Abstract
The transcription factor FOXP3 is specifically expressed in regulatory T (Treg) cells and appears to mediate immune surveillance. Indeed, FOXP3+Treg cells have been linked to disease pathogenesis, including some cancers. This study investigated the presence of FOXP3+Treg cells in colorectal cancer and the relationship of FOXP3 expression with clinicopathological features of colorectal cancer. Immunohistochemistry was used to detect expression of FOXP3 in 63 samples of colorectal cancer and 20 samples of healthy colorectal tissue; flow cytometry was used to detect FOXP3+Treg cells in peripheral blood. FOXP3 was more commonly expressed in colorectal cancer tissues than in normal colorectal tissues (P < 0.05). Similarly, the percentage of FOXP3+Treg cells in the peripheral blood was higher in patients with colorectal cancer than in control individuals (P < 0.05). The expression of FOXP3 was positively correlated with gender, Dukes staging, and lymph node metastasis. Further, expression increased with the increasing degree of malignancy (P < 0.05). Thus, FOXP3 expression may represent a valuable index in evaluating the degree of malignancy, clinicopathologic staging, and lymph node metastasis in colorectal cancer. Further, detection of FOXP3+Treg cells may be useful in predicting invasion, metastasis, and prognosis of patients with colorectal cancer.
Keywords: Colorectal cancer, fork-head/winged helix transcription factor 3, regulatory T cells, immunohistochemistry, flow cytometry
Introduction
Colorectal cancer (CRC) is a common malignant tumor of the digestive system. A staggering 1.2 million new cases were diagnosed worldwide in 2008 alone, and the mortality rate is about 8% [1]. In China, the morbidity and mortality of colorectal CRC have been increasing annually, reaching or exceeding the world averages for this cancer [2]. Thus, CRC represents a significant health burden globally, and, particularly, in China.
Many cancers develop and progress because of various dysregulation events that occur within the immune system. The fork-head/winged-helix transcription factor 3 (FOXP3 or scurfin), a member of the forkhead family, is specifically expressed on CD4+CD25+ regulatory T (Treg) cells [3]. FOXP3 has an important regulatory role in development, phenotype, and functional maintenance of Treg cells [4]. Indeed, high expression of FOXP3 in Treg cells could impede immune surveillance for cancer and inhibit an effective immune response to autologous tumor cell antigens, thereby promoting tumor growth and invasion [4,5]. Although these roles have been demonstrated in other cancer types, a potential role for FOXP3 in CRC is unknown.
In this study, immunohistochemistry and flow cytometry were used to detect FOXP3 expression in tumor tissues and Treg expression in peripheral blood, respectively, of CRC patients to investigate its clinical relevance to and significance in these tumors.
Materials and methods
Human tissue samples
Samples were obtained from 63 cases of colorectal cancer undergoing surgical excision of tumors Xijing Hospital, Fourth Military Medical University, between January and December 2013; these cases comprised 47 males and 16 females, ages 33-71 years. Control colorectal tissues were obtained from 20 individuals who had surgery to remove benign lesions during the same period. Tissue samples were archived in paraffin blocks; all CRC were histologically adenocarcinoma. Tumors were assessed for Dukes staging according to the criteria established in 2009 by the the Union for International Cancer Control (UICC) UICC and American Joint Committee on Cancer (AJCC), as follows: 9 cases were Stage A; 25 were Stage B; 26 were Stage C; and 3 were Stage D. Staging was also performed according to the criteria in the Diagnostic Histopathology of Tumors as follows: 9 cases were Grade I; 40 were Grade II; and 14 were Grade III. Further, 26 cases had lymph node metastasis, while 37 cases had no lymph node metastasis; 2 cases had distant metastases (bone, lung, liver), and 61 cases had no had distant metastasis. The cases had not received preoperative radiotherapy, chemotherapy, or other cancer treatments. Finally, 10 mL of peripheral blood were collected from participants in the morning before surgery under a fasting state. All signed consents were obtained from all subjects. This study was approved by the Ethics Committee of Xijing Hospital, Fourth Military Medical University,
Immunohistochemistry
Paraffin tissue blocks were serially sectioned at 4-μm thickness and collected onto slides by standard histological techniques. Immunohistochemistry was performed with EnVisionTM (Beijing Zhongshan Golden Bridge Biotechnology). Tissue sections were dewaxed, and the antigens were exposed by citrate treatment (pH 6.0). Sections were then incubated in 0.3% H2O2 at room temperature and washed with distilled water and 0.1 M phosphate-buffered saline (PBS) before an overnight incubation in 10% goat serum and primary antibody (1:200, mouse anti-human FOXP3 monoclonal, produced by Abcam, purchased from Wuhan Amyjet Scientific) at 4°C. Subsequently, sections were treated with the secondary antibody (1:300, Goat Anti-mouse IgG Polyclonal Antibody). Staining color was developed with DAB substrate [diaminobenzidine (DAB) color kit (Beijing Zhongshan Golden Bridge Biotechnology)] according to manufacturer’s instructions. Tissues were counterstained with hematoxylin. PBS was used as negative control for the primary antibody. Known FOXP3-positive sections were used as a positive control.
Positive staining for FOXP3 was observed as yellow to tan particles in the nucleus under light microscopy. Sections were analyzed by two experienced pathologists. The semi-quantitative accumulate-point analysis was performed using the reading results in combination with intensity of the stained cells and average count of the positive cells. Five fields were randomly selected for each section under high-power light microscopy, and the staining intensity should be synthetically determined on basis of the average percentage of positive cells (to all the cells) and accumulate points of intensity.
Isolating peripheral blood mononuclear cells (PBMC)
Peripheral blood samples were processed with heparin to prevent coagulation. An equal volume of PBS was added, then an equal volume of lymphocyte separation medium (MP Biomedical, #50494), and samples were centrifuged at 2000 rpm at room temperature for 10 minutes. Samples were rinsed twice with 4 mL PBS after removing the middle tunica albuginea layer for PBMC. The supernatants were discarded and RPMI-1640 was added. Samples were cultured at 37°C and 5% CO2, and re-suspended for use after stimulation culture.
Flow cytometry
PBMC were suspended at a concentration of 2×106 cells/L. Eighty μL of PBMC were added to a test tube and an isotype control tube for each sample. To each tube, 2.5 μL FITC-CD4 antibody and PE-CD25 antibody, respectively, were added. Samples were incubated at 4°C for 30 minutes, then rinsed with PBS twice. Membrane-rupturing solution was added for a 1-hr incubation at 4°C in the dark. Next, 2.5 μL PE-Cy5-FOXP3 antibody were added to the test tube, and the isotype control antibody was added to the control tube. Samples were incubated at 4°C for 30 minutes in the dark, rinsed twice with PBS, and placed on the flow cytometer (BD FACScan) for Treg cell analysis. FITC-CD4 antibody, PE-CD25 antibody, and PE-Cy5-FOXP3 antibodies were all purchased from eBioscience. Analysis was performed using the Cell Quest software originally attached to the instrument.
Statistical analysis
Statistical analysis was performed using SPSS18.0 statistical analysis software. A χ2 test was performed to assess the relationship between FOXP3 expression and clinicopathological parameters of colorectal cancer. A Spearman correlation analysis was performed for correlation of FOXP3 expression in colorectal cancer tissues. P < 0.05 was considered statistically significant.
Results
FOXP3 expression in colorectal tissue
FOXP3 expression is specific to Treg cells, and increased numbers of Treg cells have been identified previously in tumor tissues [6]. Immunohistochemistry revealed FOXP3 localization primarily in the nucleus of positive cells (Figure 1). FOXP3 expression was detected in 61.9% and 35.0% of samples from colorectal cancer and healthy colorectal tissues, respectively (Table 1). This difference was statistically significant (P < 0.05).
Figure 1.
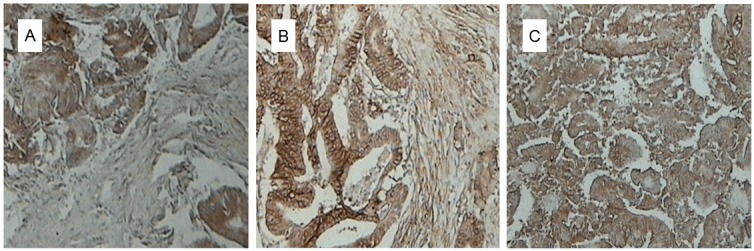
Immunohistochemical staining of FOXP3 in colorectal tissues. A: Colorectal cancer grade I (×200); B: Colorectal cancer grade II (×200); C: Colorectal cancer grade III (×200).
Table 1.
Positive expression rate of FoxP3 in colorectal cancer tissues and normal colorectal tissues [n(%)]
| Tissue | n | Positive | Negative | χ2 | P |
|---|---|---|---|---|---|
| Colorectal cancer | 63 | 39 (61.9) | 24 (38.1) | ||
| Normal tissues | 20 | 7 (35.0) | 13 (65.0) | 4.448 | 0.035 |
Correlation between FOXP3 expression and clinicopathological features of colorectal cancer
Expression of FOXP3 was positively correlated with gender, Dukes staging, and lymph node metastasis (P < 0.05; Table 2). Further, the expression of FOXP3 was more common with increasing degree of malignancy, but was not correlated with age or tumor location (P > 0.05).
Table 2.
FOXP3 expression and its relationship with clinicopathologic factors of colorectal cancer [n(%)]
| Clinicopathologic feature | n | Positive | Negative | χ2 | P |
|---|---|---|---|---|---|
| Age | |||||
| ≤ 50 years | 28 | 15 (53.6) | 13 (46.4) | 1.484 | 0.230 |
| > 50 years | 35 | 24 (68.6) | 11 (31.4) | ||
| Gender | |||||
| Male | 47 | 32 (68.1) | 15 (31.9) | 4.103 | 0.045 |
| Female | 16 | 7 (43.8) | 9 (56.2) | ||
| Histological grade | |||||
| Grade I | 9 | 4 (44.4) | 5 (55.6) | 1.708 | 0.215 |
| Grade II | 40 | 25 (62.5) | 15 (37.5) | ||
| Grade III | 14 | 10 (71.4) | 4 (28.6) | ||
| Dukes staging | |||||
| A/B | 34 | 15 (44.1) | 19 (55.9) | 9.909 | 0.001 |
| C/D | 29 | 24 (82.8) | 5 (17.2) | ||
| Lymph node metastasis | |||||
| Yes | 26 | 20 (76.9) | 6 (23.1) | 4.234 | 0.040 |
| No | 37 | 19 (51.4) | 18 (48.6) | ||
| Distant metastasis | |||||
| Yes | 2 | 2 (100.0) | 0 (0.0) | 1.271 | 0.267 |
| No | 61 | 38 (62.3) | 23 (37.7) |
Percentage of FOXP3+Treg expression in peripheral blood
Treg cells play important roles in immune surveillance, and altered function of Treg cells can promote tumorigenesis [7]. Flow cytometry was used to distinguish Treg cells in peripheral blood expressing FOXP3. The mean percentage of FOXP3+Treg cells in peripheral blood of the 63 patients with colorectal cancer was 11.27 ± 1.54%, which was significantly higher than that of healthy controls (6.31 ± 0.23%; P < 0.05) (Figure 2).
Figure 2.
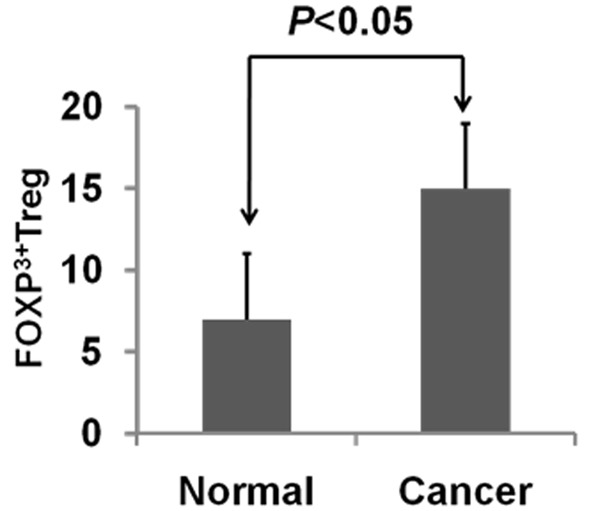
Treg expression of FOXP3 in peripheral blood of patients with and without colorectal cancer.
Discussion
This study found that the expression of FOXP3 was more common in colorectal cancer tissues than in normal colorectal tissues, likely indicating the increased likelihood of Treg cell localization within tumor tissue. Indeed, the number of Treg cells in the peripheral blood of patients with colorectal cancer was higher than that of individuals without colorectal cancer. Furthermore, the high expression of FOXP3 was positively correlated with tumor malignancy. Under normal physiological conditions, a large number of leukocytes infiltrate tumor tissues, wherein NK cells have the most effective anti-tumor effect and inhibiting effect on tumor metastasis; FOXP3+Treg cells can inhibit immune function, inducing inactivation of NK cells [8]. This can therefore result in immune escape by a tumor [8-10].
FOXP3 expression in tumor tissues from patients with lymph node metastasis was higher compared to those tissues from patients without lymph node metastasis, indicating that FOXP3 expression is correlated with lymph node metastasis. It is possible that FOXP3 inhibits function of the sentinel lymph node, inhibits antigen-presenting function of the dendritic cells, and hinders the abilities of ancillary cytotoxic T cells and cytotoxic T cells to activate an immune response, thereby promoting tumor cell lymph node metastasis [11].
In summary, a new immune therapy [12-16] should be developed, which can, through reducing or inhibiting FOXP3 expression, eliminate abnormal proliferation of FOXP3+Treg cells in patients, maintain an ideal tumor microenvironment, and block tumor immune escape, invasion, and metastasis. Thus, normal T lymphocytes and NK cells may better function in inhibiting tumor growth and proliferation, thereby improving efficiency of cancer treatment and prolonging patient survival.
Acknowledgements
Supported by the National High Technology Research and Development Program of China (863 Program), N0. SS2014AA020801.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, cancer incidence and mortality worldwide: IARC cancerbase No. 10. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 2.Littman DR, Rudensky A. Th17 and regulatory T Cells in mediating and restraining inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 4.Marzano AV, Vezzoli P, Fanoni D, Venegoni L, Berti E. Primary cutaneous T-cell lymphoma expressing FoxP3: a case report supporting the existence of malignancies of regulatory T cells. J Am Acad Dermatol. 2009;61:348–355. doi: 10.1016/j.jaad.2008.11.894. [DOI] [PubMed] [Google Scholar]
- 5.Ye J, Ma C, Wang F, Hsueh EC, Toth K, Huang Y, Mo W, Liu S, Han B, Varvares MA, Hoft DF, Peng G. Specific recruitment of γδ regulatory T cells in human breast cancer. Cancer. 2013;6:855–859. doi: 10.1158/0008-5472.CAN-13-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–11. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Garín MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Grimmig T, Grimm M, Meier E, Rosenwald A, Tsaur I, Blaheta R, Heemann U, Germer CT, Waaga-Gasser AM, Gasser M. Expression of Foxp3 in colorectal cancer but not in Treg cells correlates with disease progression in patients with colorectal cancer. PLoS One. 2013;8:e53630. doi: 10.1371/journal.pone.0053630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeuchi H, Kitajima M, Kitagawa Y. Sentinel lymph node as a target of molecular diagnosis of lymphatic micrometastasis and local immunoresponse to malignant cells. Cancer Sci. 2008;99:441–450. doi: 10.1111/j.1349-7006.2007.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HE, Park DJ, Kim WH, Lee HS. High FoxP3+ regulatory T-cell density in the sentinel lymph node is associated with downstream non-sentinel lymph-node metastasis in gastric cancer. Br J Cancer. 2011;105:413–419. doi: 10.1038/bjc.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber J. Overcoming immunologic tolerance to melanoma: targeting CTLA-4 with ipilimumab (MDX-010) Oncologist. 2008;13:16–25. doi: 10.1634/theoncologist.13-S4-16. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. 2010;70:4850–4858. doi: 10.1158/0008-5472.CAN-10-0283. [DOI] [PubMed] [Google Scholar]
- 14.Medina-Echeverz J, Fioravanti J, Zabala M, Ardaiz N, Prieto J, Berraondo P. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol. 2011;186:807–815. doi: 10.4049/jimmunol.1001483. [DOI] [PubMed] [Google Scholar]
- 15.Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs JF, Punt CJ, Lesterhuis WJ, Sutmuller RP, Brouwer HM, Scharenborg NM, Klasen IS, Hilbrands LB, Figdor CG, de Vries IJ, Adema GJ. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a Phase I/II study in metastatic melanoma patients. Clin Cancer Res. 2010;16:5067–5078. doi: 10.1158/1078-0432.CCR-10-1757. [DOI] [PubMed] [Google Scholar]


