United States (64), young adults aged 26–31 years in the Netherlands (65), and 40-year-old Nigerians who survived the famine in Biafra (66).
More important than MS or its individual components is the risk for actual cardiovascular disease. The landmark paper that fueled the study of birth weight as a marker for future disease risk came from a cohort of 5,654 men born between 1911 and 1930 in Hertfordshire, England. This study showed that men with the lowest weights at birth and at 1 year of age had the highest death rates from ischemic heart disease (67). The association between LBW and cardiovascular disease has been confirmed in different racial and ethnic study populations (68–70). Not surprisingly, the highest risk for coronary heart disease associated with LBW appears to be restricted to individuals who have a high BMI in adulthood (71, 72).
Other studies have examined the association of a suboptimal in utero environment, represented by gestational diabetes or pregnancy-related hypertension disorders, and risk of MS. Boney et al. (73) demonstrated that large-for-gestational-age offspring born to diabetic mothers were at significant risk of developing childhood MS, as were offspring of obese mothers. Even in a low-risk population of women with gestational diabetes, 6.9% of offspring had abnormal glucose metabolism (74). In contrast, studies have shown that the association between having gestational diabetes and being overweight in adolescence disappears after adjustment for birth weight and maternal BMI (75) and that prenatal exposure to diet-controlled gestational diabetes does not increase the prevalence of childhood obesity (76). Interestingly, a meta-analysis found that women with polycystic ovary syndrome (PCOS), a common endocrinopathy characterized by anovulation, insulin resistance, and androgen excess, have a higher incidence of gestational diabetes, pregnancy-induced hypertension, and preeclampsia (77). However, infant birth weight was not different between women with PCOS and controls (77). There are currently no studies evaluating long-term health of the PCOS offspring.
Last, studies have demonstrated that pregnancy-associated hypertension disorders are associated with increased systolic and diastolic blood pressure among 9-year-old offspring (78) and with an increased risk of antihypertensive drug use in offspring independent of birth weight or preterm delivery (79).
5. PHYSIOLOGICAL AND MOLECULAR MECHANISMS LEADING TO ABNORMAL REPROGRAMMING
Embryos, fetuses, and newborns show remarkable plasticity in their response to the environment. During the prenatal period, the human fetus rapidly evolves from a single cell to 10 trillion cells differentiated into more than 250 subtypes. Therefore, that various forms of stress during this time can lead to long-term problems is biologically plausible. Evidence from epidemiological studies is compelling but is limited by potential confounding variables throughout an individual’s lifetime. Animal models allow evaluations of the effects of a specific controlled stress applied over a well-defined time. However, studies in animals are not immune to bias and errors (80–82).
Multiple animal models exist, including rodent and sheep models. Sheep physiology is closer to human physiology, but sheep have a long gestation (145 days) and usually carry one or two fetuses. The rodent model is the most frequently used due to low cost and short gestation. Thus, acknowledging the different physiological characteristics of rodents and humans is important (see sidebar on differences between rodents and humans in analyzing animal models of reprogramming) (83).
The use of animal models has allowed for important observations. Importantly, there is evidence of a transgenerational effect. Female rats that are food restricted during their perinatal life but exposed to a normal diet during pregnancy deliver offspring with reduced pancreatic β-cell mass and fewer cells expressing only insulin (84). A similar phenotype occurs in F2 offspring of diabetic pregnant rats (85). A low-protein diet leads to hypertension by decreasing the number of nephrons in F1 offspring (86); this phenotype is also present in the F2 generation (87). Epigenetic changes are likely responsible for these effects.
DIFFERENCES BETWEEN RODENTS AND HUMANS TO BE CONSIDERED WHEN ANALYZING ANIMAL MODELS OF REPROGRAMMING.
First, the large litter size (n = 8–12) and relatively short gestation (21 days) in rodents imply very different energetic needs than do humans during pregnancy. For example, pregnant rodents require up to 30-fold-higher protein content in their diet than humans and large mammals do; in addition, postnatal growth in rodents is faster than in humans and sheep (144).
Second, rodents have unique physiology. They are nocturnal animals (normally more active during the dark cycle); exhibit unique dietary habits such as coprophagia, which can alter nutrient flux; and have functional brown adipose tissue throughout life, which can result in very different energy balance compared with humans (145).
Third, the HPA axis and the appetite regulatory networks develop after birth in rodents and during the third trimester of pregnancy in humans. In rodents, the kidneys and brown adipose start to develop during gestation but continue to develop in the neonatal period, whereas in humans the process is already completed at birth (83, 130). The rat pancreas develops in late gestation and undergoes an important remodeling phase at the time of weaning (2–3 weeks); however, in humans, pancreatic islet remodeling continues until age 4 (146).
Certain in utero or postnatal interventions can reverse the effects of prenatal stress. For example, undernutrition in utero leads to obesity and hyperleptinemia in adult rats (88). These effects can be reversed by injecting leptin from day 3 to day 13 of postnatal life (89). In another study, the hypertensive effects of a low-protein diet were reversed by adding glycine to the diet (90).
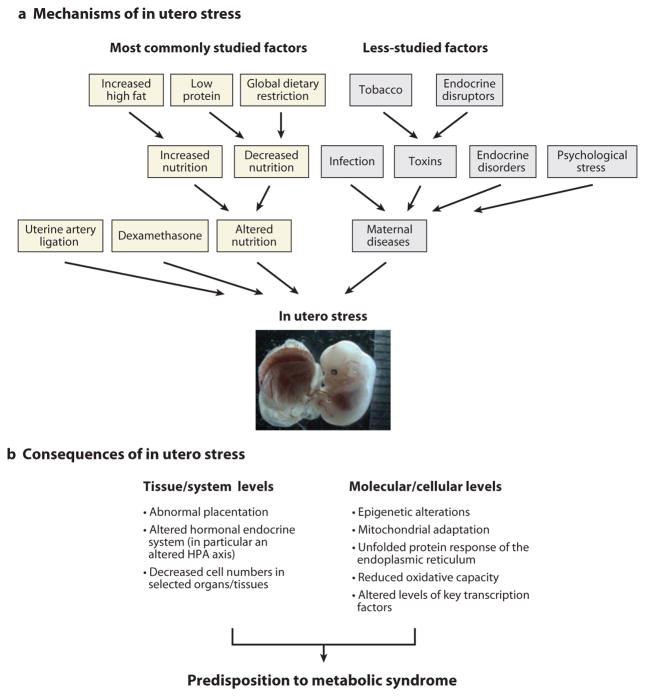
Different experimental interventions have been used to study stress during pregnancy (Figure 2) (3, 5, 91). These include (a) nutritional stress, with changes in macronutrients (global caloric restriction up to 50–70% of control, low-protein isocaloric diets, high-fat diets) or micronutrients (iron, zinc, sodium, calcium); (b) surgical stress (bilateral uterine ligation in rats or reduction of placental areas); (c) pharmacological treatments, either to alter the HPA axis with dexamethasone or steroid synthesis blockers or to induce diabetes with streptozotocin; and (d ) exposure to toxins (smoke, endocrine disruptors, medications during pregnancy).
Figure 2.
(a) Mechanisms of in utero stress. Very different types of in utero stress can affect a fetus’s health outcome. The most common experimental procedures used in animal models to study reprogramming are highlighted in yellow. The less-studied or more-difficult-to-study factors for reprogramming are in gray. (b) As a consequence of in utero stress, the organism reprograms its development at the cellular, tissue, and systemic levels. Overall, an organism stressed in utero will manifest a metabolism that favors lipid storage and increased cardiovascular reactivity, both of which are key components of metabolic syndrome. HPA axis denotes hypothalamic-pituitary-adrenal axis.
The physiological and molecular mechanisms leading to abnormal reprogramming are diverse and only partially known. Different stresses can promote reprogramming via unique and stress-specific pathways. For example, adult offspring of semistarved dams show insulin resistance in the liver but not in the peripheral tissues (92), whereas offspring of diabetic mothers exhibit both hepatic and peripheral insulin resistance (92). Importantly, multiple mechanisms can occur at the same time.
Overall, the mechanisms of stress can be broken down into several categories: (a) the cellular response to stress [e.g., epigenetic changes, mitochondrial dysfunction, the unfolded protein response (UPR), oxidative stress, the differential expression of transcription factors], (b) alterations in adult organ morphology or cell number [e.g., by adapting to a suboptimal environment, the organism trades off the development of less essential organs, such as the kidney (nephron mass) and the pancreas (β-cell mass), for the development of more essential organs such as the brain], (c) tissue or systemic responses (e.g., alterations in the placenta or in endocrine pathways, in particular the HPA axis), and (d ) a combination of the above. We discuss these mechanisms below.
5.1. Cellular Response to Stress
Changes in energy and nutrient availability modify stress signaling pathways at the cellular level. Several of these stress pathways are also altered in MS and diabetes. It is therefore tempting to postulate that these pathways can be permanently altered following suboptimal conditions in utero.
5.1.1. Epigenetic changes
Epigenetic changes are changes in gene function that occur without changes in gene sequence. Epigenetic regulation can occur at the DNA level through the mod-ification of cytosine bases (methylation of cytosine in CpG dinucleotides) or through chromatin conformational changes. Histone modifications can result in an open (active) or a closed (inactive) chromatin structure; DNA methylation of CpG results in the inability of transcription factors to bind to genes. The net result is either silencing or activation of transcription.
The preimplantation stage is particularly sensitive to epigenetic regulation. In fact, embryonic DNA methylation patterns proceed through defined phases during this stage of development. In the early murine embryo, a wave of DNA methylation is followed by remethylation at the morula or blastocyst stage. IVF and embryo culture can generate imprinting errors in mice by inducing abnormal DNA and histone methylation marks (93). The development rate and global patterns of gene expression are abnormal in mouse embryos cultured in vitro (94). Mouse blastocysts conceived in vitro have fewer trophectodermal cells than do in vivo controls. Mouse fetuses generated by IVF display delayed fetal development in comparison to controls. In particular, culture in suboptimal conditions resulted in a more severe phenotype than did culture in optimized conditions (95). Importantly, a recent paper found altered glucose parameters in adult mice conceived in vitro (96). Our group has found similar results (97). These animal studies would lend support to the metabolic findings of Ceelen et al. (23) in ART children.
Epigenetic changes follow different types of stress and different time exposure in different tissues. In the rat uterine artery ligation model, promoter methylation is increased, and expression of β-cell Pdx1 genes is decreased; these changes were associated with adult-onset diabetes (98). Uterine artery ligation is also associated with decreased methylation of the promoter of p53 genes in the kidney. The increase in p53 gene expression may lead to renal apoptosis, resulting in fewer nephrons (99). In an elegant experiment, Meaney group (100) showed changes in the promoter of the glucocorticoid receptor in offspring exposed to different postnatal care. Epigenetic changes can also explain the transgenerational effects described above (85, 87).
5.1.2. Mitochondrial changes
Mitochondria interface the environmental calorie supply and the energy requirements of each organ; they generate ATP and regulate cellular processes such as signal transduction and apoptosis. Alterations in mitochondria function may therefore be a key cellular mechanism of reprogramming. Although studies on IUGR offspring have not been reported, caloric restriction evidently increases skeletal muscle mitochondrial biogenesis (by up-regulating sirtuin 1, a protein upregulated in cases of increased insulin sensitivity) and decreases mitochondrial free-radical generation and oxidative damage to mitochondrial DNA in the rat heart (101). Mitochondrial density is reduced in insulin-resistant offspring of parents with type 2 diabetes (102). However, a definitive connection between mitochondrial dysfunction and insulin resistance is missing, and recent evidence argues against such a nexus (101).
5.1.3. Endoplasmic reticulum stress
The UPR is a cellular stress response that occurs in the lumen of the endoplasmic reticulum when there is excessive accumulation of proteins with altered tridimensional structure. Initially, the cell tries to repair the system by synthesizing molecular chaperones involved in protein folding and by halting additional protein translation. If repair does not occur, the cell initiates apoptosis. The UPR is thought to mediate inflammatory and stress signaling pathways and to be particularly important in regulating chronic metabolic diseases such as obesity, insulin resistance, and type 2 diabetes (103).
In sheep, in utero nutrient restriction and a postnatal high-fat diet cause organ-specific alteration of the UPR in different tissues. In particular, perirenal adipose tissue showed activation of the UPR and altered insulin signaling, whereas renal tissue showed reduced activation of the UPR and less histological damage (104).
5.1.4. Oxidative stress
Oxidative stress describes a condition of imbalance in the production of reactive oxygen species and the ability of antioxidant defenses to scavenge them. Oxidative stress may derive from the increased production of reactive oxygen species or from a decrease in antiox-idant capacity. Pregnancy is a state of oxidative stress because of increased metabolic activity in placental mitochondria and decreased antioxidant-scavenging ability (105). In pregnancies complicated by hypertension and diabetes, the placenta shows signs of increased hypoxia and oxidative stress and activation of the endoplasmic reticulum stress response (106). β-Cells of adult offspring whose mothers were exposed to a low-protein diet in utero show evidence of increased oxidative stress, increased lipid peroxidation, and impairment of oxidative defense (107). This study was the first to link maternal protein restriction to age-associated increased oxidative stress, impairment of oxidative defense, and fibrosis in offspring.
5.1.5. Changes in transcription factor levels
An increase in gluconeogenesis in IUGR rats can be explained by an increase in the level of peroxisome proliferator–activated receptor-γ coactivator-1 (PGC-1) or PGC-1α (108). This transcription factor regulates hepatic glucose production by increasing the mRNA levels of three key metabolic enzymes: glucose-6-phosphatase, phosphoenolpyruvate carboxykinase, and fructose-1,6-bisphosphatase (108). Hepatic levels of glu-cokinase, a key glycolytic enzyme, are also decreased in IUGR rats (109).
5.2. Organ-Specific Alterations in Response to Prenatal Stress
Prenatal stress affects different organs in different ways. However, a decrease in cell number is often present, as described in depth elsewhere (3). Interestingly, prenatal stress and the resulting reprogramming decrease insulin sensitivity (pancreas, liver, muscle, and fat tissue), increase cardiovascular reactivity, and increase HPA responses to stress in adult life. These effects lead to hyperlipidemia, obesity, glucose intolerance and hypertension—all components of MS.
5.2.1. Pancreas
Abnormal programming of the fetal pancreas may be a prime mechanism for adult-onset MS and diabetes. Prenatal stress during pregnancy can decrease both β-cell mass and β-cell function; such decreases may result in a decreased insulin response to glucose (110). Different types of stress have recognizably different effects. For example, global caloric restrictions (50% of control diet) in rats at embryonic day 15 reduce the number and size of islets; however, the existing β cells have a normal proliferative capacity (111). Conversely, an isocaloric low-protein diet results in smaller islets and a relatively smaller proportion of β cells, owing to decreased β-cell proliferation and increased apoptosis (112). Different levels of corticosterone in the two models of fetal stress may explain these differences: A low-protein diet is associated with normal corticos-terone levels, whereas global food restriction is associated with reduced corticosterone levels (113, 114). Additional mechanisms responsible for the reduced cell number include a decrease in the number of pancreatic stem cells (115) and the inappropriate expression of transcription or growth factors (e.g., insulin-like growth factor 2) (116).
5.2.2. Liver
Prenatal stress promotes increased hepatic gluconeogenesis and hepatic insulin resistance (117), which contribute to hyperglycemia. Evidence of hepatic reprogramming is important, as unsuppressed endogenous hepatic glucose production is a common component of the insulin resistance associated with type 2 diabetes. In addition, in male IUGR mice, triglyceride levels are elevated because of increased hepatic fatty acid synthesis and decreased β oxidation (33). Prenatal stress does not reduce the ratio of liver weight to total fetal weight (118) unless the prenatal stress is severe (119). However, livers of protein-restricted rats have fewer but larger lobules (118). From a mechanistic point of view, intrauterine stress may acutely decrease liver mitochondrial ATP production and NADH availability. Because ATP and NADH are two key regulators of intermediary metabolism, such changes can reduce fetal growth (120).
5.2.3. Skeletal muscle
Insulin-sensitive skeletal muscles are the major site of glucose uptake. Under euglycemic conditions, approximately 80% of total body glucose uptake occurs in skeletal muscles (121). Different experimental models in different species show that prenatal stress can alter muscle size and lead to insulin resistance (3).
Protein-restricted rat offspring show decreased muscle mass of both fast-twitch-type fibers (anterior tibialis) and slow-twitch-type fibers (soleus muscle) at postnatal day 21 (122). In contrast, sheep fetuses at embryonic day 125 had 40% higher weight of the fast-twitch plantaris muscle if they were cultured in vitro with granulosa cells from the zygote to the blastocyst. In particular, muscle of fetuses that were cultured in vitro as embryos showed both hyperplastic and hypertrophic changes, suggesting that myogenesis can be altered by preimplantation stress (123).
Muscles of IUGR rats are insulin resistant and show significantly decreased glycogen content and insulin-stimulated 2-deoxyglucose uptake (124). This insulin resistance may reflect signifi-cantly decreased pyruvate oxidation and ATP production in muscle mitochondria (124), which may decrease recruitment of the insulin-regulated glucose transporter GLUT4 to the cell surface. Muscles of IUGR rats also show increased expression of PGC-1 and its downstream gluconeoge-nesis pathway (125).
5.2.4. Adipose tissues
Worldwide, the increase in MS parallels the increase in obesity (126). Although adipose tissue contributes only approximately 3% to glucose disposal after an oral glucose tolerance test (121), it plays an important role in intermediate metabolism by releasing free fatty acids, leptin, and inflammatory cytokines (126).
Free fatty acids cause both insulin resistance and the release of proinflammatory cytokines in insulin-sensitive tissues such as skeletal muscle, liver, and endothelium. In particular, an increase in free fatty acids inhibits insulin-stimulated glucose uptake into muscle (126, 127).
Maternal undernutrition can increase retroperitoneal fat (88) and increase the proportion of large fat cells in visceral fat (128). In protein-restricted rats, insulin-stimulated glucose uptake and insulin-dependent lipolysis are reduced in adipocytes, indicating insulin resistance. These changes appear to be caused by a post–insulin receptor effect, as these rats have lower levels of phosphatidylinositol 3-kinase and protein kinase B but similar levels of insulin receptor and insulin receptor tyrosine phosphorylation compared with controls (129).
An important observation links maternal undernutrition to obesity and hyperleptinemia in adult offspring (88). Leptin is a trophic factor that is central in organizing the formation of hypothalamic circuitry. Perturbations in perinatal nutrition that alter leptin levels may therefore have lasting consequences for the formation and function of circuits regulating food intake and body weight (130).
5.2.5. Kidney
Different intrauterine stresses can lead to hypertension in later life by different mechanisms. In a classic experiment, Langley-Evans et al. (18) showed that, although a low-protein diet before conception was not associated with hypertension, blood pressure was increased in rats exposed to low protein during gestation or even for limited periods of pregnancy (days 0–7, 8–14, and 15–22). The increase elicited by these discrete periods of undernutrition was lower than that induced by feeding a low-protein diet throughout pregnancy. The effect in early gestation was significant only in males (18).
A low-protein diet or uterine artery ligation leads to hypertension by decreasing the abundance of nephrons (86). Interestingly, global caloric reduction in pregnancy has a less consistent effect on blood pressure. Brenner & Chertow (131) were the first to propose that hypertension could trail a congenital deficit of nephrons. These researchers proposed that a reduction in renal mass, and therefore in glomerular filtration surface area, leads to hypertension (131). Sustained exposure of nephrons to higher glomerular perfusion pressure gradually results in focal and segmental glomerular sclerosis, which leads to further glomerular loss, further reduced ability to excrete sodium, and a self-perpetuating cycle of increasing blood pressure and progressive kidney disease. Protein restriction also increases the expression of two ascending-limb sodium cotransporters, thereby facilitating sodium retention and hypertension (132) and suppressing the renin-angiotensin system (133).
5.2.6. Endothelium
Evidence from studies in humans and animals suggests that stress in utero can alter endothelium-dependent vasodilatation. Such a change may occur because of decreased levels of nitric oxide production (134, 135).
5.2.7. Heart
Stress in utero that leads to fetal hypoxia can alter myocardial structure (by inhibiting cardiomyocyte proliferation and increasing apoptosis) and reduce cardiac performance (136). In addition, hypoxia predisposes the developing heart to increased vulnerability to ischemia and reperfusion injury later in life (136), likely because of decreased expression of cardioprotec-tive genes like those encoding protein kinase Cε, heat shock protein-70, endothelial nitric oxide synthase (136), and insulin-like growth factor 1 (137).
5.3. Tissue and Systemic Response to Stress
The placenta, HPA axis, and appetite regulatory networks share the characteristics of regulating the development and physiological response of multiple individual organs. It is therefore particularly important to study how these systems are altered by prenatal stress.
5.3.1. Abnormal placentation
By virtue of its transport, immune, and hormonal roles, the placenta is in a key position to play a direct role in fetal programming by changing the pattern or amount of substrate transported to the fetus. Aberration in placental function is probably the most frequent cause of IUGR because optimal placental development and the ability of the placenta to compensate for stimulus-induced injury are central to the promotion of normal fetal growth.
Abnormal placentation often results from reduced trophoblast invasion, which leads to hypoxia and increased oxidative stress. In humans, IUGR placentas are not simply smaller versions of a term placenta; they display alterations in vascularization, trophoblast expression of transporters, trophoblast enzyme activity, and hormone production. Furthermore, alteration of placenta-imprinted genes may play an important role. Imprinted genes are involved in growth regulation, controlling both the supply (placental side) and the demand (fetal side) of nutrients. Paternally derived imprinted genes enhance fetal growth, whereas maternally imprinted genes suppress fetal growth. Several imprinted genes encode specific transporters in trophoblasts. The placenta may function as a nutrient sensor, matching fetal growth rate to available nutrient resources by altering transport function. Such a function would explain fetal growth restriction and possibly growth enhancement in pregnancies with gestational diabetes (138).
The placenta is vital in moderating fetal exposure to maternal factors. Glucocorticoid levels are approximately 20% lower in the fetus than in the mother because the placenta expresses high levels of 11β hydroxysteroid dehydrogenase type 2 (11β-HSD-2), which converts the active cortisol/corticosterone to the inactive 11 keto steroids. A deficiency in 11β-HSD-2 leads to overexposure of the fetus to glucocorticoids. Indeed, low levels of placental 11β-HSD-2 activity have been found in LBW in humans and rodent models, though this has not always been reported (139). Placentas of pregnancies associated with preeclampsia or unexplained IUGR have an increase of the UPR (106).
5.3.2. Abnormal activation of the HPA axis
The HPA axis is fundamental in regulating the response of the individual to stress. Increased exposure to cortisol in utero (due to stress, pharmacological treatment, or impaired function of 11β-HSD-2) (140) has long-term effects. In rats, for example, prenatal glucocorticoid exposure leads to LBW, alters cardiac development, reduces nephron number, and alters glucose-insulin homeostasis by increasing the expression of hepatic gluconeogenesis enzymes and impairing β-cell function (139). The physiological effects of a low-protein diet in utero may be mediated in part by an increase in glucocorticoid levels (113).
Epigenetic changes are involved in altering the activation of the HPA axis. Rat pups exposed to increased maternal care during the first postnatal week exhibit a persistent lack of DNA methylation of a specific CpG base in the first exon of the glucocorticoid receptor (100). This epigenetic change decreases hypothalamic corticotrophin-releasing factor expression and results in adult animals manifesting a modest HPA response to stress.
5.3.3. Appetite regulatory network
One important potential locus of reprogramming is the hypothalamic appetite regulatory network (3), in which leptin plays an important role (130). Early postnatal life plays an important role in determining future food intake. Rats from small litters tend to be heavier than those of larger litters because of increased food intake by the former (141). The experimental evidence targeted to the pregnancy period is scarce because the majority of studies limit postnatal intervention or combine a postnatal dietary manipulation with a prenatal one. Undernutrition during gestation alone does not affect the weight, length, or fat content of offspring in adulthood (142). However, adult male, but not female, offspring of rats injected with insulin during the last week of gestation develop significant obesity as adults, accompanied by elevated extracellular norepinephrine levels in the paraventricular nucleus (143).
6. CONCLUSIONS
Evidence linking severe stress in utero to fetal reprogramming is convincing, and uterine stress predisposes individuals to components of MS later in life. Undeniably, individual behavior such as diet and exercise also affects one’s predisposition to adult MS; however, this review provides compelling evidence that the short duration of in utero exposure to stress plays a crucial role of relatively great magnitude.
Although animal data provide multiple biologically plausible mechanisms for fetal reprogramming, many questions remain unanswered. For example, the mechanisms leading to adult disease need to be further studied so that novel strategies can be developed to counteract the effects of in utero stress. In addition, the use of LBW as a marker for in utero stress is of inadequate value, and new strategies to discover biomarkers of in utero stress would be of great importance.
These findings have particular relevance for the populations of developing countries, where the combination of poor nutrition in utero and overnutrition in later life may drive the observed epidemics of obesity and diabetes. In addition, given that preimplantation embryo stress can activate many reprogramming mechanisms, children conceived by ART should be increasingly monitored over time to evaluate for potential occurrence of MS. Physicians should routinely incorporate information of pregnancy course and health into the individual medical history.
SUMMARY POINTS.
There is a broadly continuous positive relationship between in utero stress and predisposition to MS or components of MS.
The use of a single marker of stress in utero (e.g., LBW) is inadequate and is potentially prone to error.
Different critical periods of sensitivity vary in different species and in males and females; overall, the prenatal and early postnatal periods represent critical periods during which reprogramming can occur. Stress occurring during these phases has a disproportionally greater effect in term of later life predisposition to diseases compared with the same stress occurring outside of a window of sensitivity.
Different types of stresses applied during the same period have different long-term effects. For example, adult rat offspring of semistarved dams show insulin resistance in the liver but not in the peripheral tissues (92). In contrast, offspring of diabetic mothers exhibit insulin resistance at both the hepatic and peripheral tissue levels (92).
Different cellular pathways are activated following in utero stress. Such activation leads to alterations in specific organs or systems.
Epigenetic changes occur following in utero stress and can explain transgenerational effects.
Health problems are amplified if there is a mismatch between the in utero and the postnatal environment; the most common scenario is the fetus exposed to reduced energy in utero followed by a postnatal life rich in food resources. This concept (14) has particular relevance in developing countries.
Patient medical history should specify if ART was used to conceive offspring and should include detailed information on the health of maternal pregnancy.
FUTURE ISSUES.
The preimplantation period is particularly sensitive to stress. ART constitutes a potential new form of preimplantation stress. Given that more than 3.5 million children have been conceived by ART, additional studies are needed to follow up children conceived by ART.
There is a need to discover novel biomarkers of in utero stress and to develop high-throughput technology to discern the cellular mechanisms that are altered after in utero stress.
Strategies to counteract the effect of in utero stress are needed. For example, the potential beneficial effect of specific nutrients (90) or hormones (89) on health should be studied. Such research could be particularly important for populations in developing countries.
Acknowledgments
This work was made possible from funding from the National Institute of Child Health and Human Development (R01 HD062803-02) and the American Diabetes Association to P.R. The authors thank Drs. Annemarie Donjacour, Marcelle Cedars, and Robert Lustig for their valuable suggestions and comments.
Glossary
- MS
metabolic syndrome
- ART
assisted reproductive technology
- NCEP/ATP III
National Cholesterol Education Program/ Adult Treatment Panel III
- IDF
International Diabetes Federation
- IUGR
intrauterine growth restriction
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–81. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, et al. Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci USA. 2007;104:12796–800. doi: 10.1073/pnas.0705667104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 4.Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol. 2009;5:604–10. doi: 10.1038/nrendo.2009.195. [DOI] [PubMed] [Google Scholar]
- 5.Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull. 2001;60:103–21. doi: 10.1093/bmb/60.1.103. [DOI] [PubMed] [Google Scholar]
- 6.Kermack WO, McKendrick AG, McKinlay PL. Death-rates in Great Britain and Sweden: expression of specific mortality rates as products of two factors, and some consequences thereof. J Hyg. 1934;34:433–57. doi: 10.1017/s0022172400043230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadsworth ME, Cripps HA, Midwinter RE, Colley JR. Blood pressure in a national birth cohort at the age of 36 related to social and familial factors, smoking, and body mass. Br Med J. 1985;291:1534–38. doi: 10.1136/bmj.291.6508.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCance RA, Widdowson EM. The determinants of growth and form. Proc R Soc Lond Ser B. 1974;185:1–17. doi: 10.1098/rspb.1974.0001. [DOI] [PubMed] [Google Scholar]
- 9.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 10.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 11.Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, et al. Developmental plasticity and human health. Nature. 2004;430:419–21. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan M, Patel MS. Metabolic programming in the immediate postnatal period. Trends En-docrinol Metab. 2008;19:146–52. doi: 10.1016/j.tem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen KM. The “fetal origins” hypothesis: challenges and opportunities for maternal and child nutrition. Annu Rev Nutr. 2001;21:73–95. doi: 10.1146/annurev.nutr.21.1.73. [DOI] [PubMed] [Google Scholar]
- 14.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 15.Kwong W, Wild A, Roberts P, Willis A, Fleming T. Maternal undernutrition during the preim-plantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 16.Edwards L, McMillen I. Periconceptual nutrition programs development of the cardiovascular system in the fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2002;283:669–79. doi: 10.1152/ajpregu.00736.2001. [DOI] [PubMed] [Google Scholar]
- 17.Gardner D, Pearce S, Dandrea J, Walker R, Ramsay M, et al. Peri-implantation undernutrition programs blunted angiotensin II evoked baroreflex responses in young adult sheep. Hypertension. 2004;43:1290–96. doi: 10.1161/01.HYP.0000126991.67203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci. 1996;91:607–15. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Gonzalez R, Moreira P, Bilbao A, Jimenez A, Perez-Crespo M, et al. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci USA. 2004;101:5880–85. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–37. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 21.Ceelen M, van Weissenbruch MM, Roos JC, Vermeiden JP, van Leeuwen FE, Delemarre–van de Waal HA. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab. 2007;92:3417–23. doi: 10.1210/jc.2006-2896. [DOI] [PubMed] [Google Scholar]
- 22.Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre–van de Waal HA. Pubertal development in children and adolescents born after IVF and spontaneous conception. Hum Reprod. 2008;23:2791–98. doi: 10.1093/humrep/den309. [DOI] [PubMed] [Google Scholar]
- 23.Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre–van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008;93:1682–88. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- 24.Law CM, de Swiet M, Osmond C, Fayers PM, Barker DJ, et al. Initiation of hypertension in utero and its amplification throughout life. Br Med J. 1993;306:24–27. doi: 10.1136/bmj.306.6869.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miles HL, Hofman PL, Peek J, Harris M, Wilson D, et al. In vitro fertilization improves childhood growth and metabolism. J Clin Endocrinol Metab. 2007;92:3441–45. doi: 10.1210/jc.2006-2465. [DOI] [PubMed] [Google Scholar]
- 26.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82:485–91. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 27.de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci USA. 2010;107:16881–86. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–77. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 29.Symonds ME. Integration of physiological and molecular mechanisms of the developmental origins of adult disease: new concepts and insights. Proc Nutr Soc. 2007;66:442–50. doi: 10.1017/S002966510700571X. [DOI] [PubMed] [Google Scholar]
- 30.Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. Br Med J. 1993;307:1519–24. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamborg M, Byberg L, Rasmussen F, Andersen PK, Baker JL, et al. Birth weight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific results from 20 Nordic studies. Am J Epidemiol. 2007;166:634–45. doi: 10.1093/aje/kwm042. [DOI] [PubMed] [Google Scholar]
- 32.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, et al. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–75. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- 33.Lane RH, Kelley DE, Gruetzmacher EM, Devaskar SU. Uteroplacental insufficiency alters hepatic fatty acid-metabolizing enzymes in juvenile and adult rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:183–90. doi: 10.1152/ajpregu.2001.280.1.R183. [DOI] [PubMed] [Google Scholar]
- 34.Tarry-Adkins JL, Joles JA, Chen JH, Martin-Gronert MS, van der Giezen DM, et al. Protein restriction in lactation confers nephroprotective effects in the male rat and is associated with increased antioxidant expression. Am J Physiol Regul Integr Comp Physiol. 2007;293:1259–66. doi: 10.1152/ajpregu.00231.2007. [DOI] [PubMed] [Google Scholar]
- 35.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome. Diabetes Care. 2005;28:1769–78. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 36.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the US. Diabetes Care. 2005;28:2745–49. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 37.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 38.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 39.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.Reaven GM. The metabolic syndrome: Is this diagnosis necessary? Am J Clin Nutr. 2006;83:1237–47. doi: 10.1093/ajcn/83.6.1237. [DOI] [PubMed] [Google Scholar]
- 41.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 42.Reinehr T, de Sousa G, Toschke AM, Andler W. Comparison of metabolic syndrome prevalence using eight different definitions: a critical approach. Arch Dis Child. 2007;92:1067–72. doi: 10.1136/adc.2006.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–97. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 44.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 45.Bayturan O, Tuzcu EM, Lavoie A, Hu T, Wolski K, et al. The metabolic syndrome, its component risk factors, and progression of coronary atherosclerosis. Arch Intern Med. 2010;170:478–84. doi: 10.1001/archinternmed.2009.551. [DOI] [PubMed] [Google Scholar]
- 46.Scuteri A, Najjar SS, Morrell CH, Lakatta EG. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care. 2005;28:882–87. doi: 10.2337/diacare.28.4.882. [DOI] [PubMed] [Google Scholar]
- 47.Sundstrom J, Vallhagen E, Riserus U, Byberg L, Zethelius B, et al. Risk associated with the metabolic syndrome versus the sum of its individual components. Diabetes Care. 2006;29:1673–74. doi: 10.2337/dc06-0664. [DOI] [PubMed] [Google Scholar]
- 48.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: Is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360:659–65. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 49.Hemachandra AH, Klebanoff MA, Duggan AK, Hardy JB, Furth SL. The association between intrauterine growth restriction in the full-term infant and high blood pressure at age 7 years: results from the Collaborative Perinatal Project. Int J Epidemiol. 2006;35:871–77. doi: 10.1093/ije/dyl080. [DOI] [PubMed] [Google Scholar]
- 50.Menezes AM, Hallal PC, Horta BL, Araújo CL, de Fátima Vieira F, et al. Size at birth and blood pressure in early adolescence: a prospective birth cohort study. Am J Epidemiol. 2007;165:611–16. doi: 10.1093/aje/kwk031. [DOI] [PubMed] [Google Scholar]
- 51.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–31. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 52.Cruickshank JK, Mzayek F, Liu L, Kieltyka L, Sherwin R, et al. Origins of the “black/white” difference in blood pressure: roles of birth weight, postnatal growth, early blood pressure, and adolescent body size: the Bogalusa heart study. Circulation. 2005;111:1932–37. doi: 10.1161/01.CIR.0000161960.78745.33. [DOI] [PubMed] [Google Scholar]
- 53.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. Br Med J. 1991;303:1019–22. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips DI, Barker DJ, Hales CN, Hirst S, Osmond C. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994;37:150–54. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- 55.Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab. 2000;85:1401–6. doi: 10.1210/jcem.85.4.6544. [DOI] [PubMed] [Google Scholar]
- 56.Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. Br Med J. 1993;307:1524–27. doi: 10.1136/bmj.307.6918.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lussana F, Painter RC, Ocke MC, Buller HR, Bossuyt PM, Roseboom TJ. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am J Clin Nutr. 2008;88:1648–52. doi: 10.3945/ajcn.2008.26140. [DOI] [PubMed] [Google Scholar]
- 58.Kajantie E, Barker DJ, Osmond C, Forsen T, Eriksson JG. Growth before 2 years of age and serum lipids 60 years later: the Helsinki Birth Cohort study. Int J Epidemiol. 2008;37:280–89. doi: 10.1093/ije/dyn012. [DOI] [PubMed] [Google Scholar]
- 59.Lauren L, Jarvelin MR, Elliott P, Sovio U, Spellman A, et al. Relationship between birthweight and blood lipid concentrations in later life: evidence from the existing literature. Int J Epidemiol. 2003;32:862–76. doi: 10.1093/ije/dyg201. [DOI] [PubMed] [Google Scholar]
- 60.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–53. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 61.Kensara OA, Wootton SA, Phillips DI, Patel M, Jackson AA, Elia M. Fetal programming of body composition: relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am J Clin Nutr. 2005;82:980–87. doi: 10.1093/ajcn/82.5.980. [DOI] [PubMed] [Google Scholar]
- 62.Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. Br Med J. 2001;323:1331–35. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Rooij SR, Painter RC, Holleman F, Bossuyt PM, Roseboom TJ. The metabolic syndrome in adults prenatally exposed to the Dutch famine. Am J Clin Nutr. 2007;86:1219–24. doi: 10.1093/ajcn/86.4.1219. [DOI] [PubMed] [Google Scholar]
- 64.Yarbrough DE, Barrett-Connor E, Kritz-Silverstein D, Wingard DL. Birth weight, adult weight, and girth as predictors of the metabolic syndrome in postmenopausal women: the Rancho Bernardo Study. Diabetes Care. 1998;21:1652–58. doi: 10.2337/diacare.21.10.1652. [DOI] [PubMed] [Google Scholar]
- 65.Ramadhani MK, Grobbee DE, Bots ML, Castro Cabezas M, Vos LE, et al. Lower birth weight predicts metabolic syndrome in young adults: the Atherosclerosis Risk in Young Adults (ARYA)-study. Atherosclerosis. 2006;184:21–27. doi: 10.1016/j.atherosclerosis.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 66.Hult M, Tornhammar P, Ueda P, Chima C, Edstedt Bonamy A-K, et al. Hypertension, diabetes and overweight: looming legacies of the Biafram famine. PLoS ONE. 2010;5:e13582. doi: 10.1371/journal.pone.0013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 68.Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, et al. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. Br Med J. 1997;315:396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stein CE, Fall CH, Kumaran K, Osmond C, Cox V, Barker DJ. Fetal growth and coronary heart disease in south India. Lancet. 1996;348:1269–73. doi: 10.1016/s0140-6736(96)04547-3. [DOI] [PubMed] [Google Scholar]
- 70.Lawlor DA, Ronalds G, Clark H, Smith GD, Leon DA. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: findings from the Aberdeen Children of the 1950s prospective cohort study. Circulation. 2005;112:1414–18. doi: 10.1161/CIRCULATIONAHA.104.528356. [DOI] [PubMed] [Google Scholar]
- 71.Rich-Edwards JW, Kleinman K, Michels KB, Stampfer MJ, Manson JE, et al. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. Br Med J. 2005;330:1115. doi: 10.1136/bmj.38434.629630.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478–80. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 73.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–96. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 74.Malcolm JC, Lawson ML, Gaboury I, Lough G, Keely E. Glucose tolerance of offspring of mother with gestational diabetes mellitus in a low-risk population. Diabet Med. 2006;23:565–70. doi: 10.1111/j.1464-5491.2006.01840.x. [DOI] [PubMed] [Google Scholar]
- 75.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–26. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 76.Whitaker RC, Pepe MS, Seidel KD, Wright JA, Knopp RH. Gestational diabetes and the risk of offspring obesity. Pediatrics. 1998;101:E9. doi: 10.1542/peds.101.2.e9. [DOI] [PubMed] [Google Scholar]
- 77.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–83. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 78.Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122:1192–99. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palmsten K, Buka SL, Michels KB. Maternal pregnancy-related hypertension and risk for hypertension in offspring later in life. Obstet Gynecol. 2010;116:858–64. doi: 10.1097/AOG.0b013e3181f3a1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neitzke U, Harder T, Schellong K, Melchior K, Ziska T, et al. Intrauterine growth restriction in a rodent model and developmental programming of the metabolic syndrome: a critical appraisal of the experimental evidence. Placenta. 2008;29:246–54. doi: 10.1016/j.placenta.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 81.Walters E, Edwards RG. On a fallacious invocation of the Barker hypothesis of anomalies in newborn rats due to mothers’ food restriction in preimplantation phases. Reprod Biomed Online. 2003;7:580–82. doi: 10.1016/s1472-6483(10)62075-5. [DOI] [PubMed] [Google Scholar]
- 82.Jansson T, Lambert GW. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3–4 months of age. J Hypertens. 1999;17:1239–48. doi: 10.1097/00004872-199917090-00002. [DOI] [PubMed] [Google Scholar]
- 83.Symonds ME, Sebert SP, Budge H. The impact of diet during early life and its contribution to later disease: critical checkpoints in development and their long-term consequences for metabolic health. Proc Nutr Soc. 2009;68:416–21. doi: 10.1017/S0029665109990152. [DOI] [PubMed] [Google Scholar]
- 84.Blondeau B, Avril I, Duchene B, Breant B. Endocrine pancreas development is altered in foetuses from rats previously showing intra-uterine growth retardation in response to malnutrition. Diabetologia. 2002;45:394–401. doi: 10.1007/s00125-001-0767-4. [DOI] [PubMed] [Google Scholar]
- 85.Holemans K, Aerts L, Van Assche FA. Evidence for an insulin resistance in the adult offspring of pregnant streptozotocin-diabetic rats. Diabetologia. 1991;34:81–85. doi: 10.1007/BF00500377. [DOI] [PubMed] [Google Scholar]
- 86.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–74. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 87.Harrison M, Langley-Evans SC. Intergenerational programming of impaired nephrogenesis and hypertension in rats following maternal protein restriction during pregnancy. Br J Nutr. 2009;101:1020–30. doi: 10.1017/S0007114508057607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:83–87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 89.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–16. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 90.Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci. 2002;103:633–39. doi: 10.1042/cs1030633. [DOI] [PubMed] [Google Scholar]
- 91.Langley-Evans SC. Nutritional programming of disease: unravelling the mechanism. J Anat. 2009;215:36–51. doi: 10.1111/j.1469-7580.2008.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holemans K, Van Bree R, Verhaeghe J, Meurrens K, Van Assche FA. Maternal semistarvation and streptozotocin-diabetes in rats have different effects on the in vivo glucose uptake by peripheral tissues in their female adult offspring. J Nutr. 1997;127:1371–76. doi: 10.1093/jn/127.7.1371. [DOI] [PubMed] [Google Scholar]
- 93.Li T, Vu TH, Ulaner GA, Littman E, Ling JQ, et al. IVF results in de novo DNA methylation and histone methylation at an Igf2-H19 imprinting epigenetic switch. Mol Hum Reprod. 2005;11:631–40. doi: 10.1093/molehr/gah230. [DOI] [PubMed] [Google Scholar]
- 94.Giritharan G, Talbi S, Donjacour A, Di Sebastiano F, Dobson A, Rinaudo P. Effect of in vitro fertilization on gene expression and development of mouse preimplantation embryos. Reproduction. 2007;134:63–72. doi: 10.1530/REP-06-0247. [DOI] [PubMed] [Google Scholar]
- 95.Delle Piane L, Lin W, Liu X, Donjacour A, Minasi P, et al. Effect of the method of conception and embryo transfer procedure on mid-gestation placenta and fetal development in an IVF mouse model. Hum Reprod. 2010;25:2039–46. doi: 10.1093/humrep/deq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scott KA, Yamazaki Y, Yamamoto M, Lin Y, Melhorn SJ, et al. Glucose parameters are altered in mouse offspring produced by assisted reproductive technologies and somatic cell nuclear transfer. Biol Reprod. 2010;83:220–27. doi: 10.1095/biolreprod.109.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rinaudo PF, Giritharan G, Delle Piane L, Donjacour A. Mice conceived by in vitro fertilization (IVF) display reduced growth curve and glucose intolerance. Presented at Soc. Gynecol. Investig; Glasgow, Scotland. 2009. [Google Scholar]
- 98.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Investig. 2008;118:2316–24. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2003;285:962–70. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- 100.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 101.Schiff M, Benit P, Coulibaly A, Loublier S, El-Khoury R, Rustin P. Mitochondrial response to controlled nutrition in health and disease. Nutr Rev. 2011;69:65–75. doi: 10.1111/j.1753-4887.2010.00363.x. [DOI] [PubMed] [Google Scholar]
- 102.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Investig. 2005;115:3587–93. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharkey D, Gardner DS, Fainberg HP, Sebert S, Bos P, et al. Maternal nutrient restriction during pregnancy differentially alters the unfolded protein response in adipose and renal tissue of obese juvenile offspring. FASEB J. 2009;23:1314–24. doi: 10.1096/fj.08-114330. [DOI] [PubMed] [Google Scholar]
- 105.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):43–48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tarry-Adkins JL, Chen JH, Smith NS, Jones RH, Cherif H, Ozanne SE. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 2009;23:1521–28. doi: 10.1096/fj.08-122796. [DOI] [PubMed] [Google Scholar]
- 108.Lane RH, MacLennan NK, Hsu JL, Janke SM, Pham TD. Increased hepatic peroxisome proliferator-activated receptor-γ coactivator-1 gene expression in a rat model of intrauterine growth retardation and subsequent insulin resistance. Endocrinology. 2002;143:2486–90. doi: 10.1210/endo.143.7.8898. [DOI] [PubMed] [Google Scholar]
- 109.Desai M, Byrne CD, Zhang J, Petry CJ, Lucas A, Hales CN. Programming of hepatic insulin-sensitive enzymes in offspring of rat dams fed a protein-restricted diet. Am J Physiol Gastrointest Liver Physiol. 1997;272:1083–90. doi: 10.1152/ajpgi.1997.272.5.G1083. [DOI] [PubMed] [Google Scholar]
- 110.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–86. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 111.Garofano A, Czernichow P, Breant B. In utero undernutrition impairs rat β-cell development. Diabetologia. 1997;40:1231–34. doi: 10.1007/s001250050812. [DOI] [PubMed] [Google Scholar]
- 112.Petrik J, Reusens B, Arany E, Remacle C, Coelho C, et al. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology. 1999;140:4861–73. doi: 10.1210/endo.140.10.7042. [DOI] [PubMed] [Google Scholar]
- 113.Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr. 1996;126:1578–85. doi: 10.1093/jn/126.6.1578. [DOI] [PubMed] [Google Scholar]
- 114.Lesage J, Blondeau B, Grino M, Breant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142:1692–702. doi: 10.1210/endo.142.5.8139. [DOI] [PubMed] [Google Scholar]
- 115.Joanette EA, Reusens B, Arany E, Thyssen S, Remacle RC, Hill DJ. Low-protein diet during early life causes a reduction in the frequency of cells immunopositive for nestin and CD34 in both pancreatic ducts and islets in the rat. Endocrinology. 2004;145:3004–13. doi: 10.1210/en.2003-0796. [DOI] [PubMed] [Google Scholar]
- 116.Hill DJ, Duvillie B. Pancreatic development and adult diabetes. Pediatr Res. 2000;48:269–74. doi: 10.1203/00006450-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 117.Holemans K, Verhaeghe J, Dequeker J, Van Assche FA. Insulin sensitivity in adult female rats subjected to malnutrition during the perinatal period. J Soc Gynecol Investig. 1996;3:71–77. doi: 10.1016/1071-5576(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 118.Burns SP, Desai M, Cohen RD, Hales CN, Iles RA, et al. Gluconeogenesis, glucose handling, and structural changes in livers of the adult offspring of rats partially deprived of protein during pregnancy and lactation. J Clin Investig. 1997;100:1768–74. doi: 10.1172/JCI119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McMillen IC, Adams MB, Ross JT, Coulter CL, Simonetta G, et al. Fetal growth restriction: adaptations and consequences. Reproduction. 2001;122:195–204. doi: 10.1530/rep.0.1220195. [DOI] [PubMed] [Google Scholar]
- 120.Ogata ES, Swanson SL, Collins JW, Jr, Finley SL. Intrauterine growth retardation: altered hepatic energy and redox states in the fetal rat. Pediatr Res. 1990;27:56–63. doi: 10.1203/00006450-199001000-00017. [DOI] [PubMed] [Google Scholar]
- 121.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin N Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 122.Desai M, Crowther NJ, Lucas A, Hales CN. Organ-selective growth in the offspring of protein-restricted mothers. Br J Nutr. 1996;76:591–603. doi: 10.1079/bjn19960065. [DOI] [PubMed] [Google Scholar]
- 123.Maxfield EK, Sinclair KD, Broadbent PJ, McEvoy TG, Robinson JJ, Maltin CA. Short-term culture of ovine embryos modifies fetal myogenesis. Am J Physiol Endocrinol Metab. 1998;274:1121–23. doi: 10.1152/ajpendo.1998.274.6.E1121. [DOI] [PubMed] [Google Scholar]
- 124.Selak MA, Storey BT, Peterside I, Simmons RA. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285:130–37. doi: 10.1152/ajpendo.00322.2002. [DOI] [PubMed] [Google Scholar]
- 125.Lane RH, Maclennan NK, Daood MJ, Hsu JL, Janke SM, et al. IUGR alters postnatal rat skeletal muscle peroxisome proliferator-activated receptor-γ coactivator-1 gene expression in a fiber specific manner. Pediatr Res. 2003;53:994–1000. doi: 10.1203/01.PDR.0000064583.40495.51. [DOI] [PubMed] [Google Scholar]
- 126.Boden G. Obesity and free fatty acids. Endocrinol Metab Clin N Am. 2008;37:635–46. doi: 10.1016/j.ecl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic β-cells. Am J Physiol Endocrinol Metab. 2000;278:1–14. doi: 10.1152/ajpendo.2000.278.1.E1. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen LT, Muhlhausler BS, Botting KJ, Morrison JL. Maternal undernutrition alters fat cell size distribution, but not lipogenic gene expression, in the visceral fat of the late gestation guinea pig fetus. Placenta. 2010;31:902–9. doi: 10.1016/j.placenta.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 129.Ozanne SE, Dorling MW, Wang CL, Nave BT. Impaired PI 3-kinase activation in adipocytes from early growth-restricted male rats. Am J Physiol Endocrinol Metab. 2001;280:534–39. doi: 10.1152/ajpendo.2001.280.3.E534. [DOI] [PubMed] [Google Scholar]
- 130.Bouret SG, Simerly RB. Minireview: leptin and development of hypothalamic feeding circuits. Endocrinology. 2004;145:2621–26. doi: 10.1210/en.2004-0231. [DOI] [PubMed] [Google Scholar]
- 131.Brenner BM, Chertow GM. Congenital oligonephropathy: an inborn cause of adult hypertension and progressive renal injury? Curr Opin Nephrol Hypertens. 1993;2:691–95. [PubMed] [Google Scholar]
- 132.Manning J, Beutler K, Knepper MA, Vehaskari VM. Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. Am J Physiol Ren Physiol. 2002;283:202–6. doi: 10.1152/ajprenal.00358.2001. [DOI] [PubMed] [Google Scholar]
- 133.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–67. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 134.Leeson CP, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation. 2001;103:1264–68. doi: 10.1161/01.cir.103.9.1264. [DOI] [PubMed] [Google Scholar]
- 135.Nuyt AM, Alexander BT. Developmental programming and hypertension. Curr Opin Nephrol Hypertens. 2009;18:144–52. doi: 10.1097/MNH.0b013e328326092c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr Mol Med. 2010;10:653–66. doi: 10.2174/156652410792630643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, Thornburg KL. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am J Physiol Regul Integr Comp Physiol. 2003;285:1481–89. doi: 10.1152/ajpregu.00232.2003. [DOI] [PubMed] [Google Scholar]
- 138.Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, et al. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42:514–19. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 139.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–77. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 140.Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993;341:355–57. doi: 10.1016/0140-6736(93)90148-a. [DOI] [PubMed] [Google Scholar]
- 141.Oscai LB, McGarr JA. Evidence that the amount of food consumed in early life fixes appetite in the rat. Am J Physiol Regul Integr Comp Physiol. 1978;235:141–44. doi: 10.1152/ajpregu.1978.235.3.R141. [DOI] [PubMed] [Google Scholar]
- 142.Stephens DN. Growth and the development of dietary obesity in adulthood of rats which have been undernourished during development. Br J Nutr. 1980;44:215–27. doi: 10.1079/bjn19800034. [DOI] [PubMed] [Google Scholar]
- 143.Jones AP, Olster DH, States B. Maternal insulin manipulations in rats organize body weight and noradrenergic innervation of the hypothalamus in gonadally intact male offspring. Brain Res Dev Brain Res. 1996;97:16–21. doi: 10.1016/s0165-3806(96)00128-9. [DOI] [PubMed] [Google Scholar]
- 144.Widdowson EM. Chemical composition of newly born mammals. Nature. 1950;166:626–28. doi: 10.1038/166626a0. [DOI] [PubMed] [Google Scholar]
- 145.Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–42. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 146.Fowden AL, Hill DJ. Intra-uterine programming of the endocrine pancreas. Br Med Bull. 2001;60:123–42. doi: 10.1093/bmb/60.1.123. [DOI] [PubMed] [Google Scholar]
- 147.Beydoun HA, Sicignano N, Beydoun MA, Matson DO, Bocca S, et al. A cross-sectional evaluation of the first cohort of young adults conceived by in vitro fertilization in the United States. Fertil Steril. 2010;94:2043–49. doi: 10.1016/j.fertnstert.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 122:1192–99. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maffeis C, Micciolo R, Must A, Zaffanello M, Pinelli L. Parental and perinatal factors associated with childhood obesity in north-east Italy. Int J Obes Relat Metab Disord. 1994;18:301–5. [PubMed] [Google Scholar]