Abstract
Objective: Bacterial sepsis in neonates is associated with elevated morbidity and mortality. A role for the pro-inflammatory Triggering Receptor Expressed on Myeloid cells-1 (TREM-1) is suspected in the innate immune response to bacteria, but little is known about its activities in infants. To begin exploring the feasibility of treating neonatal sepsis by blocking leukocyte TREM-1, we compared TREM-1 membrane expression and mRNA in newborns without clinical or microbiological evidence of infection, to that of healthy adults. The functionality of pro-inflammatory reactions in leukocyte TREM-1 of newborns was also evaluated. Methods: Twenty term newborns were enrolled in this study and cord blood samples were collected at birth. For comparison, peripheral blood specimens were collected from 20 healthy adults (control adult, CA). The expression of TREM-1 protein and mRNA in leukocytes was detected with flow cytometry and real-time qPCR, respectively. Whole cord blood was also stimulated by Escherichia coli or blocked by the TREM-1-specific peptide LP17 to identify changes in the secretion of pro-inflammatory cytokines interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α, as well as soluble TREM-1 (sTREM-1) using enzyme linked immunosorbent assay (ELISA). Results: Mean fluorescence intensity (MFI) of TREM-1 on leukocytes of newborns appeared comparable to healthy adults [monocytes: 37.5 ± 6.7 vs. 37.6 ± 8.7; polymorphonuclear cells (PMNs): 32.9 ± 6.6 vs. 33.6 ± 5.8]. However, the percentage of PMNs positive for TREM-1 was lower in newborns than in healthy adults (82.3 ± 7.1 vs. 98.6 ± 4.8; P < 0.01); the percentage of TREM-1-positive CD14-positive monocytes was comparable to that of healthy adults (97.1 ± 8.3 vs. 97.5 ± 7.4). Exposure of cord blood to E. coli resulted in increased secretion of IL-6, IL-8, TNF-α, and sTREM-1. In contrast, the concentrations of IL-6, IL-8, and TNF-α decreased by a minimum of 15% when TREM-1 was blocked by LP17 then exposed to E. coli, versus E. coli alone. In addition, the concentration of sTREM-1 was positively correlated with the levels of TNF-α (r = 0.519, P < 0.05), IL-6 (r = 0.507, P < 0.05), and IL-8 (r = 0.538, P < 0.05). Conclusions: Healthy newborns exhibit expression of TREM-1 on monocytes similar to that in healthy adults, and most PMNs express TREM-1 at the newborn stage. Detection of sTREM-1 in neonatal peripheral blood should be further investigated as a potential method for the diagnosis of neonatal infection. Finally, blocking the TREM-1 signal transduction pathway may reduce inflammatory responses of neonate leukocytes and thereby provide a new strategy for treatment of neonatal infection.
Keywords: Triggering receptor expressed on myeloid cells-1, infant, leukocytes, sTREM-1
Introduction
Despite advances in medicine, neonates have high global rates of morbidity and mortality related to bacterial sepsis [1]. The immune response in sepsis is characterized by cytokine-mediated excessive inflammation leading to multiple organ failure and hemodynamic collapse [2]. Patients often present in dramatic fashion with high spiking fevers, shock, and respiratory failure that contributes to mortality [3]. Current treatments pursue rapid diagnosis and identification of the causative organism, with tailored antibiotic use and aggressive supportive care until resolution of symptoms [4]. In particular, the regulation of neutrophils and monocytes is important under inflammatory conditions because the release of cytotoxic substances can cause collateral tissue damage [5].
Triggering Receptor Expressed on Myeloid cells (TREM)-1 is related to immunoglobulin superfamily receptors and is expressed on neutrophils, mature monocytes, and macrophages. TREM-1 is pro-inflammatory, amplifying inflammation after exposure to extracellular fungal and bacterial pathogens [6]. The role of TREM-1 in myeloid cell activation and secretion of pro-inflammatory molecules has been established in a number of microbial diseases [7]. While TREM-1-amplified responses likely aid in improved detection and elimination of pathogens, excessive production of cytokines and oxygen radicals can also severely harm the host [8]. Experimental murine models of acute or chronic inflammatory conditions are beginning to show that blocking the TREM-1 signal transduction pathway confers survival advantages during experimental murine septic shock and protects from organ damage. Furthermore, the modulation of the TREM-1 signaling pathway preserves the capacity for microbial control, suggesting that TREM-l could become an ideal potential target for the treatment of sepsis [9].
Infants typically exhibit an immature innate immunity [10]. Although modulating TREM-1 expression may protect against death while maintaining effective bacterial clearance, little is known about its expression and functionality on neonatal peripheral blood leukocytes, particularly during bacterial infection. We hypothesized that the cytokine-mediated hyper-inflammatory phase in neonatal sepsis was associated with maturity of their TREM-1 system. To test this, the expression of TREM-1 was measured in leukocytes from cord blood of healthy neonates, and blood samples were subsequently exposed to Escherischia coli to model sepsis. Pro-inflammatory cytokine secretions in response to bacteria were measured. Additiona|lly, a short ex vivo treatment with the small synthetic TREM-1-specific peptide LP17 was tested as a potential inflammatory modulator. The results provide potential new avenues for the diagnosis and treatment of neonatal sepsis.
Materials and methods
Ethics statement
The study was approved by the Bioethics Committee of Binhai Hospital (EC/2013/002), and written, informed consent was obtained from both parents of newborns and all adult participants.
Study population
Newborns delivered at our hospital were included if they were full-term and had no clinical and laboratory signs of infection. Newborns were excluded for premature delivery, low birth weight, intrauterine growth restriction, or premature rupture of membranes or if the mother had suffered from chronic infectious, respiratory, or autoimmune diseases or cancer. Twenty newborns were enrolled in this study. Cord blood samples (15 mL) were collected into sterile heparinized tubes at birth. Twenty healthy adults (9 females, 11 males; ages 26-48 years) without signs of inflammatory disease were included as controls. Peripheral blood specimens (15 mL) from these adults were collected into sterile heparinized tubes.
Flow cytometry
Flow cytometry was performed according to the manufacturers’ protocols using a monoclonal FITC-labeled CD14 antibody (BD Bioscience, USA) and a PE-labeled TREM-1 antibody (R&D, USA). Equal volumes of antibodies for the relevant isotope control were used. Whole blood were incubated with the antibodies at 4°C for 30 min in the dark. Erythrocytes were lysed using FACS lysing solution (BD Biosciences, USA). After a 10-min incubation, the cells were washed once with an excess of phosphate-buffered saline (PBS). Cell sediment was suspended in PBS. Flow cytometry analysis was performed using a FACSCalibur low laser cytometer (Becton Dickinson, USA). CellQuestPro (BD Biosciences) was used, with equivalent settings for all analyses. A minimum of 10,000 cells was analyzed in every sample.
Quantitative real-time PCR
Leukocytes were isolated from blood according to reported methodology [11]. Briefly, 5 mL of whole blood was mixed with 4.5% dextran solution, and the mixture was allowed to stand for 40 min at room temperature. The leukocytes-rich plasma was centrifuged at 400×g on a Ficoll-Paque Plus gradient (Amersham Bioscience, Medison, WI, USA) for 20 minutes. To lyse erythrocytes, hypotonic (0.2%) saline was used, and osmolality was restored using hypertonic (1.6%) saline. Leukocytes were washed twice with PBS. Total RNA was extracted from leukocytes using the RNeasy Plus Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s instructions. The quantity and quality of the total RNA samples were determined using the Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). Total RNA was reverse-transcribed to cDNA using SuperScript III First-Strand Synthesis SuperMix for reverse transcription-polymerase chain reaction (RT-PCR) (Invitrogen Life Technologies, USA). Oligo (dT) 20 Primers and PCR Nucleotide Mix were added according to the manufacturer’s protocol.
TREM-1 mRNA (GenBank accession no. NM_018643.2) was quantified using the ABI7300 real-time PCR System (Applied Biosystems, USA). β-Actin (ACTB, GenBank accession no. NM_001101.1) was used as an internal control. cDNAs were amplified with SYBR Green using the Platinum SYBR Green qPCR SuperMix UDG (Invitrogen, Carlsbad, CA, USA). The primer sequences are listed in Table 1. The cDNA amplification program was as follows: 10 min at 95°C, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. The relative expression levels of TREM-1 in each sample were calculated by the comparative Ct method (2-ΔCt formula) after normalization to β-Actin using Sequence Detection System (SDS) software (Applied Biosystems) [11].
Table 1.
Primer sets for quantitative polymerase chain reaction
| Gene names | Sequence | Amplicon size (bp) |
|---|---|---|
| TREM-1 | F: 5’-GTCTCCACTCCTGACTCTGAA-3’ | 158 |
| R: 5’-TAGGGTACAAATGACCTCAGC-3’ | ||
| ACTB | F: 5’-TTAAGGAGAAGCTGTGCTACG-3’ | 205 |
| R: 5’-TTGAAGGTAGTTTCGTGGATG-3’ |
F, forward primer; R, reverse primer.
Whole blood stimulation
Gram-negative bacteria were flagellated Escherichia coli (E. coli) strain 0111:B4 (Nantong University, China). Bacteria were grown at 37°C overnight, centrifuged, and heat-killed (50 minutes at 70°C). Inactivation was verified by plating on LB agar to check for the absence of colonies [12]. For the experiments, E. coli were opsonized with fresh 30% human serum for 30 min at 37°C and washed once with 1 mL PBS before addition to the blood [13]. LP17 (Sigma L2515, St. Louis, USA) was chemically synthesized as described by Gibot [14]. The correct peptide (LQVTDSGLYRCVIYHPP) was obtained in > 99% yield and was endotoxin free. A scrambled peptide containing the same amino acids in a different sequence order was similarly synthesized and served as a control.
Fresh heparinized blood from participants was diluted 1:2 with RPMI (RPMI-Glutamax, Invitrogen, Life Technologies, Basel, Switzerland) and incubated with heat-killed bacteria (108 bacteria/mL) for 24 h or with 100 ng/mL LP17 then bacteria (LP17 incubated with blood for 2 hours before exposure). Cell-free supernatants were removed and stored at -80°C until assayed.
ELISA
Concentrations of IL-6, IL-8, TNF-α, and sTREM-1 secreted in culture media were measured by commercially available specific enzyme-linked immunosorbent assay (ELISA) kits (DuoSet, R&D Systems, Abingdon, UK). Optical absorbance was measured at 450 nm using a microplate reader (Epoch, BioTek, Luzern, Switzerland), with a wavelength correction set at 570 nm to subtract background. A standard curve was generated using a four-parameter logistic curve fit for each set of samples assayed.
Statistical analysis
The Shapiro-Wilk’s test was used for the normal distribution of quantitative variables, and parametric statistics were used throughout the study. Data are given as mean and standard deviation (Mean ± SD). T-test for independent samples and one-way analysis of variance (ANOVA) were used to analyze differences between groups. Correlations between quantitative variables were analyzed with Pearson’s correlation coefficient. P < 0.05 was considered statistically significant. All tests were two-sided. Analysis was performed with STATA 7.0 statistical package (Stata Corp., College Station, TX, USA).
Results
TREM-1 expression on leukocytes
Demographic and descriptive data of term newborns are shown in Table 2. To begin to compare the TREM-1 system between newborns and adults, receptor surface expression was assessed in newborns and adult by flow cytometry after gating subpopulations of circulating leukocytes (Figure 1). Mean fluorescence intensity (MFI) of TREM-1 on PMNs and monocytes of newborns appeared comparable to healthy adults (P > 0.05, Table 3).
Table 2.
Demographic and descriptive data (Term newborns, N = 20)
| Gender, female/male | 10/10 | Mode of delivery, cesarean section 3 (15%) | |
|---|---|---|---|
| Mother’s age, years | 27.8 ± 5.5 | White blood cells/mL | 12654 ± 2327.8 |
| APGAR score | 9.2 ± 0.4 | Monocytes/mL | 1689 ± 249.5 |
| Birth weight, g | 3623.7 ± 553.3 | Polymorphonuclear cells/mL | 5320 ± 1264.4 |
| Gestational age, weeks | 39.3 ± 0.8 | Platelets/mL | 305834 ± 97146 |
Figure 1.
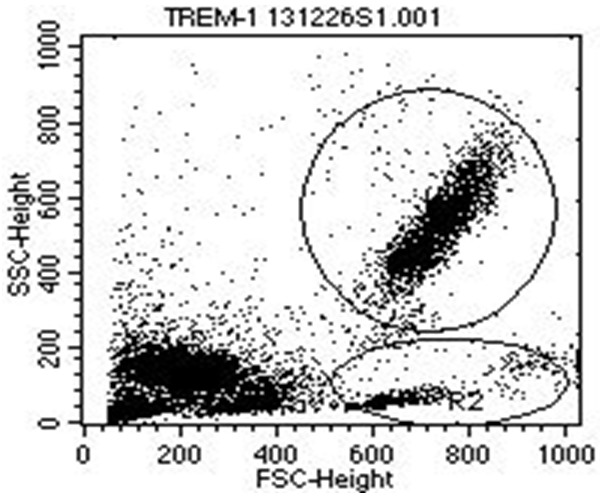
TREM-1 surface expression on leukocytes from term newborns and control adults. Representative flow cytometry plot showing forward and side-scatter characteristics with gating used to identify neutrophils (R1) and monocytes (R2).
Table 3.
TREM-1 expression on leukocytes of newborns and adult controls
| TREM-1 mean fluorescence intensity | ||||
|---|---|---|---|---|
|
|
||||
| Cell type | Newborns (N = 20) | Adults (N = 20) | t value | P value |
| PMNs | 32.9 ± 6.6 | 33.6 ± 5.8 | 0.356 | 0.724 |
| monocytes | 37.5 ± 6.7 | 36.6 ± 8.7 | 0.367 | 0.716 |
The percentage of TREM-1-positive PMNs was determined within both groups. Newborns exhibited a significantly lower percentage of TREM-1-positive PMNs than did adults (82.3 ± 7.1 vs 98.6 ± 4.8, t = 8.51, P < 0.001) (Figure 2).
Figure 2.
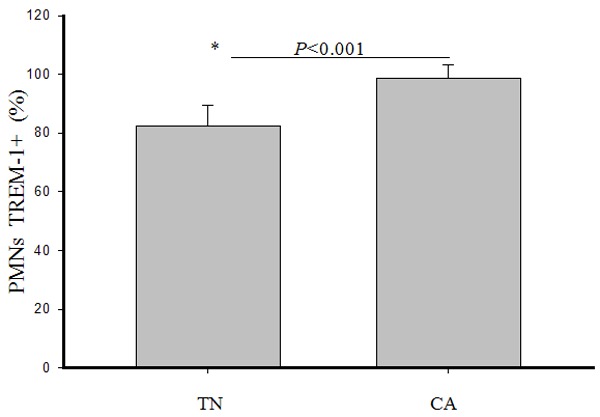
Percentage of TREM-1-positive PMNs from term newborns and control adults. Bars represent mean values ± standard errors of mean (SEM). P value determined following t-test. The asterisk represents a statistically significant difference between two groups.
CD14-positive monocyte populations were distinguished by size and granularity (Figure 3A) and by FITC-CD14 antibody (Figure 3B). The percentages of TREM-1-positive CD14+ monocytes were comparable between newborns and healthy adults (97.1 ± 8.3 vs 97.5 ± 7.4, t = 0.16, P = 0.87).
Figure 3.
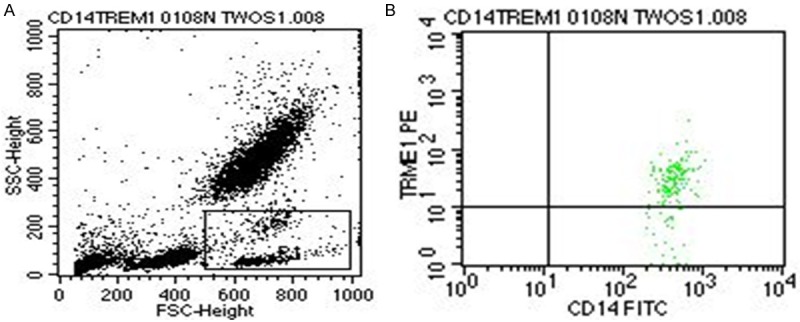
TREM-1-positive CD14+ monocytes from term newborns and control adults. A: Representative flow cytometry plot showing forward and side-scatter characteristics with gating used to identify monocytes (R1). B: TREM-1 positive CD14+ monocytes were distinguished by PE-TREM-1 and FITC-CD14 antibodies.
TREM-1 mRNA expression in leukocytes
Relative TREM-1 mRNA expression in leukocytes of term newborns was significantly lower than in control adults (1.16 ± 0.13 vs 1.63 ± 0.24, t = 7.7, P < 0.001) (Figure 4).
Figure 4.
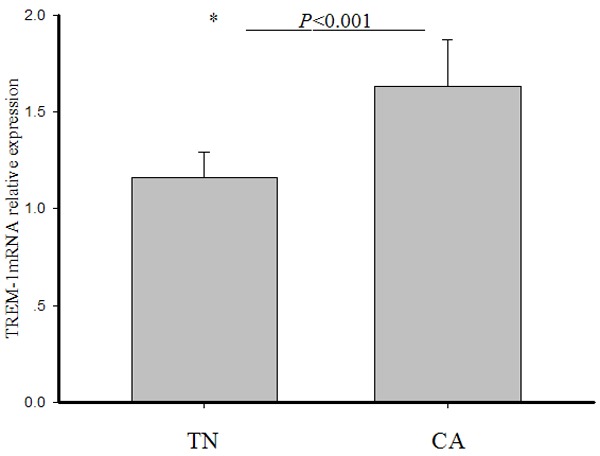
Relative mRNA expression of TREM-1 in leukocytes. Following qRT-PCR, the expression levels of TREM-1 in each sample were calculated by 2-ΔCt method. mRNA expression level was normalized to β-Actin. Bars represent mean values ± standard errors of mean (SEM). The statistical significance was determined using a t-test. The asterisk represents a statistically significant difference between the two groups.
Treatment of leukocytes from term newborns with LP17 to block TREM-1
To assess the functionality of TREM-1 in newborn leukocytes, a complementary approach was used. We investigated whether inflammatory cytokines secreted following exposure to E. coli could be blocked by adding the analogue synthetic peptide LP17, derived from the extracellular moiety of TREM-1. ELISA was used to measure the concentrations of secreted pro-inflammatory cytokines following stimulation of leukocytes with E. coli. E. coli exposure caused a significant increase in IL-8, TNF-α, and IL-6 secretion. In contrast, when leukocytes were first treated with 100 ng/mL LP17, which specifically targets TREM-1, the secretion of pro-inflammatory cytokines produced after E. coli exposure was attenuated (Table 4).
Table 4.
Leukocyte response to E. coli with or without LP17
| Group | TNF-α (ng/mL) | IL-6 (ng/mL) | IL-8 (pg/mL) |
|---|---|---|---|
| Control (n = 20) | 1.21 ± 0.23 | 0.92 ± 0.35 | 23.20 ± 3.12 |
| E. coli (n = 20) | 3.19 ± 0.23 | 12.4 ± 1.27 | 209.50 ± 21.32 |
| LP17 + E. coli (n = 20) | 2.58 ± 0.24 | 9.5 ± 1.09 | 178.20 ± 22.6 |
| scrambled peptide + E. coli (n = 20) | 3.18 ± 0.25 | 12.3 ± 1.41 | 212.62 ± 20.95 |
| F value/P value | 4.256/0.037 | 5.277/0.024 | 8.774/0.000 |
| P value (Control vs. E. coli) | 0.000 | 0.000 | 0.000 |
| P value (Control vs. LP17 + E. coli) | 0.002 | 0.003 | 0.004 |
| P value (E. coli vs. LP17 + E. coli) | 0.025 | 0.018 | 0.019 |
| P value (E. coli vs. scrambled peptide + E. coli) | 0.896 | 0.815 | 0.6433 |
Data shown as median ± SD. One-way ANOVA were used to analyze differences between three groups and Student-Newman-Keuls were used to analyze differences between two groups.
Effects of E. coli on sTREM-1 release by leukocytes
To determine if E. coli infection could elicit TREM-1 secretion from neonatal leukocytes, the leukocytes were infected with E. coli. After 24 h, the concentration of sTREM-1 in E. coli-exposed culture supernatants was significantly higher than in the unexposed control group (156.7 ± 36.3 pg/mL vs 34.6 ± 6.1 pg/mL, t = 13.6, P < 0.001) (Figure 5).
Figure 5.
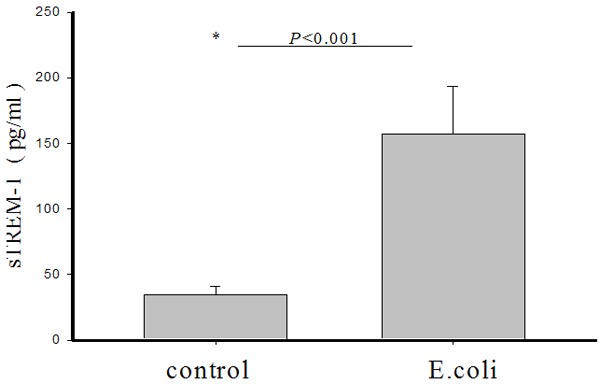
Induction of sTREM-1 secretion in leukocytes in response to E. coli exposure. Blood was exposed to E. coli. After 24 h, secretion of sTREM-1 by leukocytes into the culture supernatant was measured by ELISA. Bars represent mean values ± standard errors of mean (SEM). The asterisk represents a statistically significant difference between the E. coli-challenged and unchallenged control groups.
Interestingly, a significant correlation was found between sTREM-1 concentration and pro-inflammatory cytokine concentrations following E. coli exposure: sTREM-1 positively correlated with TNF-α (r = 0.519, P = 0.019, Figure 6A), IL-6 (r = 0.507, P = 0.022, Figure 6B) and IL-8 (r = 0.538, P = 0.014, Figure 6C).
Figure 6.
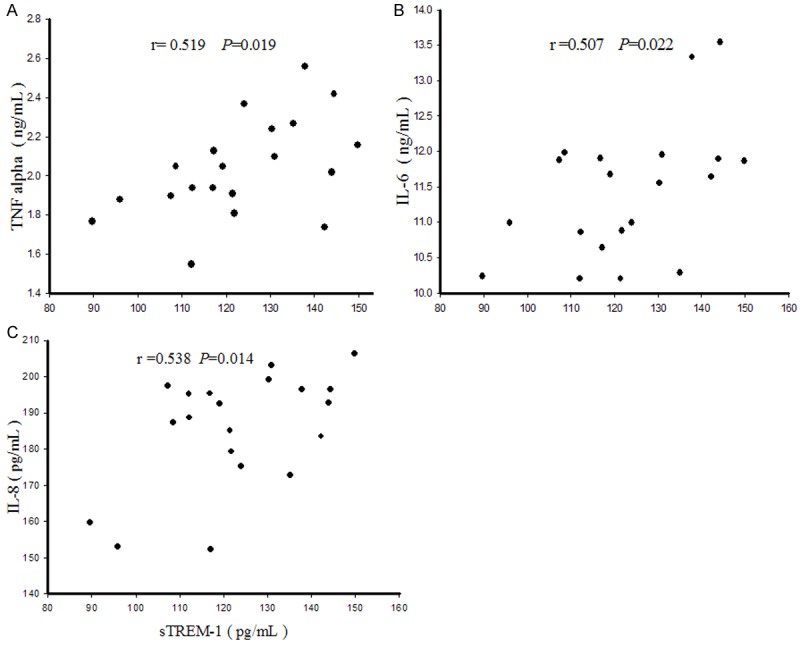
Correlation between sTREM-1 and cytokine concentrations. A: Positive correlation between TNF-α and sTREM-1 concentrations in culture media following E. coli exposure. B: Positive correlation between IL-6 and sTREM-1 concentrations in culture media following E. coli exposure. C: Positive correlation between IL-8 and sTREM-1 concentrations in culture media following E. coli exposure.
Discussion
Our study demonstrates that almost all monocytes from newborns expressed TREM-1, a finding that was consistent with that of healthy adults. Additionally, 80% of polymorphonuclear cells in newborns expressed TREM-1. Although this was slightly lower than in adults, TREM-1 expression has also been reported in leukocytes of premature infants [15] using peripheral blood samples taken from preterm newborns between days 5 and 25 after birth.
TREM-1 mRNA expression levels in neonatal leukocytes were significantly lower in infants than in adults. Several possibilities could account for this phenomenon, for example, that the sterile environment in utero may preclude activation of TREM-1, the newborn’s innate immunity is relatively immature. Further research is needed to identify the mechanism resulting in lower TREM-1 expression in infants.
Despite the slight immaturity of TREM-1, the precise role in inflammatory response to bacterial infection of TREM-1 in newborn leukocytes should be further evaluated. In particular, a TREM-l ligand has not yet been identified. Research has shown that a TREM-1 ligand is constitutively expressed on platelets and megacaryocytes. After lipopolysaccharide (LPS) stimulation, the TREM-1 ligand (expressed on platelets) mediates platelet-induced neutrophil activation, resulting in enhanced pro-inflammatory cytokine production [16]. One study showed that HMGB1 and HSP70 are TREM-1 ligands [17], but another reported that TREM-1 ligands exist on the surface of the pathogen [18].
Invading pathogens Bacterial antigen activates the innate immune system via pattern recognition receptors (PRRs) on leucocytes and epithelial cells. It has been shown that Toll-like receptors (TLRs) and Trem-1 seem to have a crucial role in immune cell activation in infection [19]. TLRs are activated via pathogen associated molecular pattern, namely LPS for gram negative and lipoteichoic acid (LTA) for gram positive bacteria, leading to an inflammatory cytokine response [20]. TREM-1 was an amplifier of the TLR4-mediated inflammatory response [17]. TREM-1 is up-regulated by TLR4 activation and induces pro-inflammatory cytokine, such as TNF-α, IL-1β, IL-8 and grain-macrophage colony stimulating factor (GM-csf), release, as has been shown in human and mouse model of sepsis [21]. We found that pro-inflammatory cytokine secretion (IL-8, IL-6, and TNF-α) was reduced when leukocytes were treated with the synthetic peptide LP17, which may act as a decoy receptor by blocking interaction of TREM-1 with its ligand [22].
Additionally, the enhanced cytokine secretion indicates that abnormal activation of TREM-1 signaling pathways occurs in neonatal sepsis. In mice, the engagement of TREM-1 with agonist monoclonal antibodies has been shown to stimulate the production of pro-inflammatory cytokines and chemokines such as TNF-α, IL-6, IL-l, GM-csf, IL-8, and monocyte chemotactic factor-l (MCP-1) [6]. Patients with E. coli-induced sepsis are characterized by an association of TREM-1 expression on blood neutrophils with cytokine inducibility [19]. These data indicate that the TREM-1 pathway on neutrophils might play a role in producing an adequate inflammatory and bactericidal response in bacterial sepsis.
To unambiguously investigate the role of TREM-1 in homeostasis and disease, Weber et al. generated mice deficient in Trem-1 [23]. In models of infection, Trem1-/- mice displayed significantly attenuated disease that was associated with reduced inflammatory infiltrates and diminished expression of pro-inflammatory cytokines, which contributed to reduced morbidity. Importantly, while immune-associated pathologies were significantly reduced, Trem1-/- mice were equally capable of controlling infections as wildtype controls. Various studies reported that blockade of TREM-1 in experimental murine infection decreased the pro-inflammatory reaction, and incomplete antibody blockade or inhibition of TREM-1 signaling reduced mortality without inhibiting bacterial clearance [9]. LP17 administration to septic mice resulted in a decreased plasma concentration of several pro-inflammatory cytokines. LP-17 treated animals were also protected against organ failure, hemodynamic disorders and finally against death [22]. These results indicate that therapeutic blocking of TREM-1 in distinct inflammatory disorders holds considerable promise by blunting excessive inflammation while preserving the capacity for microbial control. Therefore, TREM-1 has garnered interest recently as a modulator of the inflammatory response to bacterial pathogens in sepsis. Our finding of reduction in several pro-inflammatory cytokines when TREM-1 was blocked may provide a new strategy for treatment of neonatal infection.
Soluble TREM-1 (sTREM-1) is released following cleavage of the extracellular domain of TREM-1 [24]. Various studies have suggested that the concentrations of sTREM-1 in different biological fluids are significantly higher in patients with bacterial infection than in those with a non-microbial inflammatory process [25-30]. Further, serum sTREM-1 levels reflect the severity of sepsis more accurately than those of c-reactive protein (CRP) and procalcitonin (PCT) and are more sensitive for dynamic evaluations of sepsis prognosis; indeed, sTREM-1 levels and Sequential Organ Failure Assessment (SOFA) scores are positively correlated [31]. However, Su et al. [32] reported that, although sTREM-1 has high sensitivity and specificity in the diagnosis of infection, it is not better than that of PCT or CRP in predicting new infections. Thus, the clinical diagnostic value of sTREM-1 in infection remains to be determined. The current study also showed that sTREM-1 concentration increased significantly in culture supernatant after E. coli stimulation and was positively correlated with TNF-α, IL-8, and IL-6 levels. Therefore, detection of sTREM-1 in neonatal peripheral blood may provide a new method for the diagnosis of neonatal infection.
In summary, the TREM-1 expression system appears to be functional in newborn leukocytes and its stimulation results in secretion of inflammatory cytokines and sTREM-1. Blocking neonatal peripheral blood leukocyte TREM-1 resulted in reduced secretion of inflammation cytokines. Therefore, detection of neonatal peripheral blood sTREM-1 may provide a new method for the diagnosis of neonatal infection, and blocking TREM-1 may provide a new method for the treatment of neonatal infection.
Acknowledgements
This work was supported by a grant-in-aid from Nantong University.
References
- 1.Sanchez-Schmitz G, Levy O. Development of newborn and infant vaccines. Sci Transl Med. 2011;3:90ps27. doi: 10.1126/scitranslmed.3001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boomer JS, Green JM, Hotchkiss RS. The changing immune system in sepsis: Is individualized immuno-modulatory therapy the answer? Virulence. 2014;5:45–56. doi: 10.4161/viru.26516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palazzo SJ, Simpson T, Schnapp LM. Triggering Receptor Expressed on Myeloid Cells Type 1 as a Potential Therapeutic Target in Sepsis. Dimens Crit Care Nurs. 2012;31:1–6. doi: 10.1097/DCC.0b013e31823a5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 7.Bouchon A, Facchetti F, Weigand M, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 8.Arts RJW, Joosten LAB, van der Meer JWM. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukocyte Biol. 2013;93:209–215. doi: 10.1189/jlb.0312145. [DOI] [PubMed] [Google Scholar]
- 9.Derive M, Massin F, Gibot S. Triggering receptor expressed on myeloid cells-1 as a new therapeutic target during inflammatory diseases. Self/Nonself. 2010;1:225–230. doi: 10.4161/self.1.3.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tissières P, Ochoda A, Dunn-Siegrist I, Drifte G, Morales M, Pfister R, Berner M, Pugin J. Innate Immune Deficiency of Extremely Premature Neonates Can Be Reversed by Interferon-γ. PLoS One. 2012;7:e32863. doi: 10.1371/journal.pone.0032863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ubagai T, Tansho S, Ono RY. Evaluation of TREM1 Gene Expression in Circulating Polymorphonuclear Leukocytes and Its Inverse Correlation with the Severity of Pathophysiological Conditions in Patients with Acute Bacterial Infections. Jpn J Infect Dis. 2012;65:376–382. doi: 10.7883/yoken.65.376. [DOI] [PubMed] [Google Scholar]
- 12.Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood. 2007;109:1574–1583. doi: 10.1182/blood-2006-06-032961. [DOI] [PubMed] [Google Scholar]
- 13.Bostanci N, Thurnheer T, Aduse-Opoku J, Curtis MA, Zinkernagel AS, Belibasakis GN. Porphyromonas gingivalis regulates TREM-1 in Human polymorphonuclear neutrophils via its gingipains. PLoS One. 2013;8:e75784. doi: 10.1371/journal.pone.0075784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibot S, Kolopp-Sarda MN, Béné MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200:1419–1426. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garofoli F, Borghesi A, Mazzucchelli I. Preterm newborns are provided with triggering receptor expressed on myeloid cells-1. Int J Immunopathol Pharmacol. 2010;23:1297–301. doi: 10.1177/039463201002300439. [DOI] [PubMed] [Google Scholar]
- 16.Haselmayer P, Grosse-Hovest L, von Landenberg P, Schild H, Radsak MP. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood. 2007;110:1029–35. doi: 10.1182/blood-2007-01-069195. [DOI] [PubMed] [Google Scholar]
- 17.Sharif O, Knapp S. From expression to signaling: roles of TREM-l and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213:701–713. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Schenk M, Bouchon A, Seibold F, Mueller C. TREM-1-expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007;117:3097–3106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Bremen T, Drömann D, Luitjens K, Dodt C, Dalhoff K, Goldmann T, Schaaf B. Triggering receptor expressed on myeloid cells-1 (Trem-1) on blood neutrophils is associated with cytokine inducibility in human E. coli sepsis. Diagn Pathol. 2013;8:24. doi: 10.1186/1746-1596-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uehara A, Sato T, Iwashiro A, Yokota S. PR3-ANCA in Wegener’s granulomatosis prime human mononuclear cells for enhanced activation via TLRs and NOD1/2. Diagn Pathol. 2009;4:23. doi: 10.1186/1746-1596-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibot S, Alauzet C, Massin F, Sennoune N, Faure GC, Béné MC, Lozniewski A, Bollaert PE, Lévy B. Modulation of the Triggering Receptor Expressed on Myeloid Cells-1 Pathway during Pneumonia in Rats. J Infect Dis. 2006;194:975–83. doi: 10.1086/506950. [DOI] [PubMed] [Google Scholar]
- 23.Weber B, Schuster S, Zysset D, Rihs S, Dickgreber N, Schürch C, Riether C, Siegrist M, Schneider C, Pawelski H, Gurzeler U, Ziltener P, Genitsch V, Tacchini-Cottier F, Ochsenbein A, Hofstetter W, Kopf M, Kaufmann T, Oxenius A, Reith W, Saurer L, Mueller C. TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance. PLoS Pathog. 2014;10:e1003900. doi: 10.1371/journal.ppat.1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gómez-Piña V, Soares-Schanoski A, Rodríguez-Rojas A, Del Fresno C, García F, Vallejo-Cremades MT, Fernández-Ruiz I, Arnalich F, Fuentes-Prior P, López-Collazo E. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol. 2007;179:4065–73. doi: 10.4049/jimmunol.179.6.4065. [DOI] [PubMed] [Google Scholar]
- 25.Gibot S, Kolopp-Sarda MN, Béné MC, Cravoisy A, Levy B, Faure GC, Bollaert PE. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004;141:9–15. doi: 10.7326/0003-4819-141-1-200407060-00009. [DOI] [PubMed] [Google Scholar]
- 26.Gibot S, Cravoisy A, Dupays R, Barraud D, Nace L, Levy B, Bollaert PE. Combined measurement of procalcitonin and soluble TREM-1 in the diagnosis of nosocomial sepsis. Scand J Infect Dis. 2007;39:604–608. doi: 10.1080/00365540701199832. [DOI] [PubMed] [Google Scholar]
- 27.Determann RM, Millo JL, Gibot S, Korevaar JC, Vroom MB, van der Poll T, Garrard CS, Schultz MJ. Serial changes in soluble triggering receptor expressed on myeloid cells in the lung during development of ventilator-associated pneumonia. Intensive Care Med. 2005;31:1495–1500. doi: 10.1007/s00134-005-2818-7. [DOI] [PubMed] [Google Scholar]
- 28.El Solh AA, Akinnusi ME, Peter M, Berim I, Schultz MJ, Pineda L. Triggering receptors expressed on myeloid cells in pulmonary aspiration syndromes. Intensive Care Med. 2008;34:1012–1019. doi: 10.1007/s00134-008-1087-7. [DOI] [PubMed] [Google Scholar]
- 29.Jeong SJ, Song YG, Kim CO, Kim HW, Ku NS, Han SH, Choi JY, Kim JM. Measurement of plasma sTREM-1 in patients with severe sepsis receiving early goal-directed therapy and evaluation of its usefulness. Shock. 2012;37:574–578. doi: 10.1097/SHK.0b013e318250da40. [DOI] [PubMed] [Google Scholar]
- 30.Dimopoulou I, Pelekanou A, Mavrou I, Savva A, Tzanela M, Kotsaki A, Kardara M, Orfanos SE, Kotanidou A, Giamarellos-Bourboulis EJ. Early serum levels of soluble triggering receptor expressed on myeloid cells-1 in septic patients: correlation with monocyte gene expression. J Crit Care. 2012;27:294–300. doi: 10.1016/j.jcrc.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, She D, Feng D, Jia Y, Xie L. Dynamic changes of serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1)reflect sepsis severity and can predict prognosis: a prospective study. BMC Infect Dis. 2011;11:53. doi: 10.1186/1471-2334-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su L, Han B, Liu C, Liang L, Jiang Z, Deng J, Yan P, Jia Y, Feng D, Xie L. Value of soluble TREM-1, procalcitonin, and C-creative protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infect Dis. 2012;12:157. doi: 10.1186/1471-2334-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]


