Abstract
Background: Human umbilical cord mesenchymal stromal cells (UC-MSCs) have low immunogenicity and immune regulation. To investigate immunomodulatory effects of human UC-MSCs on MHC class II expression and allograft, we transplanted heart of transgenic rats with MHC class II expression on vascular endothelium. Methods: UC-MSCs were obtained from human umbilical cords and confirmed with flow cytometry analysis. Transgenic rat line was established using the construct of human MHC class II transactivator gene (CIITA) under mouse ICAM-2 promoter control. The induced MHC class II expression on transgenic rat vascular endothelial cells (VECs) was assessed with immunohistological staining. And the survival time of cardiac allograft was compared between the recipients with and without UC-MSC transfusion. Results: Flow cytometry confirmed that the human UC-MSCs were positive for CD29, CD44, CD73, CD90, CD105, CD271, and negative for CD34 and HLA-DR. Repeated infusion of human UC-MSCs reduced MHC class II expression on vascular endothelia of transplanted hearts, and increased survival time of allograft. The UC-MSCs increased regulatory cytokines IL10, transforming growth factor (TGF)-β1 and suppressed proinflammatory cytokines IL2 and IFN-γ in vivo. The UC-MSC culture supernatant had similar effects on cytokine expression, and decreased lymphocyte proliferation in vitro. Conclusions: Repeated transfusion of the human UC-MSCs reduced MHC class II expression on vascular endothelia and prolonged the survival time of rat cardiac allograft.
Keywords: Human umbilical cord mesenchymal stromal cells, cardiac allograft, vascular endothelia, rat
Introduction
Human umbilical cord mesenchymal stromal cells (UC-MSCs) do not express MHC class II and costimulatory molecules such as CD40, CD80, and CD86 [1,2]. Recent studies shown that MSCs could suppress lymphocyte proliferation induced by phytohemagglutinin (PHA) and were not restricted by MHC [3,4], and these have been tested in the treatment of autoimmune diseases, such as animal models of systemic lupus erythematosus and other autoimmune diseases [5]. Other researches have confirmed MSCs could effectively reduce the immune rejection after major organ transplantations, such as heart and kidney transplantation [6-8].
The immunomodulatory effects of MSCs have been confirmed by many experiments and clinical applications [9-12], they demonstrated that MSC modulating immunoresponsive cell cytokine production mainly [13]. However, few report mentioned about MSC regulating MHC antigen expression on transplanted solid organs.
It is well known that MHC class II molecules are critical for the generation and maintenance of immune responses. They are heterodimeric cell surface molecules that present antigens to CD4 positive lymphocytes, and thus play an important role in the regulation of allograft rejection. The MHC class II antigen distribution is greatly different in different species, for the vascular endothelial cells of human and other pirates are positive, however, they are negative in rodents [14].
Vascular endothelial cells (VECs) play an important role in immune response, especially in rejection of transplanted organ [15]. Our previous studies also showed that vascular endothelial cells are powerful stimulants of direct recognition by unprimed allogeneic CD4+ T cells. They are the powerful stimulants of xenogenic CD4+ T cells in the human anti pig model as well [16]. This capacity to stimulate CD4+ T cells is likely to be of critical importance in transplantation because the CD4+ T cell is, in almost all instances studied, the key cell in initiating allograft rejection responses. Our objective is to obtain MHC class II expression on VECs of commonly transplanted rodent organs to test if infusion of human UC-MSC alters the rejection of allograft and explore the possible immunomodulatory mechanisms.
In this study, we chosen human class II transactivator gene (CIITA) and mouse intercellular adhesion molecule 2 (ICAM2) promoter to make transgenic rat line. Because CIITA activates MHC class II expression, and ICAM-2 gene strongly expresses in all VECs in a constitutive manner. The tissue distribution of MHC class II in the transgenic rodent is favorable for the work.
Materials and methods
Ethnic consideration
This study was approved by an Institutional Review Board of Inner Mongolia Medical University and was conducted in accordance with good clinical practice, all applicable regulatory requirements and the guiding principles of the Declaration of Helsinki. Written informed consent was obtained from each subject for the use of their umbilical cords.
Human umbilical stromal cell isolation and culture
Human umbilical cords were obtained under sterile conditions from full-term infants delivered by caesarean section; residual blood was completely washed by phosphate-buffered saline (PBS). The umbilical cord membrane and blood vessels were removed to retain the Wharton’s jelly, which was cut into small pieces and then cultured in DMEM/F12 (GIBCO, Invitrogen, UK) supplemented with 10% fetal bovine serum (FBS) (GIBCO, Invitrogen). The human UC-MSCs were passaged at 80% confluence after 1-min treatment with 0.25% trypsin and 0.02% EDTA (Sigma-Aldrich, UK) at 37°C, and centrifugation at 1500 rpm for 10 min at 4°C.
Immunophenotype analysis
The third passage (P3) of UC-MSCs were trypsinized, suspended in DMEM/F12 at a concentration of 1×106/ml, and cultured until they reached 80% confluence. Aliquots of cells (100 μl) were incubated for 30 min at 4°C with mouse anti-human monoclonal antibodies conjugated with fluorescein isothiocyanate, HLA-DR-(FITC), CD29-FITC, CD44-FITC, phycoerythrin-conjugated CD34-PE, CD73-PerCP, CD90-PE, CD105-PE and CD271-PerCP (BD Biosciences, Plymouth, UK). After incubation, the cells were washed twice with PBS. Mouse isotypic antibodies PerCP-IgG1, PE-IgG1, and FITC-IgG1 (BD Biosciences, UK) were used as experimental controls. The cells were then resuspended and analyzed by flow cytometry.
Construction of human CIITA with mouse ICAM-2 promoter
Human CIITA gene was obtained from TA cloning which was our previous laboratory stock [16]. Based on the published mouse ICAM-2 gene sequence (GI: 51513), the ICAM-2 promoter was isolated from mouse liver genomic DNA with PCR. The promoter region was 620 bases in length and contained TATA inverted CAAT, and other well-known general regulatory sequences. The CMV promoter in pcDNA3-CIITA was removed and replaced by the mouse ICAM-2 promoter PCR fragment at BglII and BamHI restriction enzyme sites. The final construct was named as ICAM2-CIITA.
Production of transgenic rat
The transgene was separated from the construct ICAM2-CIITA by digestion with restriction enzymes BcI I and Bgl II, purified by electrophoresis through an agarose gel, and recovered by absorption to glass beads (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. Transgenic rats were produced by the microinjection technique in Edinburgh University. Zygotes for microinjections were obtained from superovulated Fischer (RTl1) inbred rat strain (Harlan, UK). After microinjections, the zygotes were transferred at two-cell stage into pseudopregnant foster mothers.
At the age of 3 weeks, the tails of transgenic pubs were obtained for DNA isolation using standard protocol. To scan and identify the transgenic rat offspring, ICAM-2 promoter-specific primer (GTGACTGCGTTCTAACTT) and human CIITA specific primer (CTTGGGGCTCTGACAGGTAGGAC) were employed for PCR amplification. Similarly, this pair of primers was used for generating Southern blot probe, which dUTP-digoxin (Boehringer Mannheirn, Diagnostics and Biochemicals Ltd, East Sussex, UK) was incorporated into the PCR product. After digestion with restriction enzymes Hind III and BamH I (Nippon Gene , Toyama, JP), the transgenic rat genomic DNA samples were separated under overnight electrophoresis and hybridized with the PCR generated probes. The transgenic rats were housed in a specific pathogen-free environment and studied according to UK Home Office regulations and bred with same species to generate homozygote.
Immunohistology of transgenic rat tissues
Samples of heart were obtained from transgenic and wild type rats, which were frozen in liquid nitrogen. 10 cm sections were cut and mounted onto polylysine-treated slides. The sections were fixed in acetone for 30 minutes and dried for overnight in room temperature. The staining for immunohistological staining of rat MHC class II via the immuno-alkaline phosphatase method with mouse anti-rat MHC class II RT1B antibody (Beijing Qi Wei Yi Cheng Co. Ltd, China) and then counterstaining with hematoxylin (Sino-American Biosciences Ltd, Beijing). The density of alkaline phosphatase blue staining was obtained with the previously described method [17].
Transgenic rat heterotopic heart transplantation
Twenty transgenic Fischer and 20 DA strain rats, age 20 weeks, weight 220-240 g, were defined as donors and recipients. One day before transplantation, the recipients were transfused with 107/kg UC-MSCs and then repeated every 2 days after transplantation as tested group, and the other recipients injected with same volume of DMEM/F12 supplemented with 10% FBS as control group.
Heterotopic heart transplantation was carried out. The donor ascending aorta and pulmonary artery to the recipient abdominal aorta and inferior vena cava were anastomosed respectively with 9-0 nylon, essentially as described by Wen [18]. Ischemia times were in the range of 10-15 min, fifteen heart transplantations have been obtained successfully. The beat of the transplanted heart was monitored twice a day. Whenever the grafts stopped beating, the rats were sacrificed with CO2 and their blood and grafts were harvested.
Cytokine enzyme-linked immunosorbent assay (ELISA)
The cytokine levels of rat IL2, IL10, TGF-β1 and IFN-γ were measured in the total protein isolated from transplanted hearts with ELISA kit (Tongren, Shanghai, China).
Rat lymphocyte proliferation, cytokine production and MHC class II expression
Peripheral blood lymphocytes (PBMCs) were isolated from DA rats by Ficoll-Paque (1.077 g/ml) density gradient centrifugation, and grown in 96-well plates with RPMI-1640 medium (GIBCO, Invitrogen, UK) supplemented with 10% FBS. The above prepared UC-MSCs at passage 3 were harvested and grown in RPMI-1640 medium containing 10% FBS for 24 hours. The culture supernatant of UC-MSC were collected and stored in -80°C for next step application. The isolated rat lymphocytes were cultured in the above collected media of UC-MSC.
The groups were divided as follows: 10×104, 5×104, 2.5×104, 1.25×104 lymphocytes cultured in RPMI 1640 with 2 μg/ml PHA (Sigma-Aldrich, Beijing) as a positive control, and the same number of lymphocytes was grown in the culture supernatant of UC-MSC containing 2 μg/ml of PHA. Each trial was repeated in triplicate. Cell counting kit, CCK-8 (Tongren Company, Shanghai, China) was used to assess the immunomodulatory impact of UC-MSC on rat peripheral blood lymphocytes after PHA stimulation. The procedure was carried out according to the manufacturer’s protocol. The inhibitory effects of soluble factors secreted by UC-MSC on lymphocyte proliferation were evaluated by comparing the optical density (OD620) in wells of lymphocytes grown in the positive control medium.
Proinflammatory cytokines, IL2 and IFN-γ in the above lymphocyte culture supernatant were quantified with ELISA. MHC class II expression on the rat lymphocytes was measured with flow cytometry analysis.
Statistical analysis
Data were presented as mean ± standard deviation. The statistical significance was assessed by Student’s t-test and a p value <0.05 was considered statistically significant. The Kaplan–Meier plot was used for estimating the survival function from lifetime data.
Results
Human UC-MSC markers
The cell-surface markers of the UC-MSCs were examined by FACS analysis. All cell populations were found expressing CD29, CD44, CD73, CD90, CD105 and CD271 cell markers, whereas no cells expressing HLA-DR and CD34.
Human CIITA activated MHC class II expression in transgenic rat
To find the possibility of human CIITA induce rat MHC class II expression, construct ICAM-2/CIITA was injected into fertilized rat oocytes. Total 380 oocytes were injected, 3 transgenic founder rats were obtained, however only 1 rat expressed the transgene and induced MHC class II expression in cardiac vascular endothelial cells. Most of the microinjected oocytes were either stopped developing and died or died at the foetal stage. The transgenic male rat was mated with wild-type females, and produced 86 transgenic offspring, which were confirmed with transgene PCR and Southern Blot. The transgenic rats showed variation in the MHC class II expression in different tissues. No or very weak expression of MHC-class II RT1B could be found on vascular endothelial cells of lung, kidney, brain and liver. However, the expression on vascular endothelia of heart was strong positive (Figure 1A).
Figure 1.
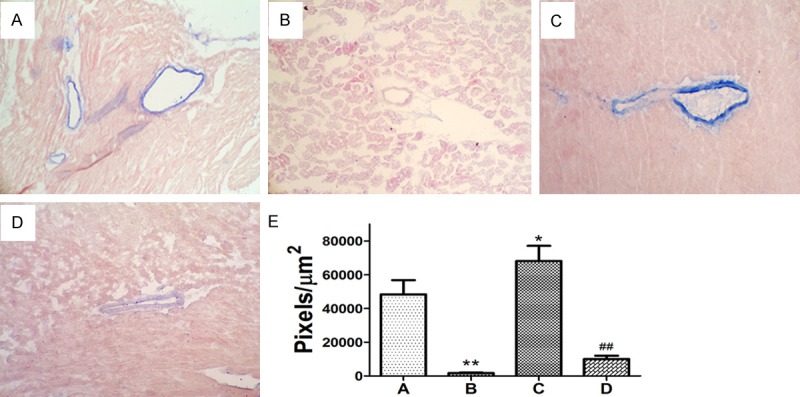
MHC class II expression on VECs of rat heart. Immunohistological staining of rat MHC class II antigens with mouse anti-rat MHC class II RT1B antibody and secondary antibody. A. Transgenic Fischer rat; B. Wildtype Fischer strain rat; C. Transgenic Fischer rat cardiac allograft, its receipt injected with control medium; D. Transgenic Fischer rat cardiac allograft, its receipt transfused with UC-MSCs; E. Semi-quantitative measurement of the immunohistological stain of MHC class II on VECs (MHC class II staining blue pixels/mm2). *P < 0.05, **P < 0.01, compared with A; ##P < 0.01, compared with D.
UC-MSCs suppressed MHC class II expression on VECs in the cardiac allografts
Immunohistological staining showed MHC-class II RT1B negative on the cardiac vascular endothelia of the inbred wild type Fischer rat (Figure 1B), but strong positive in the transgenic rats (Figure 1A). The transplanted hearts were sectioned for MHC class II staining. Its expression was suppressed on cardiac vascular endothelia in the rats by infusion of UC-MSCs compared with the animal models by injection of tissue culture medium (Figure 1C and 1D). Semiquan titative measurement of the immunohistological stain of MHC class II showed significantly different in the two groups (p < 0.001, Figure 1E).
The survival time of cardiac allograft
Before 24 hours of heart transplantation, 7 recipients were repeatedly transfused with 107/kg UC-MSCs as a tested group, and 8 of them injected with tissue culture medium (MEM/F12, 10% FBS) as a control. The survival time of allograft was recorded when the transplanted heart stopped beating. Injection of human UC-MSCs significantly promoted the mean cardiac allograft survival time (16.5+4.7 days) compared with that of control group (4.8+2.1 days) (p < 0.001, Figure 2).
Figure 2.
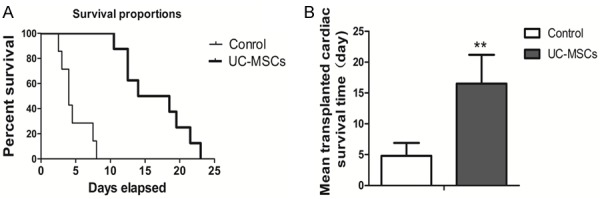
Survival comparison of cardiac allograft in control and UC-MSCs group. A. Survival curve; B. Column comparison for mean survival time, **p < 0.001.
Soluble factors secreted by human UC-MSCs suppressed rat lymphocyte proliferation and cytokine production
The experiment was designed to investigate whether soluble factors secreted by human UC-MSC could inhibit lymphocyte proliferation triggered by PHA. The PBMCs from rats were stimulated with PHA in the presence or absence of human UC-MSC culture supernatant. The lymphocyte proliferation was recorded as OD620. The results demonstrated that soluble factors of UC-MSC inhibited the proliferation of PBMC in response to mitogen treatment were 44-56% (Table 1).
Table 1.
Lymphocyte proliferation and cytokine production
| PHA 2 μg/ml | Number of PBMCs in each well | |||
|---|---|---|---|---|
|
| ||||
| 10×104 | 5×04 | 2.5×104 | 1.25×104 | |
| Control medium (OD620) | 0.92±0.12 | 0.58±0.10 | 0.28±0.08 | 0.18±0.04 |
| UC-MSC culture supernatant (OD620) | 0.52±0.08 | 0.28±0.06 | 0.13±0.05 | 0.08±0.05 |
| IL2 (pg/ml) in control medium | 1133.2±203.4 | 650.8±143.1 | 300.8±120.4 | 120.2±44.5 |
| IL2 (pg/ml) in UC-MSC medium | 212.9±73.5 | 78.1±22.1 | 20.4±3.9 | 8.6±1.6 |
| IFN (pg/ml) in control medium | 88.5±31.1 | 42.4±18.3 | 14.2±4.1 | 6.5±1.0 |
| IFN (pg/ml) in UC-MSC medium | 34.6±16.6 | 6.5±2.5 | 1.6±1.2 | 0.7±0.4 |
The proinflammatory cytokines, IL2 and IFN-γ production by lymphocytes after PHA stimulation was suppressed with human UC-MSCs culture supernatant by 81.2-92.8% and 60.9-89.2% respectively (Table 1). The anti-inflammatory cytokines, IL10 and TGF-β1 were not measured as human UC-MSCs secrete the similar cytokines which could interfere in the ELISA results.
Flow cytometry analysis showed MHC class II expression on rat lymphocytes being not suppressed significantly with human UC-MSC culture supernatant (data not shown).
UC-MSCs regulated cytokines expression in cardiac allografts
The rat cytokines in the isolated total protein in the cardiac allografts were measured with ELISA. The IL2, IFN-γ, TGF-β1 and IL10 was changing to 125.5±67.6 pg/g, 10.7+4.5 pg/g, 533.1±193.7 pg/g and 894.2±241.9 pg/g respectively in the total protein isolated from the cardiac allografts with the UC-MSC injection. However, the cytokines being 1124.7±566.3 pg/g, 122.3+43.8 pg/g, 398.1±121.7 pg/g and 123.3±54.5 pg/g in the control allografts. These results indicated that the immunomodulatory effects of UC-MSCs might be associated with inhibition of IL2 and IFN-γ and enhancing TGF-β1, IL10 secretion by different cells in the allografts.
Discussion
In current study, adoptively transferred human umbilical cord mesenchymal stromal cells (UC-MSCs) promoted rat cardiac allograft tolerance in vivo. This is the first report involving human UC-MSCs altering rat immune response through regulation of cytokine production and MHC class II expression on vascular endothelial cells in a rat cardiac transplant model. This study strengthened the fact that repeated transfusion of human UC-MSCs prolong rat allogeneic cardiac graft survival time, reduce MHC class II expression on vascular endothelium, suppress proinflammatory cytokine production and increase anti-inflammatory cytokine expression in allografts.
Human UC-MSCs express mesenchymal markers CD29, CD44, CD105, CD73, CD90 and CD271, but do not express MHC class II molecules [19,20]. They primarily suppress T cell proliferation and proinflammatory cytokine production [21], and induce organ transplantation immune tolerance [6]. The immunosuppressive property of MSCs is an attractive tool for modulating transplantation rejection.
As the rats we used here were not immune-suppressed, the transfused human UC-MSCs being rapidly eliminated by rat immune response from circulation, which means the effects of UC-MSCs being transient in vivo. The multiple administration of UCB-MSC was required to prevent allograft rejection.
Although the immunomodulatory effect of UC-MSCs has been confirmed by many experiments, the specific mechanism of immune suppression is still controversial. We found that the proliferation of lymphocytes stimulated with mitogen was inhibited by the human UC-MSC culture supernatant in vitro. The results suggest that suppression may be induced by the soluble factors secreted by the mesenchymal cells.
It is well known that IL2 and IFN-γ modulate cell-mediated immunity and immune rejection. This study revealed that the soluble factors of UC-MSC suppressed the proinflammatory cytokine expression in vitro. Because UC-MSCs also express IL10 and TGF-β1, which may affect ELISA accuracy, that is why we did not measure the anti-inflammatory cytokines in the culture supernatant of rat lymphocytes. Although MHC class II expression on B lymphocytes was not reduced significantly with UC-MSC culture supernatant, the above experimental data confirmed the unknown soluble factors secreted by the mesenchymal cells had immunoregulatory effects on rat lymphocyte.
Similarly, we found that the repeated injection of human UC-MSCs suppressed proinflammatory and increased anti-inflammatory cytokine secretion in cardiac allografts, suggesting that the combination of UC-MSCs and their secreted unknown soluble factors may play an important role in the reducing the incidence of immune rejection by these mechanisms. Because of the immune regulatory activity, UC-MSCs are implicated in roles for controlling organ transplantation rejection. The injection of human UC-MSCs down-regulated MHC class II expression on vascular endothelial cells and their secreted soluble factors had negative regulation on proinflammatory cytokines in vivo, whereas their soluble factors did not affect class II expression on lymphocytes after mitogen stimulation in vitro.
The injection of human UC-MSCs to rat recipients were able to prolong the survival of allogenic cardiac grafts, the possible mechanisms of immunosuppression are UC-MSC-mediated suppression of proinflammatory cytokine expression, such as IL2 and IFN-γ and increasing immunoregulatory cytokine expression, ie IL-10 and TGF-β1 in vivo. The change of cytokine expression pattern may partially explain why the human UC-MSCs induced the transgenic cardiac allografts immunotolerance.
MHC class II expression on vascular endothelial cells plays an important role in organ transplantation rejection [22]. The very complex and tight regulation of expression of the MHC II gene family has direct implication for T-lymphocyte activation and thus for organ transplantation rejection immune response. In this study, we employed mouse ICAM2 promoter and human CIITA gene to make transgenic murine model to induce MHC class II expression on rat VECs in vivo. As we expected, the induced MHC class II strongly expressed on VECs in some organs of the transgenic rat, especially in heart and brain. The special distribution of induced VEC MHC class II expression was favorable for our project as we know the increased MHC class II expression on VECs enhances allogenic organ transplantation rejection [23].
Human UC-MSCs inhibited constitutive MHC class II expression on vascular endothelium in allogenic cardiac allografts in vivo, which may further explain why UC-MSCs enhancing the survival of allografts. However, the exact mechanisms of suppressing MHC class II expression on vascular endothelia is not clear, more work is needed to clarify.
In addition, the experiment indicated that MHC class II positive on VECs in transgenic heart shortened the survival time of cardiac allografts compared with the similar work published recently [24]. Importantly, transfusion of human UC-MSCs inhibited MHC class II expression and reversed the effects caused by MHC class II expression on VECs. These results may have potential use for human organ transplantation in the future.
In summary, we demonstrated that the immunomodulatory effects of UC-MSCs are likely mediated by multiple factors. The human UCMSCs could reduce or even block MHC class II expressions on vascular endothelial cell surface in cardiac allografts and decrease proinflammatory cytokines IL2 and IFN-γ, at the same time, enhance anti-inflammatory cytokines, such as IL10 and TGF-β1 expression in vivo. The soluble factors secreted by human UC-MSC have immunoregulatory effects as well.
Acknowledgements
The authors wish to acknowledge the contribution of all the students and clinical fellows who participated in various aspects of this work. This research was supported by: British Heart Foundation, the Inner Mongolia Key Programme Grant (kjt10jhg) and the Science Foundation of China (81260064)
References
- 1.Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev. 2013;9:226–240. doi: 10.1007/s12015-012-9418-z. [DOI] [PubMed] [Google Scholar]
- 2.Batsali AK, Kastrinaki MC, Papadaki HA, Pontikoglou C. Mesenchymal stem cells derived from Wharton’s Jelly of the umbilical cord: biological properties and emerging clinical applications. Curr Stem Cell Res Ther. 2013;8:144–155. doi: 10.2174/1574888x11308020005. [DOI] [PubMed] [Google Scholar]
- 3.Keating A. How do mesenchymal stromal cells suppress T cells? Cell Stem Cell. 2008;2:106–108. doi: 10.1016/j.stem.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2:34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueroa FE, Carrion F, Villanueva S, Khoury M. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res. 2012;45:269–277. doi: 10.4067/S0716-97602012000300008. [DOI] [PubMed] [Google Scholar]
- 6.Casiraghi F, Perico N, Remuzzi G. Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr Opin Organ Transplant. 2013;18:51–58. doi: 10.1097/MOT.0b013e32835c5016. [DOI] [PubMed] [Google Scholar]
- 7.Reinders ME, Rabelink TJ, de Fijter JW. The role of mesenchymal stromal cells in chronic transplant rejection after solid organ transplantation. Curr Opin Organ Transplant. 2013;18:44–50. doi: 10.1097/MOT.0b013e32835c2939. [DOI] [PubMed] [Google Scholar]
- 8.Roemeling-van Rhijn M, Weimar W, Hoogduijn MJ. Mesenchymal stem cells: application for solid-organ transplantation. Curr Opin Organ Transplant. 2012;17:55–62. doi: 10.1097/MOT.0b013e32834ee676. [DOI] [PubMed] [Google Scholar]
- 9.Chao YH, Wu HP, Chan CK, Tsai C, Peng CT, Wu KH. Umbilical cord-derived mesenchymal stem cells for hematopoietic stem cell transplantation. J Biomed Biotechnol. 2012;2012:759503. doi: 10.1155/2012/759503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 11.Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, Ko AY, Roddy GW, Prockop DJ. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther. 2012;20:2143–2152. doi: 10.1038/mt.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128–134. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of Mesenchymal Stem cell-based therapy: Current status and perspectives. Cell Transplant. 2013 doi: 10.3727/096368913X667709. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Metzger R, Mempel T, Joppich I, Till H. Organ-specific distribution of major histocompatibility antigens in rats. Pediatr Surg Int. 2000;16:285–292. doi: 10.1007/s003830050746. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi Y. Reviews on mysterious vascular endothelial cells in renal allografts. Clin Transplant. 2012;26(Suppl 24):13–19. doi: 10.1111/j.1399-0012.2012.01675.x. [DOI] [PubMed] [Google Scholar]
- 16.Yun S, Rose ML, Fabre JW. The induction of major histocompatibility complex class II expression is sufficient for the direct activation of human CD4+ T cells by porcine vascular endothelial cells. Transplantation. 2000;69:940–944. doi: 10.1097/00007890-200003150-00046. [DOI] [PubMed] [Google Scholar]
- 17.Leung VW, Yun S, Botto M, Mason JC, Malik TH, Song W, Paixao-Cavalcante D, Pickering MC, Boyle JJ, Haskard DO. Decay-accelerating factor suppresses complement C3 activation and retards atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2009;175:1757–1767. doi: 10.2353/ajpath.2009.090183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen P, Wang X, Wang J, Zhang B, Qiu G, Fan Y, Xu Y, Tang H, Sun X, Peng Z. A simple technique for a new working heterotopic heart transplantation model in rats. Transplant Proc. 2013;45:2522–2526. doi: 10.1016/j.transproceed.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 19.Mundra V, Gerling IC, Mahato RI. Mesenchymal stem cell-based therapy. Mol Pharm. 2013;10:77–89. doi: 10.1021/mp3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, Zhou Z, Zhang D, Yang S, Wang J, Xue F, Yang Y, Yang R. Immunosuppressive function of mesenchymal stem cells from human umbilical cord matrix in immune thrombocytopenia patients. Thromb Haemost. 2012;107:937–950. doi: 10.1160/TH11-08-0596. [DOI] [PubMed] [Google Scholar]
- 21.Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–857. doi: 10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- 22.Taflin C, Charron D, Glotz D, Mooney N. Immunological function of the endothelial cell within the setting of organ transplantation. Immunol Lett. 2011;139:1–6. doi: 10.1016/j.imlet.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Taflin C, Favier B, Charron D, Glotz D, Mooney N. Study of the allogeneic response induced by endothelial cells expressing HLA class II after lentiviral transduction. Methods Mol Biol. 2013;960:461–472. doi: 10.1007/978-1-62703-218-6_34. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Mei YQ, Solomon MA. [Experiences of establishing an abdominal heart transplantation model in rats] . Zhonghua Yi Xue Za Zhi. 2012;92:1715–1718. [PubMed] [Google Scholar]


