Abstract
In our previous study using iTRAQ technique we found that the level of calmodulin-dependent protein kinase 2b (Camk2b) was lower in rats with hyperhomocysteinemia. We presumed that Camk2b might be involved in homocysteine-induced apoptosis and tried to explore its role in this study through the transfection with Camk2b gene. Results showed that neurons of HHcy group had lower activity measured by MTT, higher percentage of apoptotic neurons, lower expression levels of Camk2b mRNA and protein than those in normal group. Neurons with overexpression of Camk2b (Camk2b group) had lower percentage of apoptosis and higher activity than those in control group. After exposure to 2-Methoxyestradiol, the activity of neurons with overexpression of Camk2b was suppressed with more apoptotic cells observed. The expressions of BCL2, eNOS, EP300 and EPO were all elevated at both mRNA and protein levels in neurons of CamK2b group compared with other three groups. Thus, Camk2b protects neurons from Homocysteine-induced apoptosis with the involvement of HIF-1α signal pathway.
Keywords: Homocysteine, hippocampal neurons, apoptosis, Camk2b, HIF-1α
Introduction
Homocysteine (Hcy) is a sulfur-containing and non-proteinogenic amino acid which is produced during the conversion of methionine to cystine. Normally the concentration of Hcy is very low and doesn’t exceed 14 μM [1]. However, sometimes, the deficiency of vitamins B6, B12, folate and other defects in methyl group metabolism could lead to abnormal elevation of HCY level, i.e., hyperhomocysteinemia (HHcy). HHcy is a major cardiovascular risk factor, which involves in various vascular diseases including atherosclerosis and small vessel disease via complex mechanisms [2]. Besides, HHcy might also be associated with neurological abnormalities too, such as Alzheimer’s disease, seizures and epilepsy [3,4]. Now Hcy is regarded as a neurotoxic metabolite.
Since Hcy was first reported to be associated with Alzheimer’s disease, more and more studies were designed to explore the mechanism of Hcy-related neurotoxicity every year. Accumulating evidences showed that an elevated Hcy level might impair the calcium metabolism and cytoskeleton, and then lead to neural apoptosis especially the apoptosis of hippocampus neurons [5-7]. On the other side, studies about the protective agents against Hcy-related neurotoxicity are still relatively rare. In our previous study, using isobaric Tags for Relative and Absolute Quantitation (iTRAQ) technique, we detected series of proteins in the hippocampus of rats with homocysteinemia. The results showed that calmodulin-dependent protein kinase 2b (Camk2b) as a highly conserved serine/threonine kinase was obviously down-regulated in homocysteinemia rats than normal rats. Camk2b is a highly conserved serine/threonine kinase with various physiological functions in different tissues. In brains Camk2b might be associated with the neuritogenesis and myelination [8,9], with the specific mechanism not clearly elucidated yet. The results of iTRAQ suggested that Camk2b might be involved in HCY-related neurotoxicity. Besides, it also inspired us to wonder whether a normal or even higher Camk2b level in homocysteinemia could inhibit HCY-induced neural apoptosis. In order to answer this question, in this study, we transfected Camk2b gene in hippocampal neurons, and tried to explore the role of Camk2b in Hcy-induced neuron apoptosis at both genomic and proteomic levels in hippocampal neurons and the corresponding mechanisms.
Materials and methods
Hippocampus cell cultures
The procedures of rat primary hippocampus neuron cultures were just as described in our previous paper. The brains were removed from newborn mice (within 24 hours). Hippocampi were isolated, disaggregated, and then digested in Petri dishes by 0.25 tripsin at 37°C for 5-7 min. The hippocampus fragments were then collected in tubes with 10 ml DMEM medium and then mechanically disaggregated into cell suspension by a Pasture Pipette. The cell suspension was diluted with culture medium and then inoculated into Petri dishes, which were preconditioned with polylysine. Besides, L-Homocysteine (Sigma, St. Louis, MO) was added into cultures and diluted into 1 mmol/L for part of the neurons, named as HHcy group. The left neurons without exposure to HHcy were assigned to Normal group. The final cell density was 2-2.5×105/cm2.
All animal experiments were approved by the Animal Research Committee at Shanghai Tongji University and were carried out in accordance with established International Guiding Principles for Animal Research. This study was also approved by the Science and Technology Commission of Shanghai Municipality (ID: SYXK 2007-0006) with the permit number 2011-RES1.
Identification of hippocampal neurons
The hippocampal neurons used for identification were incubated for 8 days. Cells were washed by 0.1 mol/LPBS (PH7.4) for two times and then fixed by 4% paraformaldehyde for 30 min. Fixed cells were embathed in 0.01 mol/LPBST (PH7.4) for 5 min, three times in all and then oxidized by 0.3% H2O2 (diluted by 80% Methanol) for 10 min. Cells were washed by PBST for 5 min, three times in all, incubated with normal goat serum for 30 min and then incubated overnight at 4°C with anti-NSE antibody (1:100). The specimen was incubated with the appropriate peroxidase-conjugated second antibody for 90 min at room temperature. After being washed by PBST for 5 min, three times in all, the specimen was incubated with streptavidin (labeled by HRP) for 90 min at room temperature. After that, specimen was developed by 0.05% DAB-0.03% H2O2, mounted by glycerin gelatin and observed in inverted phase contrast microscope.
Camk2b gene transfection
Part of the hippocampal neurons exposed to Hcy was transfected with Camk2b gene (Camk2b group). First, we retrieved the human Camk2b gene sequence from GeneBank (NM_152997) and then designed the following primers: Camk2b -F CGTTTCACCGACGAGTACCAG and Camk2b -R GCGTACAATGTTGGAATGCTTC (Jierui Bio Co., China). Restriction endonucleases Age I (NEB, USA) and BamH I (NEB, USA) were used to digest the pGC-FU-3FLAG-IRES-Puromycin vector (GENECHEM Co., Shanghai, China). After that, the Camk2b insert and linearised plasmid were joined by T4-DNA ligase (NEB, USA) to construct pGC-FU-Camk2b. The recombinant pGC-FU-Camk2b was then characterized by restriction endonuclease digest, PCR and sequencing analysis. Lentiviral vector containing Camk2b was transfected into the packaging cells 293T (GENECHEM Co., Shanghai, China) to obtain high-level lentiviral particles packed in culture supernatant. After that, 2×105 hPDLCs were incubated in a 25 cm2 culture flask with 1:1 mix of viral supernatant: growth medium with 5 μg/ml polybrene (Sigma) for 10 h. After transfection, the cells were cultured in normal DMEM medium containing 10% FBS for 96 h. Camk2b expression levels were detected by RT-PCR and western blot assay. In addition, for some other neurons exposed to HHcy, empty plasmid without Camk2b gene were transfected following the same steps, and were assigned to Control group.
MTT assay
Neurons were seeded into 96-well plates (cultured overnight for adherent cells), and then incubated with DMEM-h with or without L-Homocysteine (1 mmol/L); part of the neurons exposed to HHcy meanwhile transfected with Camk2b gene were exposed to 2-Methoxyestradiol (25 μmol/l) which is an inhibitor of HIF1α, and were named as Inhibitor group. After 12, 24, 36, 48 or 72-hour incubation, 20 μl MTT (5 mg/ml, Sigma, America) was added into each well for another 4-hour incubation. After that, the supernatant was removed and 150 μl DMSO was added into each well in order to solubilize the blue-purple crystals of formazan. The absorbance was then measured using a model ELX800 Micro Plate Reader (Bio-Tec Instruments, Inc.) at 490 nm.
Western blot assay
Neurons were seeded into 6-well plates, washed twice with PBS solution, lysed with RIPA Lysis Buffer (Beyotime Institune of Biotechnology, Shanghai, China) and protease inhibitor (Thermo scientific). Protein concentrations were determined with Pierce BCA Protein Assay Kit (Thermo Scientific). Equivalent amounts of total protein (50 μg) were boiled and electrophoretically separated on a 10% polyacrylamide gel at 80 volts. The proteins were transferred to a nitrocellulose filter membrane. Membranes were blocked for 60 min with 5% milk solutions prepared in PBS, incubated overnight at 4°C with 1:1000 dilutions of the primary antibodies (Camk2b, BCL-2, eNOS, EPO and GAPDH), washed three times for 10 min each time with Tween 20 (1:1000 dilution)-PBS, incubated for 1 hour with the appropriate peroxidase-conjugated secondary antibody (1:1000 dilution). Membranes were washed with Tween 20-PBS three times for 10 min each and were developed using the Odyssey two-color infraed laser imaging system. The signal generated by GAPDH was used as an internal control.
Tunnel assay
Neurons in Normal group, HHcy group, Camk2b group, Control group and Inhibitor group were seeded on poly-D-lysine-coated coverslips for 48 h. After that they were fixed in 4% PFA, post-fixed in EtOH/acetic acid for 5 min at -20°C and maintained in equilibration buffer for 1 min. They were incubated with the TdT enzyme in a reaction buffer for 1 h at 37°C (Apoptosis detection kit, Chemicon, Millipore Ibe´rica, Madrid, Spain). After washing, the coverslips were incubated with the anti-DIG antibody in blocking solution and counterstained with DAPI. The data represent TUNEL + cells as a percentage of the total number of cells.
Reverse transcription-polymerase chain reaction (RT-PCR) and real-time PCR
Total RNA was extracted and first-strand cDNA was synthesized using the Omniscript RT kit (Qiagen), with 2000 ng of RNA (per 20-μl reaction) and oligo (dT) primers. cDNA was then utilized in real-time PCR reactions to analyze BCL-2, CAMK2b, eNOS, EP300, EPO, Hif1a, VEGF, P21 (CDKN1a) and GAPDH expression. PCR reactions were amplified for 40 cycles. Each cycle consisted of denaturation for 1 min at 94°C, annealing for 1 min at 60°C, and polymerization for 2 min at 72°C. PCR products were quantified using the Molecular Analyst software (Bio-Rad). The ratio of each gene against β-actin was calculated by standardizing the ratio of each control to the unit value.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (La Jolla, CA, USA). All measurement data were expressed as mean ± standard deviation (SD), and compared between two groups using Student’s t test. P < 0.05 was considered statistically significant.
Results
HCY suppresses the cellular activity of hippocampal neurons
The activity of hippocampal neurons was suppressed by the exposure to HCY (1000 μM). The living neurons were obviously less when they were exposed to HCY than those without additional HCY added into the cultures, as shown by the photographs of inverted phase contrast microscope (Figure 1B, 1C) and the results of MTT assay (Figure 1D).
Figure 1.
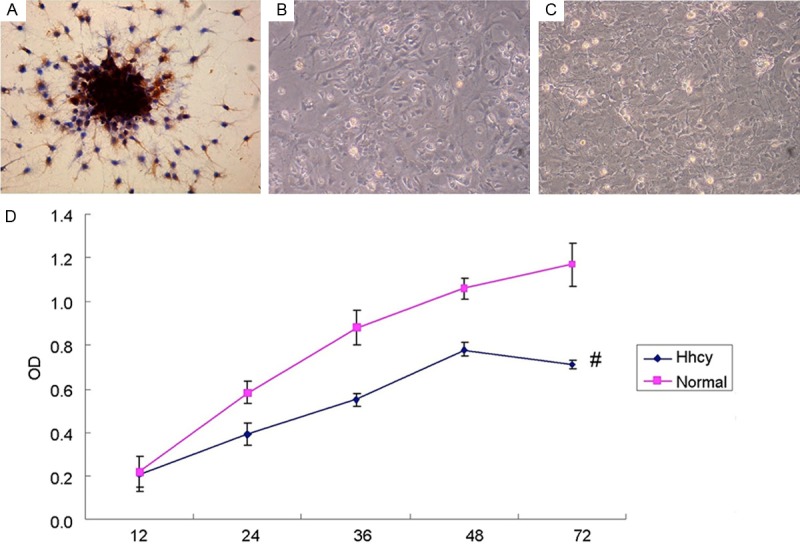
HCY suppresses the activity of cultured hippocampal neurons. A: The results of NSE immunohistochemical staining, in which the NSE immunoreactions complexes were shown as brown and in granulate. The positive NSE granulates were distributed in the cytoplasm and major nerve process, with the glia unstained. B: 1×105 neurons seeded into 6-well plates, incubated with HHcy (1000 μM) for 72 h and then photographed by inverted phase contrast microscope. C: Neurons incubated without HHcy, photographed by inverted phase contrast microscope. Compared with B1, the amount of neurons in B2 was obviously larger. D: The results of MTT Assay for the activity of neurons with or without Hhcy (normal group and Hhcy group) in 12, 24, 36, 48 and 72 hours. The activity of normal neurons in 72 h was higher than that of Hhcy group. #P < 0.05 VS normal group.
HCY increases the apoptosis of hippocampal neurons
The apoptotic neurons were obvious more when the neurons were opposed to HCY than the normal group, as shown in the results of Tunel Assay, which showed more neurons with cytoplasm stained in red in Hhcy group than normal group (Figure 2A). The percentage of apoptotic neurons was much higher in group Hhcy than normal group (P < 0.01) (Figure 2B).
Figure 2.
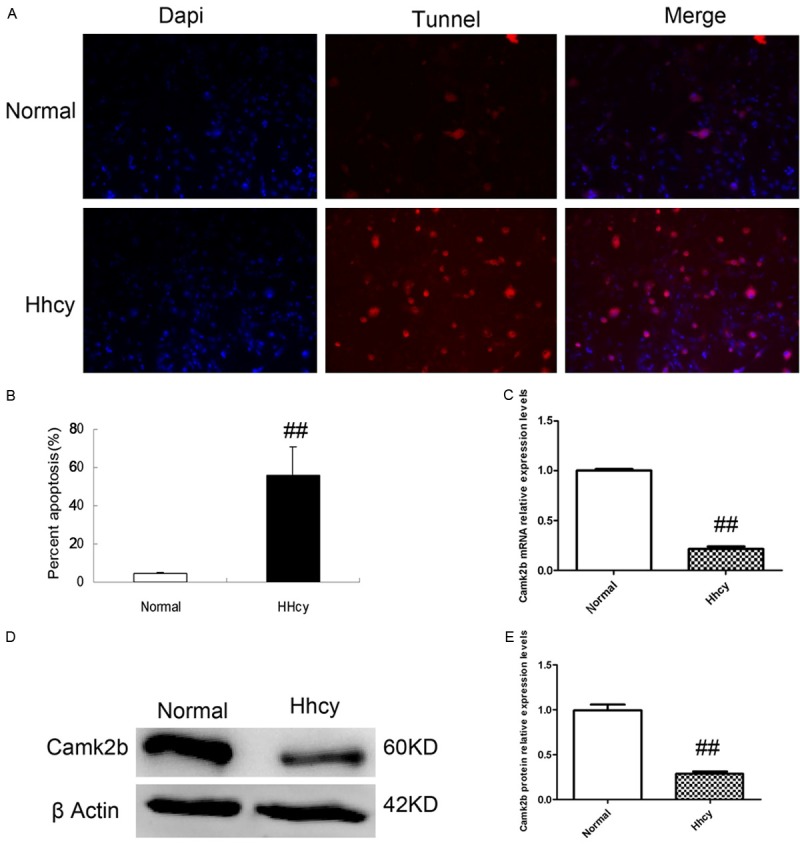
HCY increases the apoptosis of neurons and suppresses the expression of Camk2b. A, B: The results of Tunel Assay for neurons in normal and Hhcy groups. Note that the neurons with cytoplasm in red (TUNNEL +) which indicates apoptotic neurons were obviously more in Hhcy group than those in normal group, with the percentage of apoptotic neurons much higher in Hhcy group. C: The result of RT-PCR for the CamK2b mRNA in both normal and Hhcy groups. Compared with normal group, the transcription level of Camk2b mRNA in neurons exposed to Hhcy was obviously lower. D: CamK2b protein in both normal and Hhcy groups were measured by Western Blot which showed that the expression of CamK2b protein in Hhcy group was suppressed. E: CamK2b protein relative expression levels based on the results of Western Blot. ##P < 0.01 VS normal group.
HCY suppresses the expression of Camk2b
We detected the expression of Camk2b in hippocampal neurons in both mRNA and protein levels with RT-PCR and Western Blot respectively. The results showed that in Hhcy group, the expression level of Camk2b in neurons was obviously lower than that in normal group (P < 0.01) (Figure 2C). Besides, the expression of Camk2b protein was also suppressed in Hhcy group as shown in the results of Western Blot (Figure 2D, 2E).
Camk2b protects hippocampal neurons from HCY-induced apoptosis
After transfection of Camk2b successfully, the expression level of Camk2b mRNA was elevated compared with those transfected with empty plasmid (control group) as shown by the results of RT-PCR (Figure 3A), with the expression of Camk2b protein elevated too, as shown by the results of Western Blot (Figure 3B, 3C).
Figure 3.
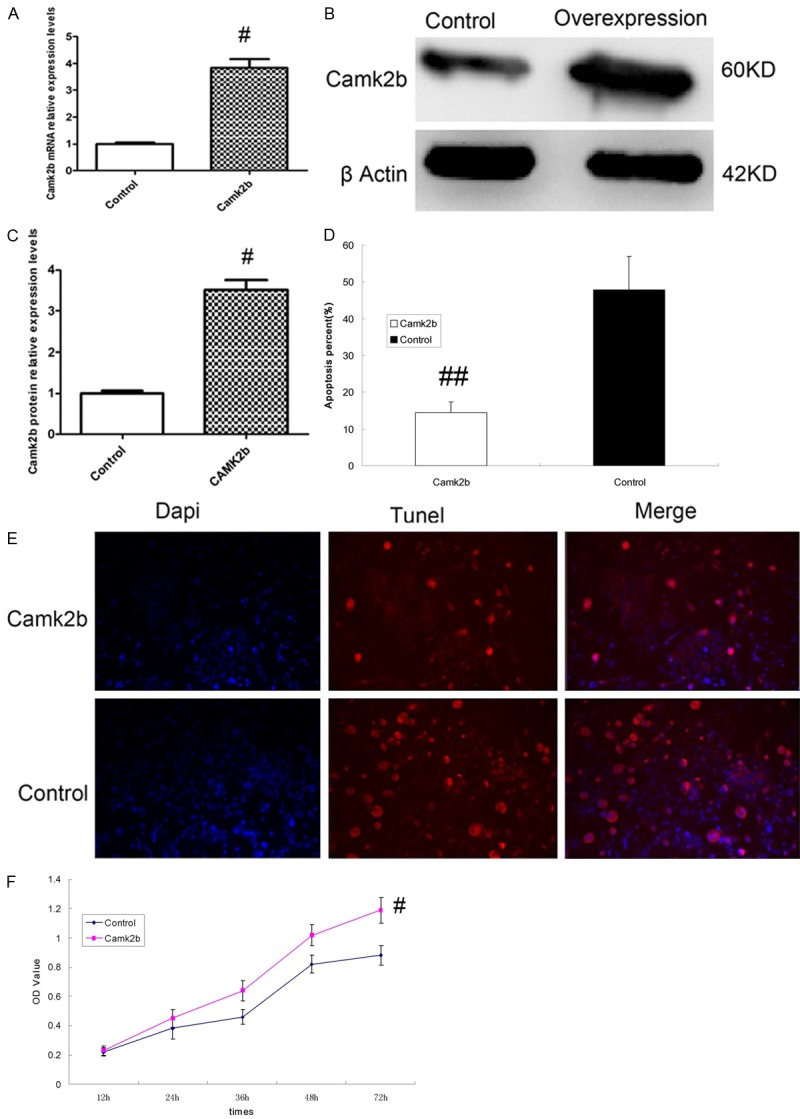
Camk2b protects hippocampal neurons from HCY-induced apoptosis. A: Result of RT-PCR for CamK2b mRNA in neurons transfected with CamK2b (Camk2b group) or empty plasmid (control group), which showed a higher transcription level of CamK2b mRNA in Camk2b group than that in control group. B: Result of Western Blot for Camk2b protein in both Camk2b (the right column) and control (the left column) groups, which showed the overexpression of Camk2b protein in Camk2b group. C: Camk2b protein relative expression levels based on the results of Western Blot. D, E: The results of Tunnel Assay for the apoptosis of hippocampal neurons in both Camk2b and control groups, which showed less apoptotic neurons with cytoplasm in red (TUNEL +) and lower percentage of apoptotic neurons in Camk2b group than that in control group. F: The results of MTT Assay for the activity of neurons in both Camk2b and control groups, which showed that neurons in Camk2b group had higher activity than those in control group. #P < 0.05, ##P < 0.01 VS control group.
The apoptosis percentage of neurons transfected with Camk2b was obviously lower than control group (P < 0.01), as shown by the results of Tunel Assay which displayed fewer apoptotic neurons with cytoplasm stained in red (Figure 3D, 3E). Besides, the results of MTT Assay also showed that neurons transfected with Camk2b had higher activity than those transfected with empty plasmid (P < 0.05) (Figure 3F).
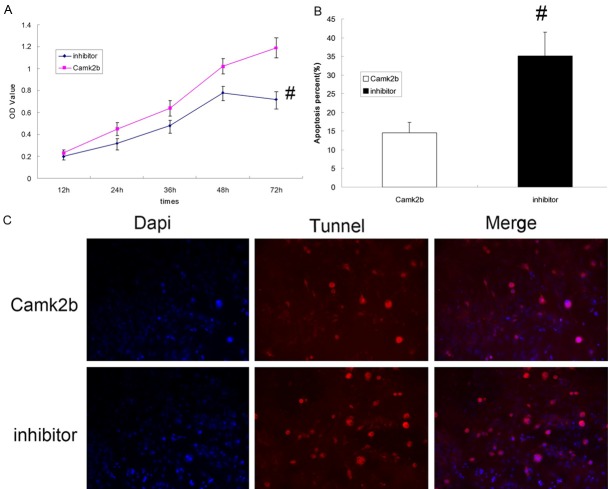
The inhibitor of HIF-1α increases the neural apoptosis in neurons transfected with Camk2b
After 2-Methoxyestradiol as a specific inhibitor of HIF-1α was added, the activity of hippocampal neurons which were already transfected with Camk2b was suppressed (P < 0.05) (Figure 4A). The results of Tunel Assay also showed that the percentage of apoptotic neurons was higher in inhibitor group (P < 0.05) (Figure 4B, 4C). The protective effect of Camk2b against HCY-induced neural apoptosis was weakened by 2-Methoxyestradiol.
Figure 4.
The inhibitor of HIF-1α increases the neural apoptosis in neurons transfected with Camk2b. A: The results of MTT Assay for the activity of neurons with or without exposure to 2-Methoxyestradiol (inhibitor group, Camk2b group) after transfected with Camk2b, which showed a higher activity of neurons in Camk2b group than that in inhibitor group. B, C: The results of Tunel Assay for the apoptosis in both inhibitor and Camk2b groups. Note that in inhibitor group the apoptotic neurons (TUNEL +) were more than those in Camk2b group. #P < 0.05 VS Camk2b group.
Expression levels of HIF-1α related co-activator and downstream elements were elevated after the transfection of CamK2b
We made a horizontal comparison among four groups (normal group, HHcy group, Camk2b group, control group) about the expression levels of HIF-1α related elements with RT-PCR and Western Blot respectively. The results showed that the expressions of BCL2, eNOS, EP300 and EPO were all elevated at both mRNA and protein levels in neurons of CamK2b group compared with other three groups. Besides, the expression levels of VEGF and P21 mRNA were also elevated in Camk2b group than HHcy and control groups, but the results of Western Blot showed no obvious difference of the protein expression among four groups (Figure 5A, 5B).
Figure 5.
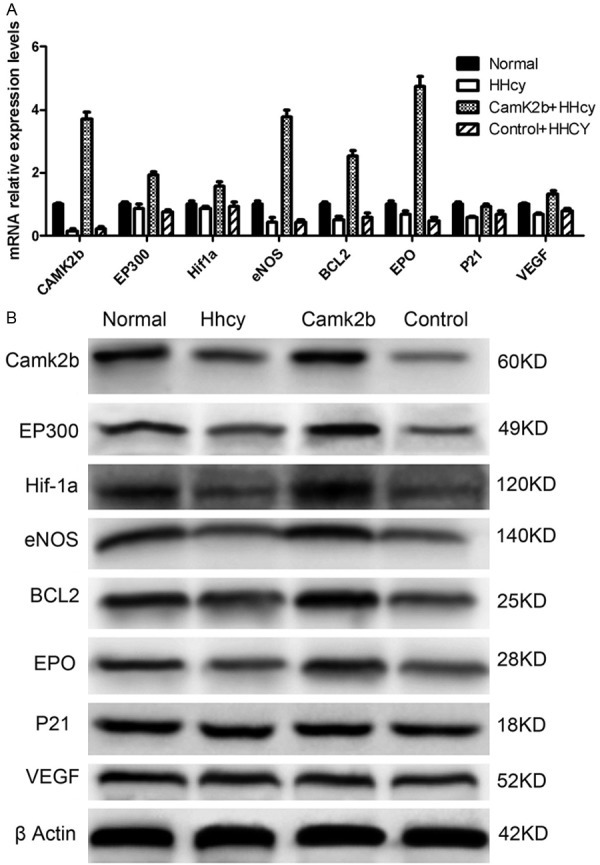
The expression levels of HIF-1α related co-activator and downstream elements were elevated after the transfection of CamK2b. A: The mRNA relative expression levels of Bcl2, Camk2b, eNOS, EP300, EPO, HIF-1α, VEGF and P21 in four groups (Normal: neurons without exposure to Hhcy, HHcy: neurons exposed to HHcy, Camk2b + HHcy: neurons exposed to HHcy and transfected with Camk2b, Control + HHcy: neurons exposed to HHcy and transfected with empty plasmid) measured by RT-PCR. Note that the transcription levels of Bcl2, eNOS, EP300 and EPO were all the highest in Camk2b group than other three groups. B: The expression levels of the corresponding proteins in four groups measured by Western Blot, which showed that the expression levels of EP300, eNOS, Bcl2 and EPO were all higher in Camk2b group than those in HHcy and control groups.
Discussion
In this study, the results indicated that HCY could inhibit the neuron activity and increase the apoptosis of neurons. The level of Camk2b was reduced in cells with homocysteinemia. After the transduction of Camk2b gene into neurons, the cellular activity was elevated while the apoptosis was inhibited. Thus it suggested that Camk2b could protect neurons from the HCY-induced apoptosis. And the specific mechanism of this protection might be related to the pathway of HIF-1α.
The apoptosis induced by HCY has been reported in plenty of studies [10-12] and was verified again in this study by both MTT and Tunel Assay which showed a low level of neural activity and more apoptotic neurons with HHcy. The specific mechanism of HCY-induced neural apoptosis is complex and might be related to the abnormal Ca2+ level and Ca2+ influx induced by the over-stimulation of N-methyl-D-aspartate (NMDA) receptors which then lead to the impairment of mitochondrial dynamics in neurons [6,7,13,14]. Nitric Oxide [15] and oxidative stress [16] might also be related to HCY related neurotoxicity too, as demonstrated in plenty of studies. On the other hand, few studies focused on the neuroprotective factors which could increase the impairment caused by HCY. In this study, based on the transduction of Camk2b gene, we found that Camk2b might just be a possible neuroprotective factor.
We further explored the possible mechanism of Camk2b related neuroprotective effect. Actually, Camk2b might be involved in various signal pathways and associated with neuritogenesis, myelination, the maturation of synapse, and plays an important role in different physiological activities including learning, memory [8,9,17,18], which could also be searched on KEGG (www.genome.jp). After searching we focused on HIF-1 signal pathway which mediates a broad, endogenous adaptive program to hypoxia and consists of a series of neuroprotective components [19,20]. In this study, after we added 2-Methoxyestradiol which is a specific inhibitor of HIF-1α [21] into the cultures, the cellular activity which was elevated by Camk2b was then decreased. Besides, the results of RT-PCR and Western Blot showed that some of the HIF-1 related proteins and the corresponding genes including HIF-1α itself were elevated in neurons transfected with Camk2b. All above just suggested that the neuroprotective effect of Camk2b might be related the HIF-1 pathway, or even functions via HIF-1 signal pathway.
Specifically, BCL2, eNOS, EP300, EPO were all elevated in both gene and protein levels. Together with CBP (or known as CREB binding protein), EP300 functions as the co-activator of HIF-1α [22,23], and could elicit the transcriptional activity and make the HIF-1α more stable, differently with the other three factors mentioned above which are all the downstream factors of HIF-1. The results of this study suggested that Camk2b might activate the expression of EP300, and then activate the other components of HIF-1 signal pathways including BCL2, eNOS and EPO. BCL2 as an anti-apoptotic gene is known to be an important inhibitor of apoptosis in various cells including neurons [24]. The activation of BCL2 could apparently reduce the apoptosis of hippocampal neurons induced by HCY. eNOS has been proved to confer protective effects during cerebral ischemia and other harmful conditions by various studies [25,26]. In homocysteinemia, it might consume nitric oxide and reduce HCY related neurotoxicity. EPO was reported to have both neuroprotective and neuroregenerative functions in various tissues including the nervous system with complex mechanisms [27,28]. Some studies demonstrated that EPO could prevent glutamate and NMDA-mediated excitotoxic brain injury [29], which was just one of the main mechanisms of HCY-induced neural apoptosis. Thus EPO could be a neuroprotector against HCY-induced neural apoptosis. Anyway, these factors may function together after the transfection of Camk2b, and then confer neuroprotective effect and reduce HCY-induced neural apoptosis.
In conclusion, this study demonstrated that Camk2b could be neuroprotective against HCY-induced neural apoptosis. The activation of HIF-1α signal pathway especially the activation of BCL2, eNOS and EPO might be involved in the mechanism for its protective effect. This was the first report about the role of Camk2b in HCY-induced neural apoptosis. This was the first report about the role of Camk2b in HCY-induced neural apoptosis, and the results suggested a potential therapeutic target for therapies against HCY-related diseases in central nervous system especially neurodegenerative diseases, i.e., reducing the neural apoptosis via the administration of Camk2b and its related effective factors. In the future, more studies are still required to explore the specific mechanism of HCY-induced neural apoptosis and the corresponding neuroprotective therapies.
Acknowledgements
This work was supported by grants from the Scientific Program of Science and Technology Committee of Shanghai (No. 11411950303) and National Natural Science Foundation of China (No. 81000492, No. 30971029, No. 81171163).
Disclosure of conflict of interest
None.
References
- 1.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 2.Feng C, Bai X, Xu Y, Hua T, Huang J, Liu XY. Hyperhomocysteinemia associates with small vessel disease more closely than large vessel disease. Int J Med Sci. 2013;10:408–412. doi: 10.7150/ijms.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippi G, Plebani M. Hyperhomocysteinemia in health and disease: where we are now, and where do we go from here? Clin Chem Lab Med. 2012;50:2075–2080. doi: 10.1515/cclm-2012-0372. [DOI] [PubMed] [Google Scholar]
- 4.Farkas M, Keskitalo S, Smith DE, Bain N, Semmler A, Ineichen B, Smulders Y, Blom H, Kulic L, Linnebank M. Hyperhomocysteinemia in Alzheimer’s disease: the hen and the egg? J Alzheimers Dis. 2013;33:1097–1104. doi: 10.3233/JAD-2012-121378. [DOI] [PubMed] [Google Scholar]
- 5.Loureiro SO, Heimfarth L, Pelaez Pde L, Vanzin CS, Viana L, Wyse AT, Pessoa-Pureur R. Homocysteine activates calcium-mediated cell signaling mechanisms targeting the cytoskeleton in rat hippocampus. Int J Dev Neurosci. 2008;26:447–455. doi: 10.1016/j.ijdevneu.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Boldyrev AA. Molecular mechanisms of homocysteine toxicity. Biochemistry (Mosc) 2009;74:589–598. doi: 10.1134/s0006297909060017. [DOI] [PubMed] [Google Scholar]
- 7.Abushik PA, Niittykoski M, Giniatullina R, Shakirzyanova A, Bart G, Fayuk D, Sibarov DA, Antonov SM, Giniatullin R. The role of NMDA and mGluR5 receptors in calcium mobilization and neurotoxicity of homocysteine in trigeminal and cortical neurons and glial cells. J Neurochem. 2014;129:264–74. doi: 10.1111/jnc.12615. [DOI] [PubMed] [Google Scholar]
- 8.Waggener CT, Dupree JL, Elgersma Y, Fuss B. CaMKIIbeta regulates oligodendrocyte maturation and CNS myelination. J Neurosci. 2013;33:10453–10458. doi: 10.1523/JNEUROSCI.5875-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito A, Miyajima K, Akatsuka J, Kondo H, Mashiko T, Kiuchi T, Ohashi K, Mizuno K. CaMKIIbeta-mediated LIM-kinase activation plays a crucial role in BDNF-induced neuritogenesis. Genes Cells. 2013;18:533–543. doi: 10.1111/gtc.12054. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Bai X, Chen Y, Zhao Y, Liu X. Homocysteine induces apoptosis of rat hippocampal neurons by inhibiting 14-3-3epsilon expression and activating calcineurin. PLoS One. 2012;7:e48247. doi: 10.1371/journal.pone.0048247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirashima Y, Seshimo S, Fujiki Y, Okabe M, Nishiyama K, Matsumoto M, Kanouchi H, Oka T. Homocysteine and copper induce cellular apoptosis via caspase activation and nuclear translocation of apoptosis-inducing factor in neuronal cell line SH-SY5Y. Neurosci Res. 2010;67:300–306. doi: 10.1016/j.neures.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Tang XQ, Shen XT, Huang YE, Ren YK, Chen RQ, Hu B, He JQ, Yin WL, Xu JH, Jiang ZS. Hydrogen sulfide antagonizes homocysteine-induced neurotoxicity in PC12 cells. Neurosci Res. 2010;68:241–249. doi: 10.1016/j.neures.2010.07.2039. [DOI] [PubMed] [Google Scholar]
- 13.Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldyrev A, Bryushkova E, Mashkina A, Vladychenskaya E. Why is homocysteine toxic for the nervous and immune systems? Curr Aging Sci. 2013;6:29–36. doi: 10.2174/18746098112059990007. [DOI] [PubMed] [Google Scholar]
- 15.Williams HM, Lippok H, Doherty GH. Nitric oxide and peroxynitrite signalling triggers homocysteine-mediated apoptosis in trigeminal sensory neurons in vitro. Neurosci Res. 2008;60:380–388. doi: 10.1016/j.neures.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Ho PI, Collins SC, Dhitavat S, Ortiz D, Ashline D, Rogers E, Shea TB. Homocysteine potentiates beta-amyloid neurotoxicity: role of oxidative stress. J Neurochem. 2001;78:249–253. doi: 10.1046/j.1471-4159.2001.00384.x. [DOI] [PubMed] [Google Scholar]
- 17.Malik BR, Gillespie JM, Hodge JJ. CASK and CaMKII function in the mushroom body alpha’/beta’ neurons during Drosophila memory formation. Front Neural Circuits. 2013;7:52. doi: 10.3389/fncir.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Zhou W, Fan J, Ren Y, Yin G. G-protein-coupled receptor kinase interactor-1 serine 419 accelerates premature synapse formation in cortical neurons by interacting with Ca(2+)/calmodulin-dependent protein kinase IIbeta. Brain Res Bull. 2013;95:70–77. doi: 10.1016/j.brainresbull.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Speer RE, Karuppagounder SS, Basso M, Sleiman SF, Kumar A, Brand D, Smirnova N, Gazaryan I, Khim SJ, Ratan RR. Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by “antioxidant” metal chelators: From ferroptosis to stroke. Free Radic Biol Med. 2013;62:26–36. doi: 10.1016/j.freeradbiomed.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Yan J, Chang Y, ShiDu Yan S, Shi H. Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Curr Med Chem. 2011;18:4335–4343. doi: 10.2174/092986711797200426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Hu Q, Chen J, Yan F, Li J, Wang L, Mo H, Gu C, Zhang P, Chen G. Inhibiting HIF-1alpha by 2ME2 ameliorates early brain injury after experimental subarachnoid hemorrhage in rats. Biochem Biophys Res Commun. 2013;437:469–474. doi: 10.1016/j.bbrc.2013.06.107. [DOI] [PubMed] [Google Scholar]
- 22.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 23.Na YR, Han KC, Park H, Yang EG. Menadione and ethacrynic acid inhibit the hypoxia-inducible factor (HIF) pathway by disrupting HIF-1alpha interaction with p300. Biochem Biophys Res Commun. 2013;434:879–884. doi: 10.1016/j.bbrc.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki T, Kitagawa K, Yagita Y, Sugiura S, Omura-Matsuoka E, Tanaka S, Matsushita K, Okano H, Tsujimoto Y, Hori M. Bcl2 enhances survival of newborn neurons in the normal and ischemic hippocampus. J Neurosci Res. 2006;84:1187–1196. doi: 10.1002/jnr.21036. [DOI] [PubMed] [Google Scholar]
- 25.Li ST, Pan J, Hua XM, Liu H, Shen S, Liu JF, Li B, Tao BB, Ge XL, Wang XH, Shi JH, Wang XQ. Endothelial Nitric Oxide Synthase Protects Neurons against Ischemic Injury through Regulation of Brain-Derived Neurotrophic Factor Expression. CNS Neurosci Ther. 2014;20:154–164. doi: 10.1111/cns.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ota H, Akishita M, Akiyoshi T, Kahyo T, Setou M, Ogawa S, Iijima K, Eto M, Ouchi Y. Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: protective role of eNOS and SIRT1. PLoS One. 2012;7:e29598. doi: 10.1371/journal.pone.0029598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller M, Yang J, Griesmaier E, Gorna A, Sarkozy G, Urbanek M, Gressens P, Simbruner G. Erythropoietin is neuroprotective against NMDA-receptor-mediated excitotoxic brain injury in newborn mice. Neurobiol Dis. 2006;24:357–366. doi: 10.1016/j.nbd.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Miljus N, Heibeck S, Jarrar M, Micke M, Ostrowski D, Ehrenreich H, Heinrich R. Erythropoietin-mediated protection of insect brain neurons involves JAK and STAT but not PI3K transduction pathways. Neuroscience. 2014;258:218–227. doi: 10.1016/j.neuroscience.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Dai H, Shi Y. Erythropoietin protects primary cultures of rat cortical neurons from hypoxia-induced toxicity through attenuating both glutamate release and NMDA receptor evoked neurotoxicity pathway. Pharmazie. 2009;64:210–213. [PubMed] [Google Scholar]