Abstract
MicroRNAs like miR-143 are increasingly linked to disease pathogenesis. miR-143 is enriched in vascular smooth muscle, and several single nucleotide polymorphisms have been identified in this miRNA. The aim of the current study was to explore a potential correlation between a polymorphism in the miR-143 promoter region, rs4705342, and essential hypertension (EH). Genotyping for miR-143 rs4705342 was performed from blood samples of 156 EH patients (case group) and 187 healthy individuals (control group) using a TaqMan assay. Participant demographic and clinical characteristics were also collected. Logistic regression was used to identify an association between genotype and EH, and odds ratios of EH risk were also determined. Frequencies of the CC, CT, and TT genotypes differed significantly between case (7.7%, 40.4%, 51.9%) and control (15.0%, 48.1%, 36.9%) groups (χ2 = 9.400, P = 0.009). Further, the frequency of the C allele was lower in the case group than in the control group (27.9% vs. 39.0%, P = 0.002). Compared with those having the TT genotype, patients carrying the CC and CT genotypes had a significantly reduced risk for EH (OR = 0.541, 95% CI = 0.351-0.834, P = 0.005), particularly for females, nonsmokers, and those not consuming alcohol (P < 0.05). Thus, the rs4705342 polymorphism in the miR-143 appears to be associated with essential hypertension, and further study is needed to understand the molecular mechanism producing this effect.
Keywords: miR-143 gene, promoter region, single nucleotide polymorphism, essential hypertension
Introduction
Essential hypertension (EH) is the major cause of cardiovascular and cerebrovascular diseases, and its prevalence has been on the rise globally. Indeed, in 2000 the global prevalence of EH was 26.4% in adults; this prevalence is predicted to reach 29.2% by 2025 [1]. EH arises through a complex interplay of both genetic and environmental factors [2]. Its pathogenesis involves upregulation of the renin- angiotensin-aldosterone system, which leads to vascular endothelial dysfunction, hypertrophy of vascular smooth muscle (VSM) and heart muscle, damaged platelet function, and changes in the development of new blood vessels [3].
In recent years, studies of microRNAs (miRNAs) have revealed their varied and numerous roles in disease pathogenesis. These small, non-coding RNAs degrade target mRNAs or inhibit their translation to negatively regulate gene expression [4,5]. One miRNA, miR-143, is enriched in the VSM [6], and can be used as a biomarker for assessing the extent of coronary atherosclerosis [7]. Further, changes in expression of this miRNA have been associated with hypertension [8]. Several polymorphisms have been identified in miR-143 [9], but it remains unclear how these polymorphisms affect miR-143 expression or disease pathogenesis. One polymorphism, rs4705342, occurs in the promoter region of miR-143 and is of interest for its potential contribution to expression of the miRNA. The current study sought to explore the contribution of this polymorphism in miR-143 to EH using a case-control study. The results provide a new avenue to investigate the prevention and treatment of EH.
Methods
Study population
The study included 156 EH patients (case group) who were admitted to The First Affiliated Hospital of Zhengzhou University between January 2012 and December 2013. All of them met WHO/ISH Hypertension Guidelines, i.e., systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg. Patients were excluded for having secondary hypertension, heart disease, nervous system abnormalities, liver or kidney dysfunction, hyperthyroidism or hypothyroidism, and chronic obstructive pulmonary disease. As a control group, the study included 187 healthy individuals who received physical examinations in the same period and were diagnosed with no hypertension, diabetes, or hyper lipoidemia. All participants provided informed consent. This study was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University.
Blood samples
Peripheral blood samples (5 mL) were collected in the morning from each fasting participant. EDTA was used as an anticoagulant; within 24 hours, blood samples were centrifuged at 4000 rpm for 10 min to separate plasma. An aliquot of each sample was used to measure serum total cholesterol (TC) by enzyme method, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and fasting blood glucose (GLU) with the reagents and kits from bioMérieux®sa (Lyon, France). The remaining blood samples were stored at -20°C.
Genotyping
DNA was extracted from blood samples by standard phenol-chloroform extraction. Ultraviolet spectrophotometry was used to determine the purity (OD 260/280 ratio) and concentration (OD260) of DNA. A TaqMan method was used to perform genotyping for rs4705342 as a polymorphic site in the promoter region of miR-143. Primer sequences were 5′-GAGGAGGAGTGGCAGAAGAA-3′ (upstream) and 5′-GTTGACCTACCTTTTGATGCC-3′ (downstream), and labeled probe sequences (Biowing Applied Biotechnology, Shanghai, China) were 5′-FAM-CATCATTCGTGGTACTT-MGB-3′ for rs4705342-P-G and 5′-HEX-CATCATTCATGGTACTT-MGB-3′ for rs4705342-P-A. TaqMan® Universal PCR Master Mix (TaKaRa Biotechnology Ltd., Dalian, China) was combined with primers and probes at appropriate concentrations to perform a polymerase chain reaction (PCR) in a 5-µL reaction volume. Thermal cycling conditions were as follows: preheating at 50°C for 2 min; initial denaturation at 90°C for 10 min; and 45 cycles of denaturation at 95°C for 15 s and annealing at 60°C for 1 min. An ABI Prism®7900HT (Applied Biosystems) was used to finish the amplification of target fragments and sequencing.
Statistical analysis
SPSS17.0 was used for statistical analysis. A chi-square goodness of fit test was used to determine whether the genotype distribution at the polymorphic site was consistent with Hardy-Weinberg equilibrium. Pearson’s χ2 test was used to compare the difference in genotype distributions in EH patients and healthy controls. An unconditional logistic regression was used to calculate the odds ratio (OR) and its 95% confidence interval (95% CI) to analyze an association between genotype and EH. These tests were two-sided, with an α = 0.05, and P < 0.05 was considered statistically significant.
Results
Comparison of population characteristics by group
No statistically significantly differences were observed between the EH group and the control group forage, sex, smoking status, or alcohol consumption (P > 0.05). However, significant differences were observed for clinical characteristics: the EH group had significantly higher TG, TC, LDL-C, GLU, BMI, SBP, and DBP and significantly lower HDL-C (P < 0.05; Table 1).
Table 1.
Demographic and clinical characteristics of the study population
| Items | EH (n = 156) | Controls (n = 187) | t/χ2 | P | |
|---|---|---|---|---|---|
| Age (years) | 55.8 ± 13.8 | 56.1 ± 13.9 | 0.229 | 0.819 | |
| Gender | 2.536 | 0.111 | |||
| Male | 104 (66.7) | 109 (58.3) | |||
| Female | 52 (33.3) | 78 (41.7) | |||
| TC (mmol/L) | 5.69 ± 0.47 | 5.18 ± 0.45 | 10.348 | 0.001 | |
| TG (mmol/L) | 1.69 ± 0.26 | 1.24 ± 0.17 | 19.337 | 0.001 | |
| HDL-C (mmol/L) | 1.53 ± 0.29 | 1.84 ± 0.30 | 9.754 | 0.001 | |
| LDL-C (mmol/L) | 3.40 ± 0.46 | 2.95 ± 0.28 | 11.223 | 0.001 | |
| GLU (mmol/L) | 5.98 ± 1.15 | 5.37 ± 1.12 | 5.016 | 0.001 | |
| BMI (kg/m2) | 25.1 ± 2.9 | 22.1 ± 2.2 | 10.844 | 0.001 | |
| SBP (mmHg) | 160.2 ± 11.0 | 113.2 ± 13.9 | 34.427 | 0.001 | |
| DBP (mmHg) | 100.1 ± 6.2 | 72.6 ± 7.9 | 35.455 | 0.001 | |
| Smoking status | 0.279 | 0.597 | |||
| Nonsmoker | 106 (67.9) | 132 (70.6) | |||
| Smoker | 50 (32.1) | 55 (29.4) | |||
| Alcohol consumption | 0.664 | 0.415 | |||
| No | 102 (65.4) | 130 (69.5) | |||
| Yes | 54 (34.6) | 57 (30.5) |
Inter-group differences in genotype at rs4705342
The frequencies of the CC, CT, and TT genotypes were compared at rs4705342in the promoter region of miR-143. In the control population, the frequencies of CC, CT, and TT genotypes were 15.0% (28/187), 48.1% (90/187), and 36.9% (69/187), respectively. In the EH case group, the frequencies of CC, CT, and TT genotypes were 7.7% (12/156), 40.4% (63/156), and 51.9% (81/156), respectively. The frequencies of C and T alleles were 39.0% (146/274 alleles) and 61.0% (228/374), respectively, in the control group and 27.9% (87/312 alleles) and 72.19% (225/312 alleles) in the EH case group (Figure 1).
Figure 1.
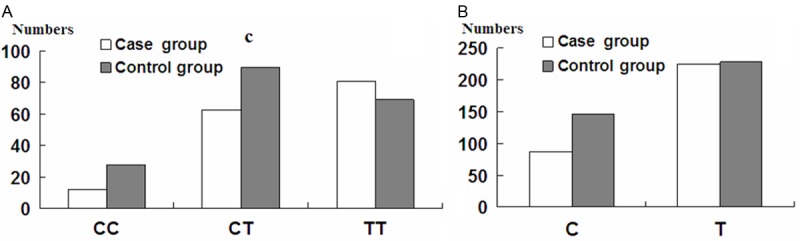
Frequency of genotype (A) and allele (B) in EH patients (case) and the control group.
Statistical analysis revealed that the genotypes for rs4705342 in miR-143 in the control group were consistent with Hardy-Weinberg equilibrium (χ2 = 5.032, P = 0.081), which indicated that the control group in this study was representative of the population. A statistically significant difference in genotype distributions was detected between the control group and the EH group (χ2 = 9.400, P = 0.009). Similarly, allele frequencies differed significantly between groups (χ2 = 9.433, P = 0.002).
Relationship between polymorphisms of rs4705342 in the gene promoter region of miR-143 and EH
Logistic regression (Table 2) identified a lower risk (OR, 0.541) of EH in individuals carrying the C allele (CC+CT) than in individuals with the TT genotype (95% CI = 0.351-0.834, P = 0.005).A stratified analysis was performed according to age, gender, smoking status, and alcohol consumption. This analysis revealed that the risk of EH was lower in individuals carrying the C allele (CT+CC) who were females, nonsmokers, or nondrinkers (P < 0.05).
Table 2.
Association between rs4705342 polymorphism in the miR-143promoter region and EH
| Characteristic | B | SE | Wald | OR | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| All | -0.614 | 0.221 | 7.738 | 0.541 | 0.351-0.834 | 0.005 | |
| Gender | |||||||
| Male | -0.517 | 0.285 | 3.296 | 0.596 | 0.341-1.042 | 0.069 | |
| Female | -0.980 | 0.374 | 6.882 | 0.375 | 0.180-0.780 | 0.009 | |
| Smoking Status | |||||||
| Nonsmoker | -1.183 | 0.273 | 18.833 | 0.306 | 0.179-0.523 | 0.001 | |
| Smoker | 0.627 | 0.402 | 2.428 | 1.872 | 0.851-4.119 | 0.119 | |
| Alcohol consumption | |||||||
| No | -0.859 | 0.271 | 10.083 | 0.423 | 0.249-0.720 | 0.001 | |
| Yes | -0.163 | 0.394 | 0.172 | 0.849 | 0.393-1.837 | 0.678 |
Discussion
Hypertension has a high incidence in the population and is a common cause of death worldwide [10]. Small RNA molecules appear to play a significant role in the development of hypertension [11]. One aspect of the physiological changes related to hypertension is within VSM cells, which are important in end arterial hyperplasia. Differentiated phenotypes of VSM cells in normal adults are mainly located in the tunica media [12]. However, under the stimulation of various factors for injury, the differentiated phenotypes may be transformed into undifferentiated phenotypes, and the cells proliferate, migrate to the intimal layer, and lead to intimal hyperplasia and stenosis. These pathological changes, i.e., intimal hyperplasia and VSM phenotype transformation, can be promoted by hypertension [13,14]. miR-143, enriched in the VSM [6], can regulate the proliferation and apoptosis of smooth muscle cells and adjust the static and proliferative phenotypes of VSM cells [15-17]. Its expression tends to be inhibited under pathological states such as trauma or atherosclerosis, thus reducing VSM differentiation. The expression of miR-143 declines with the progression of atherosclerosis, which indicates miR-143 may be involved in the pathological process of hypertensive arterial lesions [18,19].
In this case-control study analyzing the relationship between a single nucleotide polymorphism, rs4705342, in the promoter region of miR-1 and EH, genotype was found to be associated with the risk of EH. Compared with the TT genotype, carrying the C allele (CT and CC genotypes) appears to reduce the risk of getting EH, especially in females, nonsmokers, and those who do not consume alcohol. Thus, the rs4705342 single nucleotide polymorphism in miR-143 is associated with genetic susceptibility to EH, with the C allele potentially reducing risk for EH. However, the occurrence of EH is a complex process involving both polygenic factors and environmental factors. Therefore, the experimental results in this study must be verified in other populations and larger samples, and understanding the mechanisms of genetic susceptibility requires in-depth molecular and biochemical studies.
Acknowledgements
This work was supported by Science and Technology Department of Henan Province (No. 112102310180).
Disclosure of conflict of interest
None.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- 3.Okura T, Higaki J. Etiology of essential hypertension. Nihon Naika Gakkai Zasshi. 2007;96:4–8. doi: 10.2169/naika.96.4. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava K, Srivastava A. Comprehensive review of genetic association studies and meta- analyses on miRNA polymorphisms and cancer risk. PLoS One. 2012;7:e50966. doi: 10.1371/journal.pone.0050966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwai N, Naraba H. Polymorphisms in human pre-miRNAs. Biochem Biophys Res Commun. 2005;331:1439–1444. doi: 10.1016/j.bbrc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 6.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 7.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 8.Kontaraki JE, Marketou ME, Zacharis EA, Parthenakis FI, Vardas PE. Differential expression of vascular smooth muscle-modulating microRNAs in human peripheral blood mononuclear cells: novel targets in essential hypertension. J Hum Hypertens. 2014;28:510–6. doi: 10.1038/jhh.2013.117. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Pan X, Li Z, Bai P, Jin H, Wang T, Song C, Zhang L, Gao L. Association between polymorphisms in the promoter region of miR-143/145 and risk of colorectal cancer. Hum Immunol. 2013;74:993–7. doi: 10.1016/j.humimm.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Novo S, Lunetta M, Evola S, Novo G. Role of ARBs in the blood hypertension therapy and prevention of cardiovascular events. Curr Drug Targets. 2009;10:20–25. doi: 10.2174/138945009787122897. [DOI] [PubMed] [Google Scholar]
- 11.Dou XK, Xu GF, Yang W. The role of miRNAs in the occurrence and development of hypertension. International Journal of Cardiovascular Disease. 2012;39:147–149. [Google Scholar]
- 12.Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, Vatner DE, Meininger GA, Vatner SF. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1281–1287. doi: 10.1152/ajpheart.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph BK, Thakali KM, Moore CL, Rhee SW. Ion channel remodeling in vascular smooth muscle during hypertension: Implications for novel therapeutic approaches. Pharmacol Res. 2013;70:126–138. doi: 10.1016/j.phrs.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudano I, Roas S, Noll G. Vascular abnormalities in essential hypertension. Curr Pharm Des. 2011;17:3039–3044. doi: 10.2174/138161211798157766. [DOI] [PubMed] [Google Scholar]
- 15.Parmacek MS. MicroRNA-modulated targeting of vascular smooth muscle cells. J Clin Invest. 2009;119:2526–2528. doi: 10.1172/JCI40503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet. 2011;4:197–205. doi: 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 17.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rosa S, Curcio A, Indolfi C. Emerging role of microRNAs in cardiovascular diseases. Circ J. 2014;78:567–575. doi: 10.1253/circj.cj-14-0086. [DOI] [PubMed] [Google Scholar]
- 19.Pleister A, Selemon H, Elton SM, Elton TS. Circulating miRNAs: novel biomarkers of acute coronary syndrome? Biomark Med. 2013;7:287–305. doi: 10.2217/bmm.13.8. [DOI] [PubMed] [Google Scholar]


