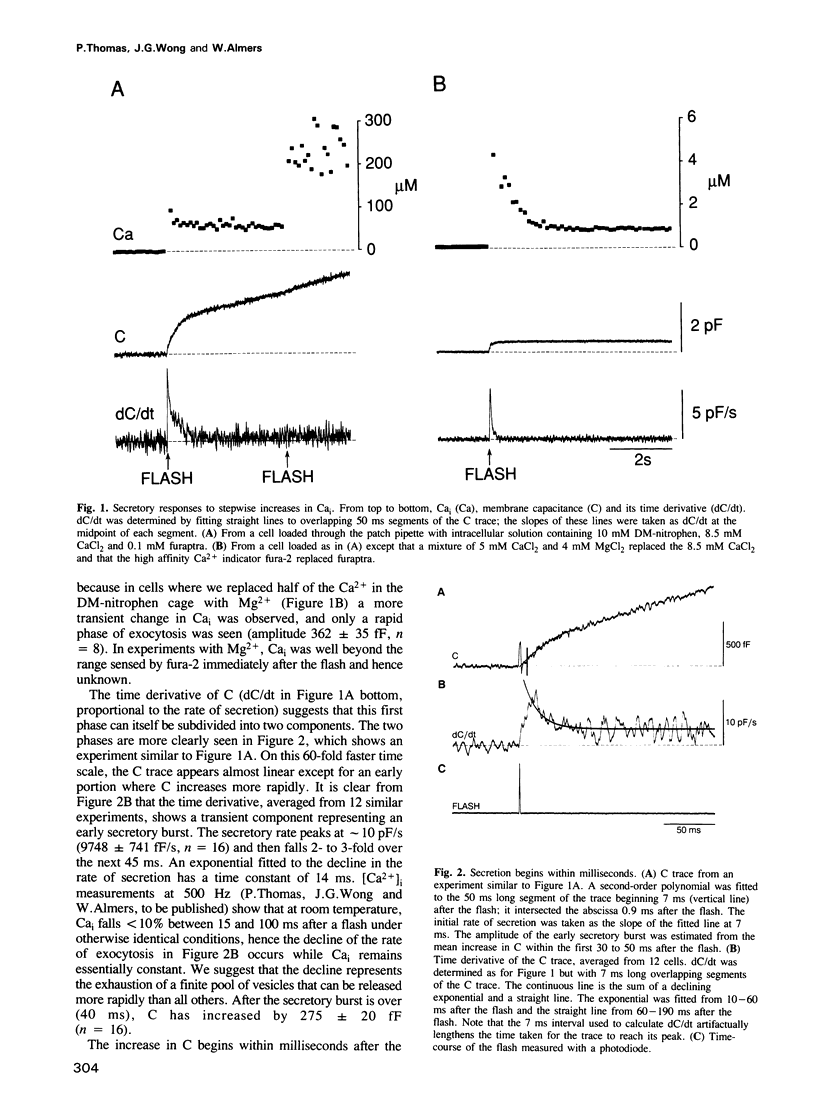
Abstract
To study the final steps in the secretory pathway of rat pituitary melanotrophs, we have monitored changes in cell surface area due to exocytosis after flash photolysis of caged Ca2+. A step increase in cytosolic [Ca2+] to 45-125 microM triggers three phases of exocytic secretion. A small cohort of a few hundred vesicles is exocytosed in 40 ms in a secretory burst with a peak rate of 17,000 vesicles/s. Next, 2700 more vesicles are released in a slower phase that is complete within 400-1000 ms. Finally, vesicles continue to be released slowly (500 vesicles/s) for > 8s. The approach described provides a way to identify and monitor the final steps in the secretory pathway at millisecond resolution. That a small portion of secretory vesicles can be released much faster than all others suggests that these vesicles are functionally equivalent to those at the presynaptic active zone of a neuron. Their release would be fast enough to be temporally correlated with single action potentials.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978 Dec 7;276(5688):620–622. doi: 10.1038/276620a0. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. 1987 Aug 27-Sep 2Nature. 328(6133):814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991 Jul 22;1071(2):174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J., Barron J. Dissection of stages in exocytosis in the adrenal chromaffin cell with use of trifluoperazine. Proc R Soc Lond B Biol Sci. 1982 Aug 23;216(1202):111–115. doi: 10.1098/rspb.1982.0064. [DOI] [PubMed] [Google Scholar]
- Chow R. H., von Rüden L., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992 Mar 5;356(6364):60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Chronwall B. M., Bishop J. F., Gehlert D. R. Rat intermediate lobe in culture: a histological and biochemical characterization. Peptides. 1988;9 (Suppl 1):169–180. doi: 10.1016/0196-9781(88)90241-0. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Action potentials in gland cells of rat pituitary pars intermedia: inhibition by dopamine, an inhibitor of MSH secretion. J Physiol. 1978 Dec;285:171–184. doi: 10.1113/jphysiol.1978.sp012565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Bittner M. A., Peppers S. C., Senter R. A., Eberhard D. A. MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. J Biol Chem. 1989 Apr 5;264(10):5412–5419. [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. Secretory granule and synaptic vesicle formation. Curr Opin Cell Biol. 1991 Aug;3(4):654–660. doi: 10.1016/0955-0674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- Konishi M., Hollingworth S., Harkins A. B., Baylor S. M. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991 Feb;97(2):271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Bartschat D. K. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun. 1991 May 31;177(1):184–191. doi: 10.1016/0006-291x(91)91966-g. [DOI] [PubMed] [Google Scholar]
- Lindau M., Stuenkel E. L., Nordmann J. J. Depolarization, intracellular calcium and exocytosis in single vertebrate nerve endings. Biophys J. 1992 Jan;61(1):19–30. doi: 10.1016/S0006-3495(92)81812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt A. D., Kelly R. B. Molecular architecture of the nerve terminal. Curr Opin Neurobiol. 1991 Oct;1(3):382–387. doi: 10.1016/0959-4388(91)90057-e. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju B., Murphy E., Levy L. A., Hall R. D., London R. E. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989 Mar;256(3 Pt 1):C540–C548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- Reynolds E. E., Melega W. P., Howard B. D. Adenosine 5'-triphosphate independent secretion from PC12 pheochromocytoma cells. Biochemistry. 1982 Sep 14;21(19):4795–4799. doi: 10.1021/bi00262a042. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack J., Surprenant A. Dopamine actions on calcium currents, potassium currents and hormone release in rat melanotrophs. J Physiol. 1991 Aug;439:37–58. doi: 10.1113/jphysiol.1991.sp018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]



