Abstract
Aims
Glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP4) inhibitors improve glucose tolerance by still incompletely understood mechanisms. Each class of antihyperglycemic drugs has also been proposed to increase pancreatitis risk. Here, we compare systematically the effects of two widely-used GLP-1 analogues, liraglutide and exendin-4, and the DPP4 inhibitor, sitagliptin, in the mouse.
Methods
C57BL6 mice were maintained for 131 days on a normal diet (ND) or a diet comprising 60% fat (HFD) before measurements of fasting blood glucose and insulin, and intraperitoneal glucose tolerance. Beta- and alpha- cell volume, and Reg3b immunoreactivity, were measured by immunohistochemical analysis of pancreatic slices.
Results
Whereas liraglutide (200 µg/kg) and exendin-4 (10 µg/kg) treatment reduced body weight and/or improved glucose tolerance, sitagliptin (10 mg/kg) was without effect on either parameter. Liraglutide caused a sharp reduction in beta-cell mass in both ND and HFD mice, whereas exendin-4 exerted no effect. By contrast, sitagliptin unmasked an action of high fat diet to increase beta-cell mass. Reg3B positive area was augmented by all three agents in normal chow-fed mice, whilst sitagliptin and exendin-4, but not liraglutide, affected this parameter in HFD animals. Correspondingly sitagliptin, but not the GLP-1 analogues, increased circulating amylase levels in ND and HFD mice.
Conclusions
Liraglutide improves glucose tolerance in the mouse whilst exerting relatively modest effects on pancreatitis risk. Conversely, exendin-4 and sitagliptin, at doses which exert, respectively, minor or no effects on metabolic parameters, lead to signs of pancreatitis.
Introduction
The incretin, glucagon-like peptide 1 (GLP-1), secreted after meal ingestion from L-cells found primarily in the distal intestine [1], promotes the secretion of insulin and somatostatin by pancreatic beta- and delta- cells, respectively, and decreases glucagon production from alpha-cells, as well as appetite and gastric emptying Together with a suggested action on beta-cell proliferation [2], and an absence of adverse effects such as weight gain and hypoglycaemic episodes [3], GLP-1 is an ideal candidate as a drug to treat type 2 diabetes (T2D)[4]. However, GLP-1 has a short circulating half-life (<2 min) due to its rapid degradation by dipeptidyl peptidase 4 (DPP-4), such that its therapeutic use requires continuous administration or the engineering of GLP-1 mimetics with longer circulating life-times [5]. Consequently the last decade has seen the development of two classes of GLP-1-based drugs for T2D: GLP-1 receptor (GLP-1R) agonists that mimic GLP-1 but are resistant to degradation by DPP-4, and DPP-4 inhibitors.
Although an efficient drug class for the treatment of T2D [6], recent data indicate that long-term administration of the GLP-1R agonists and DPP4 inhibitors may be linked to an increased risk of pancreatitis and pancreatic cancer [7]–[11]. The risk of pancreatic cancer conferred by the usage of these anti-diabetic drugs is difficult to assess as patients with a history of pancreatitis and diabetes are in any case at increased risk of developing pancreatic cancer [12], [13]. Three commonly used agents are exenatide/exendin-4 and liraglutide, which are GLP-1 mimetics, and sitagliptin, a DPP4 inhibitor.
The U.S. FDA issued a warning to healthcare professionals about the possible increased risk of pancreatitis in T2D patients taking sitagliptin following 88 cases of acute pancreatitis related with sitagliptin use in 2009; pancreatitis was also associated with exenatide use [14], [15]. Subsequently, liraglutide, exendin-4 and sitagliptin have all been variously associated with pancreatitis risk in patients and rodent models [10], [16]–[28] [29]. These results raised concerns as chronic pancreatitis has been shown to increase the risk of pancreatic cancer [26]. There is, nonetheless, a lack of information from human pancreata; data from patients on long-term treatment are not currently available, and the use of the AERS to assess drug safety is arguably imperfect [9]. The evidence for an association between GLP-1-based therapy and the development of pancreatitis is intensified by the fact that all of the developed agents which have been on the market long enough have now been linked to cases of pancreatitis [17].
Although much has been published on the potential risk of pancreatitis and pancreatic cancer from administration of GLP-1 mimetics, there have equally been studies which demonstrate no effects on this parameter [30]–[38]. A recent study by Ellenbroek and colleagues [39] demonstrated that mice administered liraglutide prior to the start of a six-week long high fat diet regimen remained normoglycaemic and exhibited decreased beta cell mass, possibly due to improved insulin sensitivity. In total liraglutide's efficacy and safety have been investigated in more than 5000 patients through 20 clinical trials; in a number of these studies markers of beta-cell function were also analysed leading to the indication of improved beta-cell function [40], [41], amongst other potential beneficial effects [41]–[43]. The FDA and EMA exert panels have also recently ruled that available data do not confirm recent concerns over an increased risk for pancreatic side effects with GLP-1-based diabetes therapies.
The aims of the present study were, therefore, to probe in a mouse model of diet-induced glucose intolerance [44]–[46] the propensity of the GLP-1 receptor agonists- liraglutide and exendin-4- and the DPP4 inhibitor-sitagliptin- to cause signs of pancreatitis, whilst comparing the action of each on weight gain, glucose homeostasis and beta-cell mass.
Methods
Materials
All general chemicals and tissue culture reagents were purchased from Sigma (Dorset, U.K.) or Invitrogen (Paisley, U.K.), unless otherwise stated.
Animals
All in vivo procedures were approved by the U.K. Home Office according to the Animals (Scientific Procedures) Act 1986 and were performed at the Central Biomedical Service, Imperial College, London, U.K. C57BL/6 male mice were purchased from Charles River (U.K.). For high fat diet treatment, mice were placed on a high fat diet at eight weeks of age for eight weeks (60% [wt/wt] fat content; Research Diet, New Brunswick, NJ, USA). Mice were housed at two to five animals per cage in a pathogen-free facility with a 12-hour light-dark cycle. Animals were fed ad libitum with a standard mouse chow diet (Research Diet, New Brunswick, NJ) unless otherwise stated. Mice were culled by cervical dislocation.
Intraperitoneal glucose tolerance test
Mice were fasted for 16 h, with water available ad libitum prior to the test. Glucose tolerance tests were conducted at 09:00 on each experimental day. Glucose tolerance was assessed by intraperitoneal administration of glucose (1 g/kg).
Administration of GLP-1 mimetics
C57BL/6 mice (eight weeks old) were maintained on a normal chow or high fat (60%; Lillico) diet for eight weeks prior to the start of the injection regime. At 16 weeks of age, mice were injected daily with saline, liraglutide (200 µg/kg [47]; Bachem, Bubendorf, Switzerland), exendin-4 (10 µg/kg [27]; Polypeptide Group SCI537 Strasbourg, France), or sitagliptin (10 mg/kg [48]; Sigma) at the start of the dark cycle.
Immunohistochemistry and widefield microscopy
Isolated pancreases were fixed in 10% buffered formalin and embedded in paraffin wax within 24 h of removal. Head-to-tail sections (5 µm lengthwise) were cut and incubated overnight at 37°C on superfrost slides. Slides were submerged sequentially in Histoclear (Sigma) followed by decreasing concentrations of industrial methylated spirits for removal of paraffin wax. Permeabilised slices were blotted with primary antibodies against insulin (DAKO, Cambridgeshire, U.K.), glucagon (Sigma) and Reg3B (R&D Systems, Abingdon, U.K.) with antigen unmasking using vector antigen unmasking solution (Vector Laboratories, Peterborough, U.K.), and visualised with Alexa Fluor 488 or 568 secondary antibodies (Invitrogen). Specimens were mounted on glass slides using Vectashield hard set with DAPI (Vector Laboratories).
Images were captured on a Zeiss Axio Observer.Z1 Motorised Inverted Widefield Microscope fitted with a Hamamatsu Flash 4.0 Camera using Plan-Apochromat 20×/0.8 M27 air objective with Colibri.2 LED illumination. Data acquisition was controlled by Zeiss Zen Blue 2012 software configured at a bit depth of 16-bit and binning mode 2×2.
Whole tissue tiled preview scans were obtained using an EC Plan-Neofluar 10×/0.3 Ph1 air objective with phase contrast. For Reg3B analysis, a 20 point array was chosen at random with point focusing achieved with the Plan-Apochromat 20×/0.8 M27 air objective using EGFP 488 filter as the reference channel for local surface focusing.
Fluorescence quantification was achieved using Fiji [49]. Threshold measurements were taken for total tissue area using particle size 200-infinity and fluorescence area using particle size 20-infinity for each of the 20 point arrays. Ratios were subsequently calculated with an in-house macro for comparative analyses between sample groups. We verified that our macro was providing a measure of change in fluorescence through comparison with manual scoring of the slides using an Nikon TS100-F microscope fitted with a LED light source and appropriate filters (Fig. S1; Nikon, London, U.K.). All image capture and analysis were performed with the operator blinded to the identity of the slides.
Measurement of plasma amylase, lipase and GLP-1
Blood (200 µl) was removed by cardiac puncture from mice killed by cervical dislocation. Plasma was collected using high speed (2000 g, 5 min at 4°C) centrifugation in heparin-coated Microvette tubes containing EDTA (Sarstedt, Leicester, U.K.). Plasma amylase and lipase levels were assessed using the lipase and amylase assay kits from Abcam (Cambridge, U.K.). Plasma GLP-1 levels were assessed as previously described [45].
Histopathology
Slices from pancreases prepared as detailed under ‘Immunohistochemistry and widefield microscopy’ were stained with eosin and haemotoxylin and subjected to histopathological analysis. Qualitative analysis of the following parameters were performed- endocrine islet size variation and inflammation; cytoplasmic vacuole, nucleus size variation, necrolysis/autolysis, autolysis/fibrosis, lobular inflammation, septal inflammation in exocrine pancreas; peripancreatic fat inflammation.
Statistical analysis
Data are the means ± S.E. for the number of observations indicated. Statistical significance and differences between means were assessed by Student's t-test with Bonferroni correction for multiple analyses as appropriate in Excel (Microsoft).
Results
Liraglutide and exendin-4 restrict weight gain, but liraglutide is more effective at maintaining glucose tolerance in high fat diet-fed mice
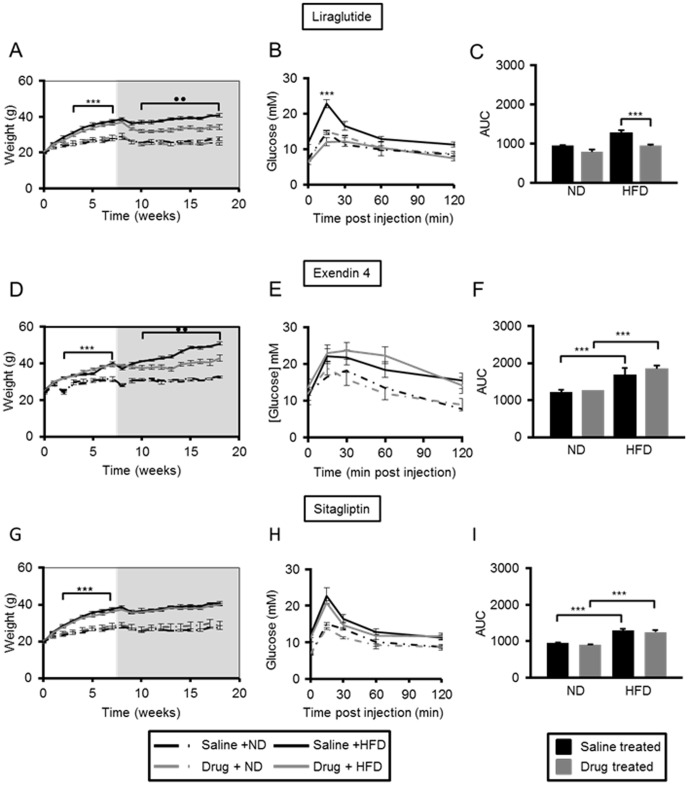
Administration of a high fat (60% fat) diet (HFD) led to marked weight gain in mice as expected (Fig. 1A, B, C;). Correspondingly, glucose tolerance was impaired, with an increase in the area under the curve (AUC) of 56.8±4.9% (p≤0.001, n = 16 per group) vs mice maintained on a normal chow diet (Fig. S2). We chose doses and administration routes of liraglutide, exendin-4 and sitagliptin that were previously shown to be effective in mice [27], [47], [48]. Neither liraglutide, exendin-4, nor sitagliptin exerted any effect on weight gain or glucose tolerance in mice maintained on a normal chow diet (Fig. 1). Administration of liraglutide and exendin-4 led to a 23.9±3.0% (p≤0.01, n = 4 per group) and a 37.4±5.0% (p≤0.01, n = 3 per group), decrease in weight gain in mice on a high fat diet over the course of the drug regimen, respectively (Fig. 1A, B). Administration of liraglutide led to an improvement in glucose tolerance in HFD mice at the end of the 75 days' treatment period (26.3±2.1% decrease in AUC vs mice high fat diet that had been administered saline, p≤0.01), such that glucose tolerance was indistinguishable from that observed in mice maintained on a normal diet (Fig. 1D, G). In our hands, neither exendin-4 nor sitagliptin improved glucose tolerance in mice maintained on HFD by the end of the treatment period (Fig. 1E–F, H–I).
Figure 1. Liraglutide and exendin-4 are effective weight management drugs in high fat-fed C57BL/6 mice, but liraglutide is more effective at improving glucose tolerance.
The weight of male C57BL/6 mice was monitored on a weekly basis over a period of eight weeks (white area) on high fat (HFD) or normal chow diet (ND), followed by 75 days (grey area) of daily intraperitoneal injections of saline, or a GLP-1 mimetic- liraglutide (panels A–C), exendin-4 (panels D–F), or sitagliptin (panels G–I). Intra-peritoneal glucose tolerance tests were performed after 75 days of treatment (B–C, E–F, H–I). Time courses (B, E, H) and the corresponding areas under the curve (C, F, I) are shown. p≤0.001, ***; p≤0.01, **, n = 3–7 mice.
Long term adminstration of liraglutide, exendin-4 and sitagliptin exert differing effects on beta-cell mass but no effect on alpha-cell mass
Pancreatic beta- and alpha-cell mass (Fig. 2A) were quantified in pancreatic slices as described under Materials and Methods. Administration of liraglutide markedly lowered beta-cell mass in mice maintained on a normal chow diet (39±9.8%, p≤0.01, n = 3 per group) or high fat diet (62.2±4.5%, p≤0.001, n = 4 per group) vs control mice (Fig. 2B).Administration of exendin 4 had effect on beta cell mass (Fig. 2C). Interestingly, whilst a HFD alone exerted no effects on beta-cell mass in the absence of drug treatment, administration of sitagliptin led to the unmasking of an action of HFD to increase beta cell mass (40.6%, p≤0.01, n = 4 per group), when compared with mice treated with the drug on a normal chow diet (Fig. 2D). No observable differences in alpha-cell mass were found between any of the cohorts (Fig. 2E–G).
Figure 2. Long term adminstration of exendin-4, liraglutide and sitagliptin exerts different effects on beta-cell mass but no effect on alpha-cell mass.
Representative images of pancreatic sections stained for insulin (green), glucagon (red) and nuclei (DAPI, blue) (A). Pancreatic sections from mice treated with liraglutide (B, E), exendin-4 (C, F) and sitagliptin (D, G) were examined. Beta-cell mass (B, C, D), and alpha-cell mass (E, F, G) were measured from whole section scans as described in ‘Materials and Methods’. p≤0.001, ***; p≤0.01, n = 3–7 mice.
Long term administration of liraglutide, exendin-4 and sitagliptin lead to increased Reg3b immunoreactivity
Administration of liraglutide, exendin-4 and sitagliptin led to increased Reg3b immunoreactivity to differing extents in mouse pancreata, and depending on diet (Fig. 3A–D). Thus, the increase in Reg3b signal area associated with administration of liraglutide to mice on a normal diet was 3.3±0.6- fold (p≤0.001, n = 3 per group) (Fig. 3B). Interestingly, administration of liraglutide did not exacerbate the effects of high fat diet alone (Fig. 3B). Administration of exendin-4, on the other hand, led to a signal increase of 5.8±0.8-fold (p≤0.001, n = 4 per group) in the Reg3b signal in mice on high fat diet (Fig. 3C), as well as in ND animals. Administration of sitagliptin to mice on normal diet resulted in a 3.9±0.9-fold increase in Reg3b signal (p≤0.05, n = 3 per group), and this increase was of 18.6±6.6-fold (p≤0.01, n = 4 per group) in mice on high fat diet (Fig. 3D).
Figure 3. C57BL/6 mice do not display clinical signs of pancreatitis following 75 days' treatment with liraglutide, exendin-4, or sitagliptin.
Reg3b immunoreactivity was assessed in pancreatic sections from mice treated with saline (Ai), liraglutide (B), exendin-4 (Aii, C) and sitagliptin (D), and normalised to Reg3b area observed in pancreata from saline treated mice, as described in ‘Materials and Methods’. Plasma amylase (E) and lipase (F) levels were measured after 75 days treatment with saline, liraglutide, exendin-4 or sitagliptin. ND, normal chow diet; HFD, high fat diet. p≤0.05, *; p≤0.01, **, n = 3–7 mice.
Clinical measures of pancreatitis
There were no statistically significant changes in plasma amylase activity in mice that were administered liraglutide or exendin-4 vs mice administered saline (Fig. 3E). However, administration of sitagliptin to animals on normal diet led to a 1.4-fold increase in amylase activity (p≤0.01, n = 3 per group) and a 1.3- fold increase in mice on a high fat diet (p≤0.01, n = 4 per group) (Fig. 3E).
There were no detectable differences in plasma lipase activity in mice on a normal chow diet administered any of the three drugs when compared to animals administered saline (Fig. 3F). Likewise, there was no significant change in plasma lipase activity in mice that were administered saline on a high fat diet vs normal diet (Fig. 3F). Furthermore, administration of liraglutide and exendin-4 in combination with a high fat diet also failed to affect plasma lipase activity. We observed no detectable changes in plasma lipase activity in animals maintained on a normal chow diet and administered any of the three drugs when compared to animals administered saline (Fig. 3F).
Histopathological analysis revealed no significant differences in pathological status of pancreatic slices from the different treatment groups, with a potential indication of an increase in inflammatory response in mice subjected to a high fat diet vs mice that have been maintained on a normal chow diet (Fig. S3).
Discussion
Animal studies do not necessarily predict with certainty what will happen in humans during similar treatment protocols [50]. Nonetheless, given the poor prognosis for patients diagnosed with pancreatic cancer, and the lack of risk data from long term studies of patients on these treatments, there is a need to examine the risk of pancreatitis following long-term treatment with GLP-1 receptor agonists in model systems. To circumvent some of the potential problems involved with studying human disease risk in mouse models, we have selected a model which reflects at least several key elements of T2D in humans [44]. Importantly, we have administered drugs to mice that already exhibit glucose dyshomeostasis, in contrast to a recent study [39] whereby liraglutide was administered prior to the start of the high fat diet regimen.
Of the three agents tested, liraglutide, at the dose tested here, was overall the most effective at decreasing weight gain and improving glucose tolerance by the end of the 75 days' treatment (Fig. 1). Interestingly, this change was accompanied by a decrease in beta-cell mass in mice on both normal chow diet and high fat diet (Fig. 2B). These data are consistent with a recent report showing that mice administered liraglutide and maintained on a high fat diet for six weeks, exhibited decreased beta-cell mass possibly due to improved insulin sensitivity [39]. Interestingly, mice on a high fat diet in the earlier study by Ellenbroek and colleagues [39] remained normoglycaemic throughout the study, since they were given liraglutide prior to starting the high fat diet regime. More closely mimicking the use of the drug in man, our mice were rendered hyperglycaemic prior to drug administration and the drug regimen was longer than that reported in [39]. Suggesting these differences in protocol may be important [39], we saw no changes in alpha-cell mass during liraglutide treatment in the present studies. By contrast, neither exendin-4 nor sitagliptin exerted any effect on beta-cell mass in the present study, albeit under conditions here where neither drug exerted significant effects on glucose homeostasis.
In this study we administered sitagliptin by intraperitoneal injection as daily oral gavages were impractical and would mean increasing the number of mice we needed to use per cohort, with the associated ethical issues. Administration by admixture was prohibitively costly. To assess the efficacy of adminstration of sitagliptin by intraperitoneal injection, we measured plasma GLP-1 levels one hour after co-injection of glucose (1 g/kg) and sitagliptin (10 mg/kg), and demonstrated that this was effective at raising plasma GLP-1 content, vs saline control (Fig. S1C).
An important prompt for the present investigation was the controversy that exists over whether long term administration of GLP-1 mimetics may lead to pancreatitis and pancreatic cancer. One confounding factor may be the propensity for rodent models to develop spontaneous pancreatic lesions [51]. Our observations indicate that rodents may indeed manifest signs of spontaneous pancreatic lesions but that some of the signals for pancreatic disease are exaggerated by exposure to the GLP-1 mimetics and DDP 4 inhibitor drug class (Fig. 3, Fig. S1). We have also observed that prolonged exposure to high fat diet has a tendency to lead to increased Reg3B immunoreactivity (Fig. 3B–D) even though there were no signs of overt clinical pancreatitis. Clinical pancreatitis is indicated by increases in the activity of both plasma amylase and lipase. Although we saw increased pancreatic content of Reg3B, a marker of pancreatitis, by immunohistochemical analysis, with all three GLP-1 mimetics used in this study (Fig.3A–D), we did not observe increases in plasma amylase or lipase with administration of liraglutide and exendin-4 in conjunction with the high fat diet (Fig. 3E-F). Administration of sitagliptin led to an increase in amylase activity when administered to mice on both normal chow diet and high fat diet (Fig. 3E). Histopathological analysis did not indicate an increased signal for pancreatitis from drug treatment (Fig. S3). The apparent discrepancy from the four methods of assessing pancreatitis may be explained by the relative sensitivities of the methods. Histopathology is a subjective method of analysis of the pancreas and, whilst we think it is a valuable assessment tool, we wanted 1) a more quantitative way for assessing pancreatitis, and 2) more than one method to assess pancreatitis. We therefore chose to also assess pancreatitis by measuring pancreatic Reg3b content, and plasma amylase and lipase content. Reg3b is an indicator of tissue regeneration and is upregulated when pancreata are damaged e.g. by pancreatitis. Thus, a change in the content of this protein in the pancreas can be a measure for mild pancreatitis, where pancreatic tissue is available for analysis. Assessment of changes in plasma amylase and lipase in mild pancreatitis is difficult as these can be cleared by the renal system; this is problematic for early diagnosis of pancreatitis, but is the only measure available in the clinic where pancreatic material is not available for analysis.
Thus, our current data do not suggest a clinical lesion, as defined in the human setting. However, as we observed an effect of sitagliptin to increase two out of our three measures for pancreatitis, longer term studies which follow larger cohorts of mice until the end of their natural life may shed more light on the risk for pancreatitis and pancreatic cancer in this model.
Supporting Information
Mice were rendered glucose intolerant following 8 weeks on a high fat diet. Time course (A) and area under the curve (B) of 16 week old male C57BL/6 mice on high fat diet (HFD) and normal diet (ND) after 8 weeks on differential diet. (C) Plasma was extracted from mice 1 h following intraperitoneal injection of glucose (1 g/kg) and saline or sitagliptin (10 mg/kg), and plasma GLP-1 was measured as described in [45].
(TIF)
Manual verification of macro calculations. The percentage of Reg3b positive areas out of 10 (manual; A) and 20 (macro; B) randomly chosen fields from pancreatic sections from mice treated with saline or exendin-4 were scored by visual examination. In the manual verification, a field was considered positive when there was at least one positive signal within the optical field regardless of area of signal. ND, normal chow diet; HFD, high fat diet.
(TIF)
Histopathology report. Analyses were carried out as described in ‘Materials and Methods’. Numbers in brackets indicate number of positive observations by the total number of observations made.
(TIF)
Acknowledgments
We thank the personnel at the Facility for Imaging by Light Microscopy (FILM, Imperial College London) for their help with image acquisition, and Ms. Lorraine Lawrence from the Histopathology Service for preparation of pancreatic sections.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Relevant data are included within the paper.
Funding Statement
The research leading to this report was funded by grants to GdSX and GAR from the European Foundation for the Studies of Diabetes (Diabetes and Cancer grant scheme 2012), and Diabetes U.K. (Diabetes UK Alec and Beryl Warren Award, Project grant 13/0004672), and to GAR from the Wellcome Trust (Senior Investigator Award WT098424AIA), and MRC (UK) (Programme Grant MR/J0003042/1). GAR is the recipient of a Royal Society Wolfson Research Merit Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Marathe CS, Rayner CK, Jones KL, Horowitz M (2013) Glucagon-like peptides 1 and 2 in health and disease: a review. Peptides 44: 75–86 S0196-9781(13)00033-8 [pii]; 10.1016/j.peptides.2013.01.014 [doi] [DOI] [PubMed] [Google Scholar]
- 2. Arulmozhi DK, Portha B (2006) GLP-1 based therapy for type 2 diabetes. Eur J Pharm Sci 28: 96–108 S0928-0987(06)00020-0 [pii]; 10.1016/j.ejps.2006.01.003 [doi] [DOI] [PubMed] [Google Scholar]
- 3. Turner RC (1998) The U.K. Prospective Diabetes Study. A review. Diabetes Care 21 Suppl 3: C35–C38. [DOI] [PubMed] [Google Scholar]
- 4. Aroda VR, Ratner R (2011) The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: a review. Diabetes Metab Res Rev 27: 528–542 10.1002/dmrr.1202 [doi] [DOI] [PubMed] [Google Scholar]
- 5. Lovshin JA, Drucker DJ (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5: 262–269 nrendo.2009.48 [pii]; 10.1038/nrendo.2009.48 [doi] [DOI] [PubMed] [Google Scholar]
- 6. Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, et al. (1997) Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol 273: E981–E988. [DOI] [PubMed] [Google Scholar]
- 7. Butler PC, Matveyenko AV, Dry S, Bhushan A, Elashoff R (2010) Glucagon-like peptide-1 therapy and the exocrine pancreas: innocent bystander or friendly fire? Diabetologia 53: 1–6 10.1007/s00125-009-1591-5 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butler PC, Dry S, Elashoff R (2010) GLP-1-based therapy for diabetes: what you do not know can hurt you. Diabetes Care 33: 453–455 33/2/453 [pii]; 10.2337/dc09-1902 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drucker DJ, Sherman SI, Bergenstal RM, Buse JB (2011) The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab 96: 2027–2031 96/7/2027 [pii]; 10.1210/jc.2011-0599 [doi] [DOI] [PubMed] [Google Scholar]
- 10. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC (2011) Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 141: 150–156 S0016-5085(11)00172-7 [pii]; 10.1053/j.gastro.2011.02.018 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, et al. (2010) Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care 33: 428–433 33/2/428 [pii]; 10.2337/dc09-1499 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, et al. (1993) Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 328: 1433–1437 10.1056/NEJM199305203282001 [doi] [DOI] [PubMed] [Google Scholar]
- 13. Jura N, Archer H, Bar-Sagi D (2005) Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res 15: 72–77 10.1038/sj.cr.7290269 [doi] [DOI] [PubMed] [Google Scholar]
- 14. Denker PS, Dimarco PE (2006) Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care 29: 471.29/2/471 [pii] [DOI] [PubMed] [Google Scholar]
- 15. Tripathy NR, Basha S, Jain R, Shetty S, Ramachandran A (2008) Exenatide and acute pancreatitis. J Assoc Physicians India 56: 987–988. [PubMed] [Google Scholar]
- 16. Garg SK (2010) The role of basal insulin and glucagon-like peptide-1 agonists in the therapeutic management of type 2 diabetes—a comprehensive review. Diabetes Technol Ther 12: 11–24 10.1089/dia.2009.0127 [doi] [DOI] [PubMed] [Google Scholar]
- 17. Butler PC, Elashoff M, Elashoff R, Gale EA (2013) A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe? Diabetes Care 36: 2118–2125 dc12-2713 [pii]; 10.2337/dc12-2713 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, et al. (2013) Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med 173: 534–539 1656537 [pii]; 10.1001/jamainternmed.2013.2720 [doi] [DOI] [PubMed] [Google Scholar]
- 19. Franks AS, Lee PH, George CM (2012) Pancreatitis: a potential complication of liraglutide? Ann Pharmacother 46: 1547–1553 aph.1Q789 [pii]; 10.1345/aph.1Q789 [doi] [DOI] [PubMed] [Google Scholar]
- 20. Lando HM, Alattar M, Dua AP (2012) Elevated amylase and lipase levels in patients using glucagonlike peptide-1 receptor agonists or dipeptidyl-peptidase-4 inhibitors in the outpatient setting. Endocr Pract 18: 472–477 V27172GKL8586K52 [pii]; 10.4158/EP11290.OR [doi] [DOI] [PubMed] [Google Scholar]
- 21. Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, et al. (2012) Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model. Diabetes 61: 1250–1262 db11-1109 [pii]; 10.2337/db11-1109 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knezevich E, Crnic T, Kershaw S, Drincic A (2012) Liraglutide-associated acute pancreatitis. Am J Health Syst Pharm 69: 386–389 69/5/386 [pii]; 10.2146/ajhp110221 [doi] [DOI] [PubMed] [Google Scholar]
- 23. Jeong KH, Yoo BK (2011) The efficacy and safety of liraglutide. Int J Clin Pharm 33: 740–749 10.1007/s11096-011-9552-8 [doi] [DOI] [PubMed] [Google Scholar]
- 24. Lee PH, Stockton MD, Franks AS (2011) Acute pancreatitis associated with liraglutide. Ann Pharmacother 45: e22 aph.1P714 [pii]; 10.1345/aph.1P714 [doi] [DOI] [PubMed] [Google Scholar]
- 25. Iyer SN, Drake AJ III, West RL, Mendez CE, Tanenberg RJ (2012) Case report of acute necrotizing pancreatitis associated with combination treatment of sitagliptin and exenatide. Endocr Pract 18: e10–e13 P160158P076H2250 [pii]; 10.4158/EP11264.CR [doi] [DOI] [PubMed] [Google Scholar]
- 26. Bhanot UK, Moller P (2009) Mechanisms of parenchymal injury and signaling pathways in ectatic ducts of chronic pancreatitis: implications for pancreatic carcinogenesis. Lab Invest 89: 489–497 labinvest200919 [pii]; 10.1038/labinvest.2009.19 [doi] [DOI] [PubMed] [Google Scholar]
- 27. Nachnani JS, Bulchandani DG, Nookala A, Herndon B, Molteni A, et al. (2010) Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia 53: 153–159 10.1007/s00125-009-1515-4 [doi] [DOI] [PubMed] [Google Scholar]
- 28. Matveyenko AV, Dry S, Cox HI, Moshtaghian A, Gurlo T, et al. (2009) Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 58: 1604–1615 db09-0058 [pii]; 10.2337/db09-0058 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, et al. (2013) Marked Expansion of Exocrine and Endocrine Pancreas With Incretin Therapy in Humans With Increased Exocrine Pancreas Dysplasia and the Potential for Glucagon-Producing Neuroendocrine Tumors. Diabetes 62: 2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nyborg NC, Molck AM, Madsen LW, Knudsen LB (2012) The human GLP-1 analog liraglutide and the pancreas: evidence for the absence of structural pancreatic changes in three species. Diabetes 61: 1243–1249 db11-0936 [pii]; 10.2337/db11-0936 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ (2009) Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 58: 2148–2161 db09-0626 [pii]; 10.2337/db09-0626 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vrang N, Jelsing J, Simonsen L, Jensen AE, Thorup I, et al. (2012) The effects of 13 wk of liraglutide treatment on endocrine and exocrine pancreas in male and female ZDF rats: a quantitative and qualitative analysis revealing no evidence of drug-induced pancreatitis. Am J Physiol Endocrinol Metab 303: E253–E264 ajpendo.00182.2012 [pii]; 10.1152/ajpendo.00182.2012 [doi] [DOI] [PubMed] [Google Scholar]
- 33. Aston-Mourney K, Subramanian SL, Zraika S, Samarasekera T, Meier DT, et al. (2013) One year of sitagliptin treatment protects against islet amyloid-associated beta-cell loss and does not induce pancreatitis or pancreatic neoplasia in mice. American Journal of Physiology-Endocrinology and Metabolism 305: E475–E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Monami M, Dicembrini I, Martelli D, Mannucci E (2011) Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Current Medical Research and Opinion 27: 57–64. [DOI] [PubMed] [Google Scholar]
- 35. Garg R, Chen W, Pendergrass M (2010) Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care 33: 2349–2354 dc10-0482 [pii]; 10.2337/dc10-0482 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Engel SS, Williams-Herman DE, Golm GT, Clay RJ, Machotka SV, et al. (2010) Sitagliptin: review of preclinical and clinical data regarding incidence of pancreatitis. International Journal of Clinical Practice 64: 984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tatarkiewicz K, Polizzi C, Villescaz C, D'Souza LJ, Wang Y, et al. (2013) Combined antidiabetic benefits of exenatide and dapagliflozin in diabetic mice. Diabetes Obes Metab. 10.1111/dom.12237 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forest T, Holder D, Smith A, Cunningham C, Yao X, et al. (2014) Characterization of the Exocrine Pancreas in the Male Zucker Diabetic Fatty Rat Model of Type 2 Diabetes Mellitus Following 3 Months of Treatment with Sitagliptin. Endocrinology en20131781. 10.1210/en.2013-1781 [doi] [DOI] [PubMed] [Google Scholar]
- 39. Ellenbroek JH, Tons HA, Westerouen van Meeteren MJ, de GN, Hanegraaf MA, et al. (2013) Glucagon-like peptide-1 receptor agonist treatment reduces beta cell mass in normoglycaemic mice. Diabetologia 56: 1980–1986 10.1007/s00125-013-2957-2 [doi] [DOI] [PubMed] [Google Scholar]
- 40. Peters KR (2013) Liraglutide for the treatment of type 2 diabetes: a clinical update. Am J Ther 20: 178–188 10.1097/MJT.0b013e3182204c16 [doi] [DOI] [PubMed] [Google Scholar]
- 41. Dai Y, Mehta JL, Chen M (2013) Glucagon-like Peptide-1 Receptor Agonist Liraglutide Inhibits Endothelin-1 in Endothelial Cell by Repressing Nuclear Factor-Kappa B Activation. Cardiovasc Drugs Ther. 10.1007/s10557-013-6463-z [doi] [DOI] [PubMed] [Google Scholar]
- 42. Wideman RD, Kieffer TJ (2009) Mining incretin hormone pathways for novel therapies. Trends Endocrinol Metab 20: 280–286 S1043-2760(09)00071-X [pii]; 10.1016/j.tem.2009.02.005 [doi] [DOI] [PubMed] [Google Scholar]
- 43. Gaspari T, Welungoda I, Widdop RE, Simpson RW, Dear AE (2013) The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE(-/-) mouse model. Diab Vasc Dis Res 10: 353–360 1479164113481817 [pii]; 10.1177/1479164113481817 [doi] [DOI] [PubMed] [Google Scholar]
- 44. Ahren B, Simonsson E, Scheurink AJ, Mulder H, Myrsen U, et al. (1997) Dissociated insulinotropic sensitivity to glucose and carbachol in high-fat diet-induced insulin resistance in C57BL/6J mice. Metabolism 46: 97–106. S0026-0495(97)90175-X [pii] [DOI] [PubMed] [Google Scholar]
- 45. da Silva Xavier G, Mondragon A, Sun G, Chen L, McGinty JA, et al. (2012) Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2-null mice. Diabetologia 55: 2667–2676 10.1007/s00125-012-2600-7 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun G, Tarasov AI, McGinty J, McDonald A, da Silva Xavier G, et al. (2010) Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia 53: 924–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, et al. (2009) GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58: 975–983 db08-1193 [pii]; 10.2337/db08-1193 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poucher SM, Cheetham S, Francis J, Zinker B, Kirby M, et al. (2012) Effects of saxagliptin and sitagliptin on glycaemic control and pancreatic beta-cell mass in a streptozotocin-induced mouse model of type 2 diabetes. Diabetes Obes Metab 14: 918–926 10.1111/j.1463-1326.2012.01619.x [doi] [DOI] [PubMed] [Google Scholar]
- 49. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682 nmeth.2019 [pii]; 10.1038/nmeth.2019 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, et al. (2010) Can animal models of disease reliably inform human studies? PLoS Med 7: e1000245 10.1371/journal.pmed.1000245 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chadwick KD, Fletcher AM, Parrula MC, Bonner-Weir S, Mangipudy RS, et al. (2013) Occurrence of Spontaneous Pancreatic Lesions in Normal and Diabetic Rats: A Potential Confounding Factor in the Nonclinical Assessment of Glucagon-Like Peptide (GLP)-1-Based Therapies. Diabetes. db13–1268 [pii]; 10.2337/db13-1268 [doi] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mice were rendered glucose intolerant following 8 weeks on a high fat diet. Time course (A) and area under the curve (B) of 16 week old male C57BL/6 mice on high fat diet (HFD) and normal diet (ND) after 8 weeks on differential diet. (C) Plasma was extracted from mice 1 h following intraperitoneal injection of glucose (1 g/kg) and saline or sitagliptin (10 mg/kg), and plasma GLP-1 was measured as described in [45].
(TIF)
Manual verification of macro calculations. The percentage of Reg3b positive areas out of 10 (manual; A) and 20 (macro; B) randomly chosen fields from pancreatic sections from mice treated with saline or exendin-4 were scored by visual examination. In the manual verification, a field was considered positive when there was at least one positive signal within the optical field regardless of area of signal. ND, normal chow diet; HFD, high fat diet.
(TIF)
Histopathology report. Analyses were carried out as described in ‘Materials and Methods’. Numbers in brackets indicate number of positive observations by the total number of observations made.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Relevant data are included within the paper.