Abstract
The sonic hedgehog (SHH) and STAT3 signaling pathways play important roles during carcinogenesis with possible interaction. To determine the association of the activation of SHH signaling pathway and STAT3 pathway in carcinogenesis of human papillary thyroid cancer (PTC), we examined the expression of SHH signaling pathway molecules including SHH, Patched (PTCH), Smoothened (SMO) and GLI1 (glioma-associated oncogene homolog 1), as well as p-STAT3 (phosphorylation at Tyr705) by immunohistochemistry in 164 cases of PTC. In PTC, 70.12%, 64.02%, 68.90%, 64.02%, and 56.71% and in the adjacent normal thyroid tissues, 18.29%, 18.90%, 26.83%, 14.63%, and 10.98% of the specimens stained positive for SHH, PTCH, SMO, GLI1, and p-STAT3, respectively. Significant difference were found for the positive rate of SHH, PTCH, SMO, and GLI1 as well as p-STAT3 expression between PTC and adjacent normal thyroid tissues. There was a high accordance rate between SHH, PTCH, SMO, and GLI1 expression and all of them positively correlated with larger tumor size, the presence of ETE and LNM, and higher TNM stage. P-STAT3 expression positively correlated with the presence of ETE and LNM, and higher TNM stage but not age, gender, tumor size of the PTC patients. Signifi cant positive correlation between p-STAT3 and SHH, PTCH, SMO and GLI expression was found in PTC. These findings suggest that the SHH and STAT3 signaling pathways are frequently activated in PTC, interact with each other and may therefore be indicators for prognosis or potential targets for therapy against PTC.
Keywords: Thyroid cancer, hedgehog, p-STAT3, immunohistochemistry
Introduction
Papillary thyroid cancer (PTC) is the most common endocrine malignancy, which has a fast growing incidence during recent years. PTC is usually indolent and curable, but it can spread early to local lymph nodes. In addition, disease persistence and/or recurrence are common and associated with increased mortality [1]. Thus, it is essential to explore the molecular mechanism in early events of carcinogenesis of PTC and search new targets for cancer therapy.
The Sonic Hedgehog (SHH) signaling pathway controls a myriad of key development processes and its malfunction causes many human disorders, including birth defects and cancer [2]. The core components of this signaling pathway are the morphogen SHH and its receptor patched 1 (PTCH) that inhibits the transmembrane protein Smoothened (SMO) [3]. SHH exerts its biological influence through a conserved signaling cascade that culminates at the regulation of the latent transcription factor Ci/Gli, that is, SHH binding to PTCH inhibits the repression of SMO, which results in the activation of the only known transcriptional mediators of the SHH response, zinc-finger proteins of the Gli (also known as Cubitus interruptus (Ci) in D. melanogaster) family [4-6]. Three Gli genes have been identified in vertebrate - GLI1, GLi2, and GLI3 [7]. Among them, GLI1 is a strong positive activator of downstream target genes, and it is itself a transcriptional target of the SHH signaling pathway. Therefore, GLI1 is considered to be the only loyal marker of the SHH signaling pathway activity [8]. Dysregulation of the components of the SHH signaling pathway can lead to the development and progression of several malignancies, including basal cell carcinomas (BCCs), medulloblastomas, leukemia, gastrointestinal, lung, ovarian, breast and prostate cancers [9,10]. Pathway-blockage of the SHH pathway resulted in a significant dose-dependent reduction of tumor cell growth in vitro and in vivo. Moreover, combined treatment with cyclopamine, an antagonist that binds SMO, and the conventional antimetabolite gemcitabine revealed a synergistic effect on the reduction of tumor growth in pancreatic adenocarcinoma xenografts [11]. A recent study reveals that vismodegib is the first Hedgehog pathway inhibitor to be approved in the USA, where it is indicated for the treatment of adults with metastatic basal cell carcinoma (BCC), or with locally advanced BCC that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation. In an ongoing, noncomparative, phase II trial, oral vismodegib was effective in and had an acceptable tolerability profile in the treatment of patients with locally advanced or metastatic BCC [12]. Therefore, a thorough understanding of the SHH molecular pathway could prove beneficial in the early diagnosis of these cancers and in the development of therapeutic drugs aimed at interfering with this signaling.
The signal transducer and activator of transcription STAT3 is a cytoplasmic transcription factor that gets phosphorylated upon activation, translocates into the nucleus and activate target genes. Besides its normal functions, this protein plays an important role in cellular transformation and tumorigenesis and is constitutively activated in about 70% of solid and hematological cancers [13-16]. Inhibition of the signal transducer and activator of transcription 3 (STAT3) signaling pathway has been considered a novel therapeutic strategy to treat human cancers that harbor aberrantly-active STAT3 [17]. Constitutive activation of STAT3 in tumors can result from upstream activated signaling components, including increased cytokines (IL-6 and IL-10) production, activated receptor (cytokine receptors, VEGFR and EGFR) and non-receptor tyrosine kinases (including JAKs, Src and Abl). Several strategies have been developed to target p-STAT3, including IL-6 and IL-6R blocking antibodies and JAK inhibitors [18].
As mentioned above, both the SHH signaling pathway and STAT3 activation play an important role in the development and progression of cancer. In addition, recent studies have shown an association between the SHH signaling pathway and STAT3 activation in chronic myeloid leukemia and pulmonary adenocarcinoma [15,16]. However, the roles of the SHH signaling pathway and STAT3 activation and their association in PTC have not yet been well documented. Thus, the present study was undertaken to examine the expression of the SHH signaling pathway molecules and p-STAT3 to elucidate their clinical significance in PTC and further explore their association.
Materials and methods
Patients and tissue specimens
Thyroid tissue specimens were obtained from 164 Chinese PTC patients, consisting of 43 men (mean 40.2 years; range 7-74) and 121 women (mean 44.6 years; range 14-82). All of these patients were admitted to our hospital for a standard thyroidectomy between 2008 and 2011. Patient records were obtained from the Medical Records Department at our hospital and also from pathology reports. Their diagnoses were confirmed by histopathological examination. None of these patients had a history of familial thyroid cancer or neck external irradiation. Clinicopathological data, such as tumor size, presence of extra thyroidal extension (ETE), and lymph node metastasis (LNM), were retrieved from patients’ medical records. ETE was defined as invasion of adjacent organs or skeletal muscle outside the isthmus [19]. The cancer stage was defined according to the 7th edition of tumor, node and metastasis system classification by the American Joint Committee on Cancer [20]. The study protocol was performed according to the declaration of Helsinki and approved by the Medical Ethics Committee of China Medical University. Written and verbal informed consent was obtained from all participants.
Immunohistochemical staining
Immunohistochemical staining was performed to analyze the expression of 4 molecules in the SHH signaling pathway, SHH, PTCH, SMO, and GLI1, as well as p-STAT3 in the above mentioned PTC tissue samples. Paraffin-embedded tissue sections fixed in formalin were used. Slides were deparaffinized, rehydrated, and subjected to antigen retrieval by pressure cooking in 10 mM citrate buffer (pH 6.0) for 8 min. After blocking endogenous peroxidase activity, sections were incubated with primary antibodies against SHH (1:100, EP1190Y, Novus Biologicals, Inc. Littleton, CO, USA), PTCH (1:100, H0267, Santa Cruz Biotechnology Inc.), SMO (1:100, H-300, Santa Cruz Biotechnology Inc.), GLI1 (1:100, H-300, Santa Cruz Biotechnology Inc.), and p-STAT3 (1:50, B-7, Santa Cruz Biotechnology Inc.) overnight at 4°C. After washing with PBS, staining was performed by the Elivision™ plus two-step system (Maixin Bio, China). Immunoreactivity was visualized using the chromogen 3,3’-diamino-benzidine (DAB) (Maixin Bio, China). Slides were then counterstained with hematoxylin, washed, dehydrated with alcohol and xylene, and mounted onto coverslips. Appropriate positive and negative controls were run simultaneously with the patient specimen.
Review and scoring of stained tissue sections
We used the Kim KH et al. scoring method to evaluate both the intensity of the IHC staining and the proportion of the stained epithelial cells [21]. The staining intensity was classified as 1, weak; 2, moderate; and 3, strong. The positive cells were quantified as a percentage of the total number of epithelial cells and assigned to one of five categories (0, < 5%; 1, 5-25%; 2, 26-50%; 3, 51-75%; 4, > 75%). The percentage of positivity of the tumor cells and the staining intensities were then multiplied in order to generate the IHC score. Three investigators (Dong WW, Cui JS and Tian XS) independently evaluated the tissue sections and graded SHH, PTCH, SMO, GLI1, and p-STAT3 expression as 0-3, negative (-); 4~6, positive (+); 7~9, strongly positive (++); and 10~12, very strongly positive (+++) in a blinded fashion. Cases with a discrepancy in scores were discussed to obtain a consensus.
Statistical analysis
Descriptive statistics were used according to the distribution of the variables. χ2 or Fisher’s exact test was used to determine whether there were significant differences in the expression of SHH, PTCH, SMO, GLI1, and p-STAT3 between PTC and its adjacent normal thyroid tissues; and whether there were significant differences in SHH, PTCH, SMO, GLI1, and p-STAT3 expression among patients of different age and gender, and whether SHH, PTCH, SMO, GLI1, and p-STAT3 expression correlated with tumor size, the presence of ETE and LNM, and TNM stage. The spearman correlation test was used to assess the association between the expression of the SHH signaling pathway molecules and p-STAT. All statistical analyses were conducted by using the statistical program SPSS 17.0 for windows (SPSS, Chicago, IL, USA). A P value below 0.05 was considered statistically significant.
Results
Expression of the SHH signaling pathway components and p-STAT3 in PTC patients
We conducted IHC staining to analyze the expression of four molecules in the SHH signaling pathway SHH, PTCH, SMO, and GLI1, as well as p-STAT3 in PTC patients. The staining for SHH, PTCH, and SMO was localized predominantly to the cytoplasm; GLI1 appeared to be localized to both cytoplasm and nuclei; whereas p-STAT3 was localized to the nuclei (Figure 1). The positive rate of SHH, PTCH, SMO, and GLI1, as well as p-STAT3 expression in PTC and adjacent normal thyroid tissues were 70.12%, 64.02%, 68.90%, 64.02%, 56.71% and 18.29%, 18.90%, 26.83%, 14.63%, 10.98%, respectively (Table 1). Significant difference were found for the positive rate of SHH, PTCH, SMO, and GLI1 as well as p-STAT3 expression between PTC and adjacent normal thyroid tissues (all P < 0.05).
Figure 1.
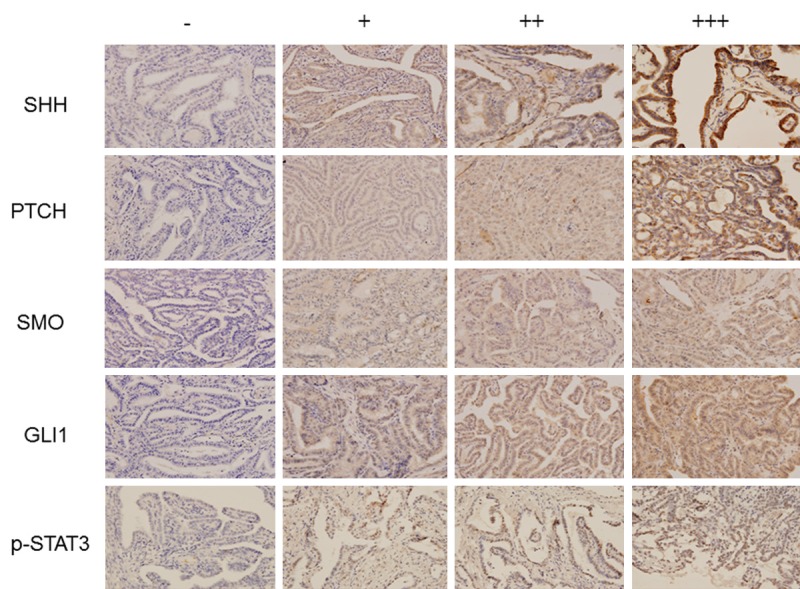
Immunohistochemical staining of SHH, PTCH, SMO, GLI1, and p-STAT3. The SHH signaling molecules and p-STAT3 expression was analyzed by IHC staining with their specifi c antibodies in PTC. Aberrant expression of the SHH signaling molecules and p-STAT3 was detected in PTC and graded as negative (-), positive (+), strongly positive (++), and very strongly positive (+++) (400 X).
Table 1.
Expression of the SHH signaling molecules SHH, PTCH, SMO, and GLI1, as well as p-STAT3 in PTC
| SHH | PTCH | SMO | GLI1 | p-STAT3 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||||||||
| - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | |
| PTC (n = 164) | 49 | 58 | 41 | 16 | 59 | 54 | 39 | 12 | 51 | 56 | 41 | 16 | 59 | 54 | 42 | 9 | 71 | 57 | 32 | 4 |
| adjacent normal tissues (n = 164) | 134 | 27 | 12 | 1 | 133 | 20 | 11 | 0 | 120 | 32 | 10 | 2 | 140 | 17 | 7 | 0 | 146 | 15 | 3 | 0 |
Concomitant upregulation of the SHH pathway molecules and its relationship to clinicopathologic parameters in PTC
It has been well established that activation of the SHH signaling pathway leads to an autocrine upregulation of SHH, PTCH, and GLI1 [22]. We found that the accordance rate between SHH and PTCH expression and between SHH and SMO expression was 75% and 80% respectively, the accordance rate between PTCH and SMO expression was 76%. There was an accordance rate of 77%, 72%, 82% between GLI1 and SHH, PTCH, or SMO expression among 164 patients with PTC. We did not find that the expression of SHH, PTCH, SMO, and GLI1 in PTC correlated with patients’ age and gender. However, all of them positively correlated with larger tumor size, the presence of ETE and LNM, and higher TNM stage (all P < 0.05) (Table 2).
Table 2.
Correlation of the expression of the SHH signaling molecules SHH, PTCH, SMO, and GLI1, as well as p-STAT3 with clinicopathological features in PTC
| SHH | PTCH | SMO | GLI1 | p-STAT3 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||||||||
| Total | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | |
| Gender | |||||||||||||||||||||
| Female | 121 | 34 | 40 | 33 | 14 | 42 | 39 | 31 | 9 | 37 | 39 | 32 | 13 | 41 | 43 | 31 | 6 | 47 | 47 | 26 | 1 |
| Male | 43 | 15 | 18 | 8 | 2 | 17 | 15 | 8 | 3 | 14 | 17 | 9 | 3 | 18 | 12 | 10 | 3 | 24 | 10 | 6 | 3 |
| Age | |||||||||||||||||||||
| ≤ 45 | 87 | 25 | 32 | 21 | 9 | 30 | 28 | 22 | 7 | 27 | 29 | 22 | 9 | 29 | 32 | 24 | 2 | 41 | 26 | 17 | 3 |
| > 45 | 77 | 24 | 26 | 20 | 7 | 29 | 26 | 17 | 5 | 24 | 27 | 19 | 7 | 30 | 23 | 17 | 7 | 30 | 31 | 15 | 1 |
| Tumor Size | |||||||||||||||||||||
| ≤ 2 cm | 84 | 31 | 29 | 16 | 8 | 41 | 24 | 14 | 5 | 37 | 25 | 15 | 7 | 38 | 22 | 20 | 4 | 39 | 29 | 14 | 2 |
| > 2 cm | 80 | 18 | 29 | 25 | 8 | 18 | 30 | 25 | 7 | 14 | 31 | 26 | 9 | 21 | 33 | 21 | 5 | 32 | 28 | 18 | 2 |
| ETE | |||||||||||||||||||||
| Yes | 19 | 4 | 7 | 8 | 0 | 2 | 5 | 9 | 3 | 2 | 6 | 8 | 3 | 2 | 11 | 5 | 1 | 4 | 7 | 6 | 2 |
| No | 145 | 45 | 51 | 33 | 16 | 57 | 49 | 30 | 9 | 49 | 50 | 33 | 13 | 57 | 44 | 36 | 8 | 67 | 50 | 26 | 2 |
| LNM | |||||||||||||||||||||
| Yes | 98 | 13 | 52 | 24 | 9 | 25 | 36 | 29 | 8 | 16 | 42 | 30 | 10 | 18 | 47 | 29 | 4 | 30 | 41 | 23 | 4 |
| No | 66 | 36 | 6 | 17 | 7 | 34 | 18 | 10 | 4 | 35 | 14 | 11 | 6 | 41 | 8 | 12 | 5 | 41 | 16 | 9 | 0 |
| TNM Stage | |||||||||||||||||||||
| I+II | 112 | 39 | 32 | 28 | 13 | 48 | 42 | 17 | 5 | 41 | 43 | 23 | 5 | 46 | 33 | 27 | 6 | 57 | 34 | 18 | 3 |
| III+IV | 52 | 10 | 26 | 13 | 3 | 11 | 12 | 22 | 7 | 10 | 13 | 18 | 11 | 13 | 22 | 14 | 4 | 14 | 23 | 14 | 1 |
ETE, extra thyroidal extension; LNM, lymph node metastasis.
Correlation between STAT3 activation and clinicopathologic parameters in PTC
The expression of activated STAT3 in human thyroid tumors has been poorly characterized. We analyzed nuclear p-STAT3 levels by IHC in a panel of 164 PTC lesions. We found that p-STAT3 expression positively correlated with the presence of ETE and LNM, and higher TNM stage (all P < 0.05) whereas no significant associations were found between p-STAT3 expression and age, gender, tumor size of the PTC patients (Table 2).
Correlation between STAT3 activation and aberrant SHH signaling pathway in PTC
The significant positive correlation between p-STAT3 and SHH, PTCH, SMO and GLI1 expression was found in PTC (r = 0.428, P < 0.001; r = 0.382, P < 0.001; r = 0.407, P < 0.001; r = 0.166, P < 0.05, respectively) (Table 2).
Discussion
As a highly conserved developmental signaling pathway, the SHH signaling pathway is critical for thyroid development. It indirectly governs the symmetric bilobation of the thyroid during late organogenesis and also seems to repress inappropriate thyroid differentiation in nonthyroidal embryonic tissues [23]. It is important to clarify the role of reactivation of this pathway in the process of carcinogenesis and progression in PTC. In this study, we examined the expression of four components of the SHH signaling pathway, SHH, PTCH, SMO, and GLI1 in PTC. We found that these molecules were widely expressed in PTC and more than 64% of PTC tissue samples were positive for these four molecules. The expression of these molecules was increased in PTC tissues compared with the adjacent normal thyroid tissues. These observations suggest that the SHH signaling pathway is activated in thyroid tumorigenesis. This notion is in line with several other studies showing increased expression of the members of the SHH pathway in malignant tumour, such as breast cancer, endometrial adenocarcinomas, and ovarian carcinomas [9,21,24,25]. Our findings are also consistent with the results of the SHH pathway molecules expression reported in other studies of thyroid malignant tumors, including PTC, medullary thyroid carcinoma, and anaplastic thyroid cancer [26-29]. We further found that SHH, PTCH, SMO, and GLI1 were concomitantly upregulated in PTC and positively correlated with larger tumor size, the presence of ETE and LNM, and higher TNM stage, suggesting the activation of the SHH signaling pathway may be involved in thyroid tumor progression. The correlation between the expression of the SHH signaling pathway molecules and the invasive or metastatic potential of malignant tumors has been well documented [25,30-34]. However, Xu et al. did not find any correlation between the status of the expression of the SHH signaling pathway molecules and any clinicopathologic parameters, including the age, gender, the status of BRAF gene mutation, tumor stage, local invasion, and metastasis in PTC. We propose the different sample size might be one of the reasons leading to this inconsistency.
The STAT3 pathway clearly has an important role in the development and progression of many different human tumors [35]. We also detected the STAT3 pathway by the protein expression of p-STAT3 which can be considered as the indicator for this pathway. In our current study, the expression of p-STAT3 was increased in PTC tissues compared with the adjacent normal thyroid tissues. Trovato1 also reported that p-STAT3 expression was higher than the follicular tumors, including normal thyroids, colloid nodules, follicular hyperplasia, oncocytic adenomas, and follicular adenomas. We also found p-STAT3 positively correlated with the presence of ETE and LNM, and higher TNM stage, but not age, gender, tumor size of the PTC patients. Our fingdings are consistent with the report by Zhang et al. that p-STAT3 expression did not correlate with tumor size, but p-STAT3 was significantly upregulated in PTC tumors with metastatic disease. However, other studies show that, in the context of thyroid cancer, STAT3 is paradoxically a negative regulator of tumor growth [36,37]. Kim et al. found basal STAT3 activities are negatively correlated with tumor size in PTC. Couto et al. also observed an inverse relationship between p-STAT3 expression with tumor size and the presence of distant metastases in PTC. These findings suggest that a better understanding of the mechanisms and contexts that predict the dual-edged function of STAT3 in thyroid tumorigenesis is necessary.
Constitutive activation of STAT3 could lead to altered regulation of a number of genes involved in cell cycle and angiogenesis, such as cyclin D1, MMP-2, and VEGF and thus may also be important in neoplastic transformation and metastatic spread [38-40]. STAT3 could also enhance the occurrence and development of tumors through the interaction with other signaling pathways, such as Skp2/p27/p21 pathway and androgen receptor pathway [41,42]. As mentioned above, both the STAT3 and SHH signaling pathway play an important role in the development and progression of many different human tumors and both of them positively correlated with the presence of ETE and LNM, and higher TNM stage in PTC in our study. We then correlated the protein expression of p-STAT3 and the SHH pathway molecules. We found the significant positive correlation between p-STAT3 and SHH, PTCH, SMO and GLI expression, which suggested the internal relationship between the two pathways. The interesting interaction between them was first reported by Sengupta et al. in chronic myeloid leukemia [16]. The authors propose that paracrine SHH activates STAT3, which then turns on the expression of autocrine SHH and Wnt3a in CML cells, making the cells less dependent on the BM for these important self-renewal factors. Then, Yang et al. also found the interaction in pulmonary adenocarcinoma and speculated that activation of STAT3 could up-regulate SHH gene expression directly or indirectly, and thereby activated SHH signaling resulting in lung tumor cell ontogeny [15]. However, we know little about that in PTC before. Thus, we would further verify the internal relationship between the two pathways in in vitro experiments and explore its possible molecular mechanisms in the future study.
In conclusion, our study showed the SHH and STAT3 signaling pathways are frequently activated in PTC and confirmed a crosstalk between these two pathways. The findings may provide rationale to antagonize the interaction of SHH with STAT3 signaling pathway in the prevention and treatment of PTC.
Acknowledgements
This work was supported by the Innovation Team Project of Education Department of Liaoning Province (No. LT2010102), the Liaoning Baiqianwan Talents Project (No. 2010921070), the Scientific and Technological Project of Liaoning Province (No. 2012225087), and Specialized Research Fund for the Doctoral Program of Higher Education (No. 20122104110006).
Disclosure of conflict of interest
None.
References
- 1.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 2.Shi Q, Li S, Jia J, Jiang J. The Hedgehog-induced Smoothened conformational switch assembles a signaling complex that activates Fused by promoting its dimerization and phosphorylation. Development. 2011;138:4219–4231. doi: 10.1242/dev.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oue T, Yoneda A, Uehara S, Yamanaka H, Fukuzawa M. Increased expression of the hedgehog signaling pathway in pediatric solid malignancies. J Pediatr Surg. 2010;45:387–392. doi: 10.1016/j.jpedsurg.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 4.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson CW, Chuang PT. Mechanism and evolution of cytosolic Hedgehog signal transduction. Development. 2010;137:2079–2094. doi: 10.1242/dev.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 7.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 8.Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Hui M, Cazet A, Nair R, Watkins DN, O’Toole SA, Swarbrick A. The Hedgehog signalling pathway in breast development, carcinogenesis and cancer therapy. Breast Cancer Res. 2013;15:203. doi: 10.1186/bcr3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 11.Bahra M, Kamphues C, Boas-Knoop S, Lippert S, Esendik U, Schuller U, Hartmann W, Waha A, Neuhaus P, Heppner F, Pietsch T, Koch A. Combination of hedgehog signaling blockage and chemotherapy leads to tumor reduction in pancreatic adenocarcinomas. Pancreas. 2012;41:222–229. doi: 10.1097/MPA.0b013e31822896dd. [DOI] [PubMed] [Google Scholar]
- 12.Lyseng-Williamson KA, Keating GM. Vismodegib: a guide to its use in locally advanced or metastatic basal cell carcinoma. Am J Clin Dermatol. 2013;14:61–64. doi: 10.1007/s40257-012-0004-6. [DOI] [PubMed] [Google Scholar]
- 13.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J. Clin. Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank DA. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 2007;251:199–210. doi: 10.1016/j.canlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Yang Q, Shen SS, Zhou S, Ni J, Chen D, Wang G, Li Y. STAT3 activation and aberrant ligand-dependent sonic hedgehog signaling in human pulmonary adenocarcinoma. Exp Mol Pathol. 2012;93:227–236. doi: 10.1016/j.yexmp.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta A, Banerjee D, Chandra S, Banerji SK, Ghosh R, Roy R, Banerjee S. Deregulation and cross talk among Sonic hedgehog, Wnt, Hox and Notch signaling in chronic myeloid leukemia progression. Leukemia. 2007;21:949–955. doi: 10.1038/sj.leu.2404657. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Zhu W, Li Y. Discovery of novel inhibitors of signal transducer and activator of transcription 3 (STAT3) signaling pathway by virtual screening. Eur J Med Chem. 2013;62:301–310. doi: 10.1016/j.ejmech.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Sansone P, Bromberg J. Environment, inflammation, and cancer. Curr Opin Genet Dev. 2011;21:80–85. doi: 10.1016/j.gde.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Mete O, Rotstein L, Asa SL. Controversies in thyroid pathology: thyroid capsule invasion and extrathyroidal extension. Ann Surg Oncol. 2010;17:386–391. doi: 10.1245/s10434-009-0832-7. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 21.Kim KH, Kim JM, Choi YL, Shin YK, Lee HC, Seong IO, Kim BK, Chae SW, Chung YS, Kim SH. Expression of sonic hedgehog signaling molecules in normal, hyperplastic and carcinomatous endometrium. Pathol Int. 2009;59:279–287. doi: 10.1111/j.1440-1827.2009.02366.x. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 23.Fagman H, Grande M, Gritli-Linde A, Nilsson M. Genetic deletion of sonic hedgehog causes hemiagenesis and ectopic development of the thyroid in mouse. Am J Pathol. 2004;164:1865–1872. doi: 10.1016/S0002-9440(10)63745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Horiuchi A, Kikuchi N, Osada R, Yoshida J, Shiozawa T, Konishi I. Hedgehog signal pathway is activated in ovarian carcinomas, correlating with cell proliferation: it’s inhibition leads to growth suppression and apoptosis. Cancer Sci. 2007;98:68–76. doi: 10.1111/j.1349-7006.2006.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding YL, Wang QS, Zhao WM, Xiang L. Expression of smoothened protein in colon cancer and its prognostic value for postoperative liver metastasis. Asian Pac J Cancer Prev. 2012;13:4001–4005. doi: 10.7314/apjcp.2012.13.8.4001. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Ding H, Rao G, Arora S, Saclarides CP, Esparaz J, Gattuso P, Solorzano CC, Prinz RA. Activation of the Sonic Hedgehog pathway in thyroid neoplasms and its potential role in tumor cell proliferation. Endocr Relat Cancer. 2012;19:167–179. doi: 10.1530/ERC-11-0305. [DOI] [PubMed] [Google Scholar]
- 27.Nelson KK, Gattuso P, Xu X, Prinz RA. Expression of the sonic hedgehog pathway molecules in synchronous follicular adenoma and papillary carcinoma of the thyroid gland in predicting malignancy. Surgery. 2010;148:654–660. doi: 10.1016/j.surg.2010.07.030. discussion 660. [DOI] [PubMed] [Google Scholar]
- 28.Bohinc B, Michelotti G, Diehl AM. Hedgehog signaling in human medullary thyroid carcinoma: a novel signaling pathway. Thyroid. 2013;23:1119–1126. doi: 10.1089/thy.2012.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinterseher U, Wunderlich A, Roth S, Ramaswamy A, Bartsch DK, Hauptmann S, Greene BH, Fendrich V, Hoffmann S. Expression of hedgehog signalling pathway in anaplastic thyroid cancer. Endocrine. 2014;45:439–47. doi: 10.1007/s12020-013-0015-y. [DOI] [PubMed] [Google Scholar]
- 30.Mori Y, Okumura T, Tsunoda S, Sakai Y, Shimada Y. Gli-1 expression is associated with lymph node metastasis and tumor progression in esophageal squamous cell carcinoma. Oncology. 2006;70:378–389. doi: 10.1159/000098111. [DOI] [PubMed] [Google Scholar]
- 31.Cavicchioli Buim ME, Gurgel CA, Goncalves Ramos EA, Lourenco SV, Soares FA. Activation of sonic hedgehog signaling in oral squamous cell carcinomas: a preliminary study. Hum Pathol. 2011;42:1484–1490. doi: 10.1016/j.humpath.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Kim TJ, Lee JY, Hwang TK, Kang CS, Choi YJ. Hedgehog signaling protein expression and its association with prognostic parameters in prostate cancer: a retrospective study from the view point of new 2010 anatomic stage/prognostic groups. J Surg Oncol. 2011;104:472–479. doi: 10.1002/jso.21988. [DOI] [PubMed] [Google Scholar]
- 33.Chen XL, Cheng QY, She MR, Wang Q, Huang XH, Cao LQ, Fu XH, Chen JS. Expression of sonic hedgehog signaling components in hepatocellular carcinoma and cyclopamine-induced apoptosis through Bcl-2 downregulation in vitro. Arch Med Res. 2010;41:315–323. doi: 10.1016/j.arcmed.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 34.He HC, Chen JH, Chen XB, Qin GQ, Cai C, Liang YX, Han ZD, Dai QS, Chen YR, Zeng GH, Zhu JG, Jiang FN, Zhong WD. Expression of hedgehog pathway components is associated with bladder cancer progression and clinical outcome. Pathol Oncol Res. 2012;18:349–355. doi: 10.1007/s12253-011-9451-2. [DOI] [PubMed] [Google Scholar]
- 35.Devarajan E, Huang S. STAT3 as a central regulator of tumor metastases. Curr Mol Med. 2009;9:626–633. doi: 10.2174/156652409788488720. [DOI] [PubMed] [Google Scholar]
- 36.Kim WG, Choi HJ, Kim WB, Kim EY, Yim JH, Kim TY, Gong G, Kim SY, Chung N, Shong YK. Basal STAT3 activities are negatively correlated with tumor size in papillary thyroid carcinomas. J Endocrinol Invest. 2012;35:413–418. doi: 10.3275/7907. [DOI] [PubMed] [Google Scholar]
- 37.Couto JP, Daly L, Almeida A, Knauf JA, Fagin JA, Sobrinho-Simoes M, Lima J, Maximo V, Soares P, Lyden D, Bromberg JF. STAT3 negatively regulates thyroid tumorigenesis. Proc Natl Acad Sci U S A. 2012;109:E2361–2370. doi: 10.1073/pnas.1201232109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang JH, Kim DW, Suh JM, Kim H, Song JH, Hwang ES, Park KC, Chung HK, Kim JM, Lee TH, Yu DY, Shong M. Activation of signal transducer and activator of transcription 3 by oncogenic RET/PTC (rearranged in transformation/papillary thyroid carcinoma) tyrosine kinase: roles in specific gene regulation and cellular transformation. Mol Endocrinol. 2003;17:1155–1166. doi: 10.1210/me.2002-0401. [DOI] [PubMed] [Google Scholar]
- 39.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21(Suppl 2):S37–43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang SA, Schachtschneider P, Moser C, Mori A, Hackl C, Gaumann A, Batt D, Schlitt HJ, Geissler EK, Stoeltzing O. Dual targeting of Raf and VEGF receptor 2 reduces growth and metastasis of pancreatic cancer through direct effects on tumor cells, endothelial cells, and pericytes. Mol Cancer Ther. 2008;7:3509–3518. doi: 10.1158/1535-7163.MCT-08-0373. [DOI] [PubMed] [Google Scholar]
- 41.Wei Z, Jiang X, Qiao H, Zhai B, Zhang L, Zhang Q, Wu Y, Jiang H, Sun X. STAT3 interacts with Skp2/p27/p21 pathway to regulate the motility and invasion of gastric cancer cells. Cell Signal. 2013;25:931–938. doi: 10.1016/j.cellsig.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda T, Junicho A, Yamamoto T, Kishi H, Korkmaz K, Saatcioglu F, Fuse H, Muraguchi A. Cross-talk between signal transducer and activator of transcription 3 and androgen receptor signaling in prostate carcinoma cells. Biochem Biophys Res Commun. 2001;283:179–187. doi: 10.1006/bbrc.2001.4758. [DOI] [PubMed] [Google Scholar]


