Abstract
Background
Autosomal recessive, loss-of-function mutations in DOCK8 cause a combined immunodeficiency characterized by atopy, recurrent infections, and cancer susceptibility. A genotype-phenotype explanation for the variable disease expression is lacking.
Objective
We investigated whether reversions contributed to the variable disease expression.
Methods
Patients followed at the NIH Clinical Center were studied. We performed detailed genetic analyses and intracellular flow cytometry to detect DOCK8 protein expression within lymphocyte subsets.
Results
We identified 17 out of 34 DOCK8-deficient patients who had germline mutations with variable degrees of reversion due to somatic repair. Somatic repair of the DOCK8 mutations resulted from second-site mutation, original-site mutation, gene conversion, and intragenic crossover. Higher degrees of reversion were associated with recombination-mediated repair. DOCK8 expression was restored primarily within antigen-experienced T cells or in NK cells, but less so in naïve T cells or B cells. Several patients exhibited multiple different repair events. Patients who had reversions were older and had less severe allergic disease, although infection susceptibility persisted. No patients were cured without hematopoietic cell transplantation.
Conclusions
In DOCK8 deficiency, only certain combinations of germline mutations supported secondary somatic repair. Those patients had an ameliorated disease course with longer survival, but still had fatal complications or required hematopoietic cell transplantation. These observations support the concept that some DOCK8 immunodeficient patients have mutable mosaic genomes that may modulate disease phenotype over time.
Keywords: DOCK8, reversion, somatic repair, recombination, gene conversion, intragenic single crossover, T cell, NK cell, allergy, immunodeficiency
INTRODUCTION
DOCK8 immunodeficiency is caused by autosomal recessive mutations in the DOCK8 gene, which encodes an atypical guanine-nucleotide exchange factor for CDC42 and RAC activation.1, 2 Initially described as a hyper-immunoglobulinemia E syndrome, this combined immunodeficiency features atopy, recurrent cutaneous and sinopulmonary infections, and cancer susceptibility.3 Typically, patients develop diffuse eczematous dermatitis with bacterial skin infections early in life, along with respiratory tract infections and severe food allergies accompanied by anaphylaxis, asthma, elevated serum IgE, and eosinophilia. Intractable viral infections of the skin are caused by herpes simplex virus (HSV), molluscum contagiosum virus (MCV), varicella-zoster virus (VZV), and/or human papillomavirus (HPV).4 Mucocutaneous candidiasis can also occur. Death from infections or cancers usually occurs by late adolescence or early adulthood. However, in some patients the disease course is more aggressive, with severe skin disease and life-threatening infections developing at an earlier age.5, 6 Furthermore, patients have been identified who lack atopic dermatitis, food allergies, elevated serum IgE, and/or eosinophilia. As known pathogenic mutations in DOCK8 cause loss of protein expression, a molecular explanation for the phenotypic variability remains lacking.
Loss of DOCK8 expression within T cells, B cells, NK cells, and NKT cells can cause abnormal cytokine production including T helper type 2 (TH2) skewing, as well as defects in activation, proliferation, survival, affinity maturation, and cytotoxicity.1–3, 7–12 T cells play a major role in disease pathogenesis, as the infection susceptibility is cured by hematopoietic cell transplantation (HCT) when nearly complete donor T-cell chimerism is achieved, even when other leukocyte subsets are of partial donor origin.13, 14 HCT also cures or significantly ameliorates atopic dermatitis, food allergies, elevated serum IgE, and hypereosinophilia.13, 15–17 However, the minimal level and type of T-cell reconstitution required for cure, as well as the relative contributions of other lymphocytes, are unknown.
Naturally arising somatic reversions of germline mutations have been observed in several primary immunodeficiency disorders, including the Wiskott-Aldrich syndrome, severe combined immunodeficiencies, and X-linked lymphoproliferative disease.18–20 Such cases have provided insights into the relative contributions of loss-of-function mutations in different cell types. Here we sought to determine the circumstances by which reversions occurred in DOCK8 immunodeficiency, and whether they could explain phenotypic differences among patients.
METHODS
Study subjects
Patients and their relatives provided written informed consent and were investigated under NIAID Institutional Review Board approved research protocols. Patients 2, 3, 4, 5, 13, 18, and 21 were previously reported as 8–2, 4–1, 4–2, 5–2, 6–1, 2–1, and 1–1, respectively.1 Patient 1 was reported as ARH011.3.2 Patients 9, 10, 11, 19, 22, 23, 24, and 27 were also reported elsewhere.4, 11, 21 The median ages of patients were calculated from the age of living patients at most recent evaluation at the NIH or when transplanted, or age at death of deceased patients. Disease severity was scored according to criteria listed in Table E1.
Detailed procedures regarding cell preparation, array comparative hybridization, immunoblotting, flow cytometry, sequencing, and statistical analyses are provided in the Methods section of this article’s Online Repository.
RESULTS
Identification of patients who had somatically repaired their germline DOCK8 mutations
DOCK8 immunodeficiency is caused by autosomal recessive, loss-of-function mutations in the DOCK8 gene.1, 2 We have followed 34 DOCK8-deficient patients from 23 families at the Clinical Center of the National Institutes of Health. Seventeen patients from 11 families formed the core of this study. Clinical diagnoses of DOCK8 immunodeficiency were confirmed by mutational analyses showing germline loss-of-function mutations in both DOCK8 alleles (Table 1, columns 3 and 5; Fig 2; Fig E1–E8).
Table 1.
DOCK8 mutational analyses in the NIH patients who have somatic repair
| Patient | Germline mutations | Nomenclature | Mechanism of somatic repair | Supporting evidence |
|---|---|---|---|---|
| 1 | Homozygous splicing mutations (exon 11) | c.1214A>G, p.K405RfsX15 | Second-site mutations differing in T cells and NK cells | Ref (2); Fig E1 |
| 2 | Not determined – second-site mutation or original-site reversion | Ref (1), (2) | ||
| 3 | Large deletion (entire gene) + nonsense mutation (exon 11) | Chr9:g.(163,190_204,193) _ (538,588_544,450)del, plus c.1153G>T, pE385X | Second-site mutation | Ref (1); Fig 2A |
| 4 | Original-site reversion | |||
| 5 | Large deletion (promoter to exon 17) + nonsense mutation (exon 8) | Chr9:g.(163,190_204,193) _ (361,777_370,184)del, plus c.745C>T, p. R249X | Not determined – second-site mutation or original-site reversion | Ref (1) |
| 6 | Homozygous nonsense mutations (exon 19) | c.2044G>T, p.E682X | Not determined – second-site mutation or original-site reversion | Fig E2 |
| 7 | Homozygous nonsense mutations (exon 41) | c.5182C>T, p.R1728X | Not determined – second-site mutation or original-site reversion | Fig E2 |
| 8 | Not determined – second-site mutation or original-site reversion | |||
| 9 | Small indel (exon 19) + missense mutation (exon 44) | c.2174_2175delinsAC>T, p.H725LfsX45, plus c.5627C>T, p.P1876L | Intragenic single crossover | Fig 2C; Fig E3 |
| 10 | Large deletion (exon 21 to end of gene) + small indel with frameshift mutation (exon 12) | Chr9:g.(383,073_383,756) _ (474,634_474,667)del, plus c.1266delC, p.W423TfsX18 | Gene conversion (exon 12) | Fig 2B; Fig E4 |
| 11 | ||||
| 12 | Large deletion (promoter to exon 13) + small indel with frameshift mutation (exon 32) | Chr9:g.(1_163,131)_ (368,288_ 368,361)del, plus c.4031_4032insT, p.D1344RfsX2 | Intragenic single crossover. Additional intragenic double crossover. | Fig E5 |
| 13 | Large deletion (exons 13 to 26) + splicing mutation (intron 5) | Chr9:g.(340,142_356,076) _ (405,056_416,292)del, plus c.538-18C>G, p.E180VfsX4 | Intragenic single crossover, or gene conversion (exons 13 to 26) | Ref (1); Fig E6 |
| 14 | Nonsense mutation (exon 17) + small indel with frameshift mutation (exon 36) | c.1895G>A, p.W602X, plus c.4540delG, p.E1514KfsX8 | Gene conversion differing in T cells (exon 17) and NK cells (exon 36) | Fig E7 |
| 15 | Not determined – all possible | |||
| 16 | Large deletion (exons 5 to 9) + splicing point mutation (intron 23) | Chr9:g.(300,972_301,582) _(323,232_323,291)del, c.(325_921del), p.A109_K307del, plus c.2767-1G>A, p.K924TfsX15 | Intragenic single crossover | Fig E8 |
| 17 | Gene conversion (intron 23), or original-site reversion (intron 23) |
Germline mutational analyses were performed on genomic DNA isolated from neutrophils, and in some cases also Herpesvirus saimiri (HVS)-transformed T cells or Epstein-Barr Virus (EBV)-transformed B cells. Somatic mutational analyses were performed on genomic DNA and cDNA as indicated. Parenthetical information indicates where the germline mutation or somatic repair occurred. See Fig 2 and Fig E1-E8 for supporting genetic data. Patients 1–8 had point mutation-mediated repair, whereas patients 9–17 had probable recombination-mediated repair.
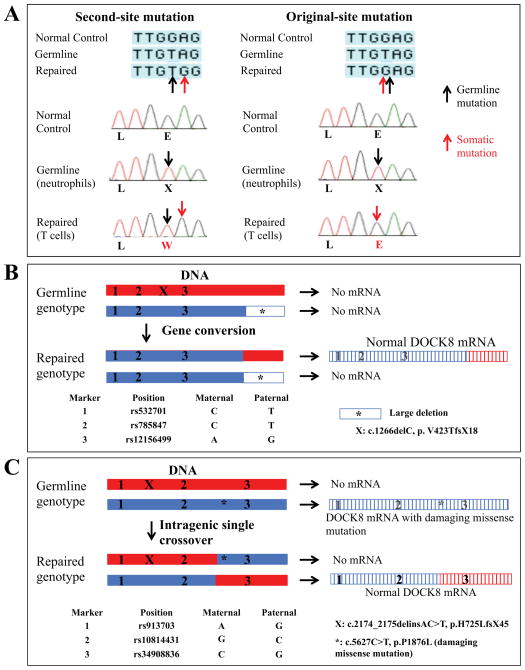
FIG 2.
Mechanisms underlying somatic repair of DOCK8 mutations. Representative examples. A, Second-site mutation in Patient 3 (left). Original-site reversion in Patient 4 (right). Black and red arrows designate germline and somatic mutations, identified from DNA of neutrophils and primary T cells, respectively. B, Gene conversion in Patients 10 or 11. C, Intragenic single crossover in Patient 9. Red and blue designate maternally- and paternally-derived alleles, respectively, as inferred by the genotyped SNPs and mutations. Hatching indicates mRNA. Additional details are provided in Fig E3 and E4.
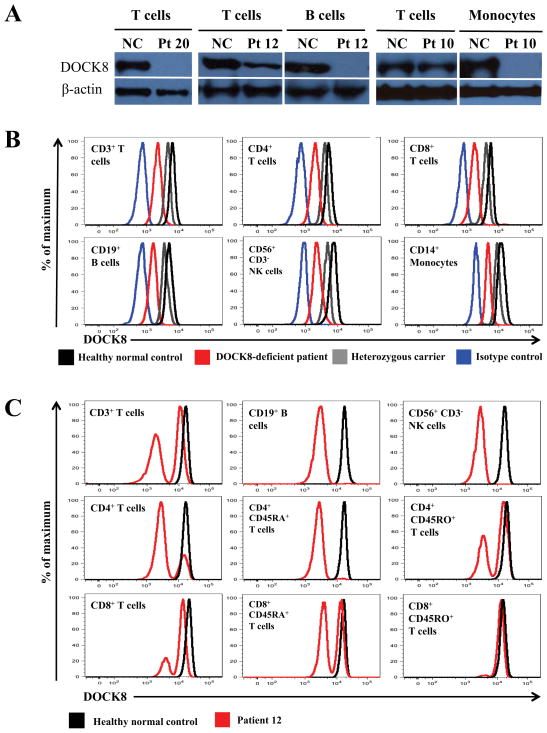
DOCK8-deficient patients normally express no DOCK8 protein in lysates from purified primary T cells (Fig 1, A, left panel). As expected, patients also expressed no DOCK8 in B cells (Fig 1, A, middle panel) or monocytes (Fig 1, A, right panel). However, in some patients, normal or near normal levels of DOCK8 were detected in primary T cells (Fig 1, A, middle and right panels). The discrepancy between germline mutations and actual protein expression suggested somatic mosaicism occurring within T cells. The germline mutations had been identified by sequencing genomic DNA from neutrophils. When we compared these against mutational analyses performed on primary T cells and in some cases NK cells, we discovered somatic repair in 17 patients (Table 1, column 4).
FIG 1.
DOCK8 immunodeficient patients having T cell reversions. A, Immunoblotting for DOCK8 or β-actin proteins in primary T cells, B cells, or monocytes. NC, normal healthy control. Patient 20 has a homozygous large deletion. Patients 12 and 10 have somatic repair. B, Representative flow cytometry histograms showing intracellular DOCK8 expression in gated subsets from a normal healthy control (black), DOCK8 heterozygous carrier (grey), Patient 19 with a large homozygous deletion (red), or isotype control staining (blue). C, Histograms are from Patient 12 who has somatic repair.
Somatic repair could be categorized into one of three groups. In the first group, somatic repair resulted from point mutations, which corrected for germline-encoded deleterious single base substitutions. Patients 1 and 3 had second-site mutations (Fig E1; Fig 2, A, left panel), whereas Patient 4 had an original-site mutation (Fig 2, A, right panel). These abolished use of the germline-encoded cryptic splice site or premature stop codon. Patients 2, 5, 6, 7, and 8 were obligate for either a second-site mutation or original-site mutation.
In the second group, somatic repair resulted from recombination-mediated gene conversion. For example, in Patients 10 and 11, genotyping of single nucleotide polymorphisms (SNPs) throughout the DOCK8 gene indicated which portions of the DOCK8 alleles were derived from each parent (Fig 2, B; Fig E4). In DNA from primary T cells, the paternally-inherited large deletion was present, but the maternally-inherited indel was absent. Furthermore, maternal SNPs upstream of the deletion were also absent. Thus, we inferred that gene conversion repaired the indel on the maternally-inherited allele using the intact undeleted portion of the paternally-inherited allele. Gene conversion was likely responsible for somatic repair in T cells from Patients 14 and 17 (Fig E7–E8).
In the third group, somatic repair resulted from recombination-mediated intragenic single crossover. For example, analysis of genomic DNA from primary T cells of Patient 9 showed that both maternally- and (presumed) paternally- inherited mutations and SNPs were present throughout the entire DOCK8 gene (Fig 2, C). However, when sequencing was performed after cloning PCR-amplified regions of cDNA prepared from primary T cells, neither the indel nor the missense mutation was detected. A single wildtype transcript was present, whose 5’ portion contained non-maternal SNPs and 3’ portion contained maternal SNPs. Thus, we inferred that an intragenic single crossover event generated a new allele that lacked both mutations, while simultaneously generating a second new allele that contained both mutations and underwent nonsense-mediated decay. Intragenic single crossover was also responsible for somatic repair in T cells from Patients 12 and 16, and probably Patient 13 (Fig E5, E8, and E6).
To summarize, 17 DOCK8-immunodeficient patients had somatic mosaicism, which resulted from repair of germline DOCK8 mutations through compensatory point mutations or recombination. Recombination-mediated gene conversion or intragenic crossover occurred in all patients from our cohort who had a germline mutation on one allele, plus an intact region corresponding to this mutation on the other allele (Table 1, column 2). By contrast, in patients with overlapping deletions on both alleles, repair was not possible and was not observed (Table E3; data not shown).
Reversions are enriched in T cells
To determine in which cells somatic repair occurred, we developed an intracellular flow cytometric method to quantify DOCK8 protein. In PBMC from normal healthy controls, we detected high levels of DOCK8 in T cells, B cells, and NK cells (Fig 1, B). As expected, patients who had unrepaired germline DOCK8 mutations expressed minimal DOCK8. Heterozygous carriers expressed intermediate levels. A similar expression pattern occurred in monocytes, despite higher non-specific background. By contrast, we observed DOCK8 within T cells from patients who had somatic repair, at levels slightly decreased or similar to normal healthy controls (Fig 1, C). DOCK8-positive cells ranged up to 94% of total T cells (Table 2). Proportions of DOCK8-expressing NK cells were generally lower or absent, but reached 84% in one patient (Table 2). Low proportions of DOCK8-expressing B cells were also observed (Table 2). These trends were mirrored at the genetic level in Patients 10 and 11, as determined by estimating proportions of repaired lymphocyte subsets after PCR amplification, cloning, and sequencing of transformants (Table E4).
Table 2.
Proportions of lymphocyte subsets expressing DOCK8
| Patient | CD3+ T cells | CD4+ T cells | CD4+ CD45RA+ T cells | CD4+ CD45RO+ T cells | CD8+ T cells | CD8+ CD45RA+ T cells | CD8+ CD45RO+ T cells | CD19+ B cells | CD56+ CD3− NK cells |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | 43 | 4 | 56 | 49 | 10 | 82 | 3 | 43 |
| 2 | 7 | 6 | 0 | 9 | 10 | 7 | 14 | 1 | 1 |
| 3 | 22 | 28 | 1 | 48 | 23 | 8 | 30 | 10 | 4 |
| 4 | 26 | 26 | 15 | 38 | 53 | 59 | 34 | 5 | 3 |
| 5 | 10 | 6 | 2 | 15 | 24 | 2 | 59 | 1 | 0 |
| 6 | 5 | 4 | 0 | 9 | 4 | 0 | 5 | 4 | 0 |
| 7 | 1 | 1 | 0 | 6 | 0 | ND | ND | 1 | 1 |
| 8 | 1 | 1 | 0 | 4 | 1 | ND | ND | 1 | 2 |
| 10 | 90 | 83 | 23 | 87 | 96 | 91 | 97 | 1 | 1 |
| 11 | 83 | 80 | 14 | 86 | 95 | 94 | 95 | 1 | 1 |
| 12 | 60 | 23 | 3 | 62 | 76 | 46 | 96 | 0 | 0 |
| 13 | 69 | 56 | 3 | 82 | 68 | 60 | 61 | 4 | 0 |
| 14 | 86 | 57 | 3 | 72 | 88 | 79 | 89 | 8 | 44 |
| 15 | 94 | 85 | 15 | 88 | 96 | 97 | 95 | ND | 84 |
| 16 | 94 | 96 | 28 | 99 | 96 | 96 | 97 | 1 | 3 |
| 17 | 75 | 67 | 11 | 96 | 93 | 91 | 97 | 2 | 1 |
| Normal controls | 98 ± 2 | 99 ± 1 | 100 ± 1 | 99 ± 1 | 99 ± 2 | 99 ± 2 | 99 ± 2 | 99 ± 2 | 98 ± 2 |
Flow cytometric analyses for intracellular DOCK8 protein and the indicated cell surface markers were performed on PBMC. Percentages of the different lymphocyte subsets that were positive for DOCK8 are shown. Patient 15 had previously received rituximab. ND, not determined. Patients 1–8 had point mutation-mediated repair, whereas patients 10–17 had probable recombination-mediated repair. The means ± SD for 14 normal healthy controls are shown, based upon gates established from patient cells.
To characterize further the revertant T cells, we costained for additional cell surface markers along with intracellular DOCK8 protein (Table 2; Fig 1, C). DOCK8 was expressed in CD4+ and especially CD8+ T cells. Expression was also enriched in T cells bearing the effector/memory phenotypic marker CD45RO, but less frequently in CD45RA+ T cells. Up to 28% of CD4+ CD45RA+ T cells expressed DOCK8. Several patients showed DOCK8 in > 90% of their CD8+ CD45RA+ T cells (Table 2), which includes highly differentiated effector T cells that share CD45RA with naïve T cells but lack CCR7 expression. Such effector memory CD45RA+ CD8+ T cells (TEMRA), which are seen in states of chronic viral infections, are expanded in DOCK8 immunodeficient patients.8 These data were corroborated by calculated frequencies of repaired sequences in CD4+ or CD8+ T-cell subsets that had been further sorted based upon CD45RA expression (Table E4).
Thus, among our 17 patients, reversions accumulated to the highest extent in T cells, particularly antigen-experienced T cells. Because somatic repair occurred in less than ~1/3 of naïve CD4+ T cells, this process inefficiently corrected the defect throughout the full T-cell repertoire. Repair also occurred to variable degrees in other lymphocyte subsets, and in some patients reached high levels in NK cells. Interestingly, recombination-mediated repair was associated with higher levels of reversion, as compared to the somatic repair caused by compensatory point mutations (Table 2). Recombination often targets repetitive sequences within a gene but exact breakpoints cannot be resolved at the nucleotide level. Because of this limitation, the actual numbers of different recombination events we observed may be underestimations.
Reversions occurring in multiple lymphocyte lineages
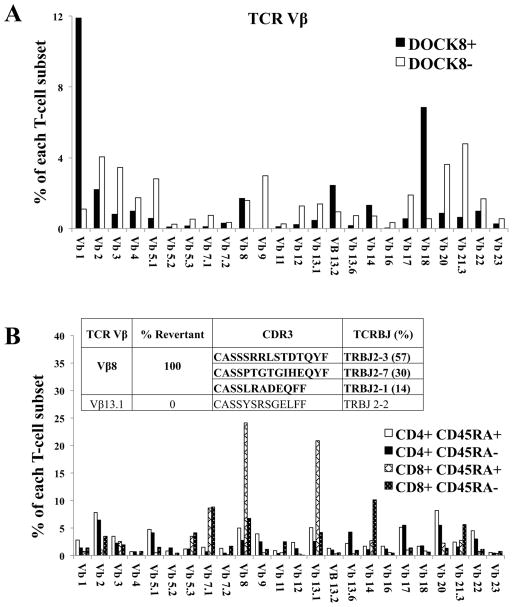
We next analyzed the repertoire of revertant T cells. In Patient 12, cells were stained for intracellular DOCK8, and co-stained with a panel of antibodies to identify rearranged T-cell receptor (TCR) (Fig 3, A). Of the DOCK8-expressing T cells, ~40% were TCRγδ and ~60% TCRαβ. Among the latter, Vβ1 (TRBV9) and Vβ18 (TRBV30) subsets were preferentially expanded, in contrast to T cells lacking DOCK8 expression or T cells from normal healthy controls (Fig E9). These results suggested that reversion conferred a survival advantage for DOCK8-expressing T cells, as had been seen in adoptive transfer studies in mice.7, 8 However, the markedly increased frequency of revertants in antigen-experienced cells, along with TEMRA expansion, suggested that chronic antigenic stimulation also contributed to the expansion of such repaired cells. This was supported by studies in Patient 10, who had Vβ8 (TRBV12) or Vβ13.1 (TRBV6-5) subsets expanded to more than 20% each of total CD8+ CD45RA+ cells (Fig 3, B). After sorting these two expanded subsets, the clonotype(s) they contained were identified by DNA sequencing of CDR3. The Vβ8-expressing T-cell subset contained three clonotypes, and the Vβ13-expressing T-cell subset contained one clonotype. DOCK8 mutational analysis showed somatic repair in all three Vβ8-expressing clonotypes but not in the single Vβ13-expressing clonotype.
FIG 3.
Reversions in multiple T cell clonotypes. TCR Vβ repertoire analyses performed on purified T cells. A, Patient 12 cells also stained for intracellular DOCK8. Normal healthy controls are shown in Fig E9. B, Patient 10 cells also stained for the indicated cell surface markers. Vβ-subsets are expressed as a percentage of each T-cell subset as indicated in the key. Insets indicate the CDR3-sequenced clonotypes contained within the sorted cell subsets, with DOCK8-repaired clonotypes bolded.
DOCK8 reversions occurring in different T cells could be explained by a single recombination event that had occurred early in a hematopoietic progenitor, followed by selective outgrowth in certain clones. However, additional analyses supported the possibility that multiple recombination events had occurred in separate lymphocyte lineages. Patient 14 had two different gene conversion events, with the nonsense mutation repaired in T cells and indel repaired in NK cells (Fig E7). T cells from Patient 12 also had intragenic single crossover, and at least one other repair event (Fig E5). Finally, Patient 1 had different second-site mutations in DOCK8-expressing T cells and NK cells (Fig E1), indicating that non-recombination-mediated somatic repair had occurred independently in different cell lineages.
Disease course in patients having reversions
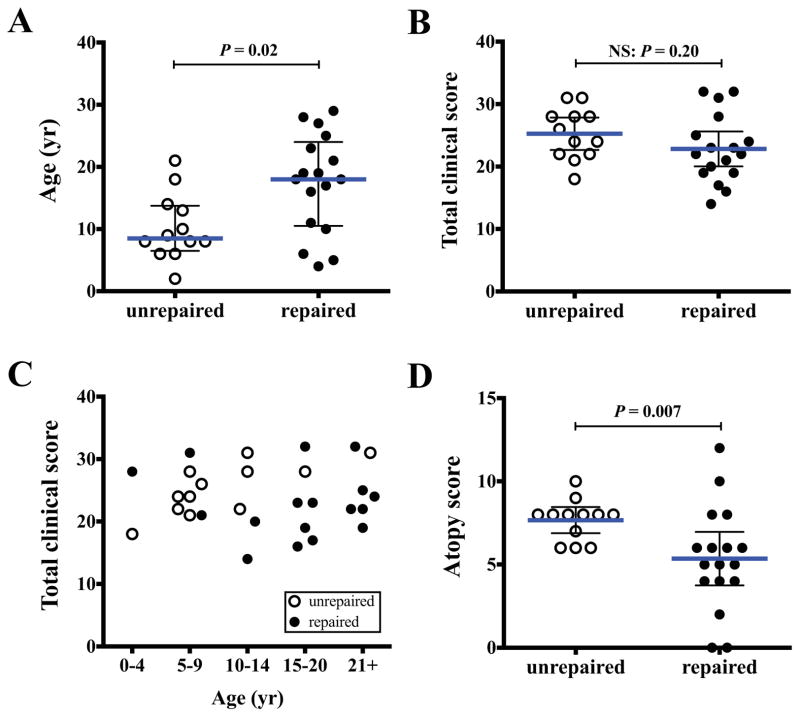
Spontaneously arising somatic reversions have been likened to “natural gene therapy,” and have been associated with improved disease in some primary immunodeficiencies and inherited skin diseases.18, 19, 22, 23 To investigate whether patients having reversions had less severe disease, we devised a scoring system that gauged severity of accumulated disease features among patients who had somatic repair, as compared to those who did not (Table E1). Patients with reversions had a median age that was 9.5 years older at last evaluation, suggesting an improved overall survival (Fig 4, A; Mann-Whitney test: p = 0.02). Although total disease scores were similar (Fig 4, B; Mann-Whitney test: p = 0.20), when scores were stratified by age, they decreased with age for patients who had somatic repair, but increased with age for the patients without repair (Fig 4, C). However, improvement remained insufficient for disease elimination, as six patients underwent HCT for uncontrolled viral infections and a seventh patient died; these outcomes were comparable to patients without reversions (Table E1).
FIG 4.
Clinical scoring in patients with reversions. Patients with reversions (filled circles) were compared to those without (open circles). A, Age, median ± quartiles. B, Total clinical score. C, Clinical score stratified by age groups. D, Atopic score. Scoring criteria are provided in Table E1. B and D show means ± 95% confidence intervals. P-values calculated by Mann-Whitney test.
Total infectious disease burden, including viral disease burden, respiratory tract infections, other invasive or serious bacterial infections, fungal or opportunistic infections increased with age. These measures were similar between both groups, although fewer patients with reversions had staphylococcal skin and soft tissue infections (50% vs. 92%; Fisher’s exact test: p = 0.002). The severity of functional antibody impairment was decreased (impaired specific antibodies to protein and polysaccharide antigens: 62% vs. 92%; Chi square test: p = 0.037), whereas the extent of lymphopenia did not differ. Failure to observe significant differences in overall infections may be partially due to the effectiveness of overall prophylactic management, including immune globulin and antibiotics.
Interestingly, atopic disease burden was decreased in patients who had reversions (Fig 4, D; Mann-Whitney test: p = 0.007). Although eczema, asthma, and eosinophilic gastrointestinal disease were similar (Table E1), the frequency and severity of food allergies were decreased (food allergies without anaphylaxis: 6% vs. 40%; food allergies with anaphylaxis: 38% vs. 50%; Chi square test: p = 0.03) and growth was also improved (poor growth: 19% vs. 77%; Fisher’s exact test: p = 0.003). Furthermore, the severity of peripheral eosinophilia was decreased (absent: 37% vs. 0%; mild-moderate eosinophilia: 44% vs. 8%; severe eosinophilia: 19% vs. 92%; Chi square test p < 0.001). Several patients also had normal serum IgE levels, although this did not reach statistical significance (33% vs. 0%; Fisher’s exact test: p = 0.14). Finally, other disease features, including vascular abnormalities, autoimmunity, or malignancy, were similar in both patient groups.
Discussion
DOCK8 deficiency usually leads to death by late adolescence or early adulthood, unless curative HCT is performed.13, 14 Nevertheless, the HCT risk-to-benefit ratio may not be obvious for some patients who have less severe disease. We now identify one important source for the phenotypic variation among patients: revertant mosaicism. Reversions have been observed in several primary immunodeficiencies, including the Wiskott-Aldrich syndrome, where it occurs in ~11% of patients.18–20 Here, we found reversions in half of the DOCK8 immunodeficient patients whom we follow, most of whom have a non-consanguineous background. This high prevalence probably reflects DOCK8’s location within a recombination hotspot that is characterized by many subtelomeric repetitive sequences.2 Such locations are known to contribute to large intragenic germline deletions found in other human diseases,24 and could also contribute to the recombination-mediated somatic repair seen here.
Among our patients, reversions occurred most frequently within T cells. In other diseases, reversion is often associated with a survival or growth advantage.18, 22, 23, 25 Thus, our observations suggest that DOCK8 confers a selective advantage especially in T cells. This is consistent with published findings in mice showing preferential outgrowth of Dock8-expressing T cells after bone marrow adoptive transfers.7–9 DOCK8 may also confer a selective advantage in NK cells, as we observed that the proportion of NK cells that were revertant increased in Patient 1 from ~15 to 43% over a period of 2.5 years (data not shown). Revertant T cells and NK cells could theoretically correct the TH2 skewing, defective NK cell cytotoxicity, and other T-cell abnormalities in this disease, thereby modulating phenotype over time. That reversion predominates in these cell types probably also reflects their higher proliferative rates in the periphery.26–28
Although cure may also require correction in multiple lineages including dendritic cells, a key role for T cells was previously suggested by a report of a DOCK8-deficient patient in whom HCT established donor engraftment of 98% of T cells, but only 35% of B cells, 53% of mononuclear cells, and 6% of granulocytes.13 Those levels completely cured infectious complications and markedly improved atopic disease. By contrast, despite high reversion frequencies in T cells, our patients still had pronounced infections even though atopy was improved and median age increased. The different outcomes could be explained by differences in the repertoire of DOCK8-expressing T cells following transplantation as compared to spontaneous repair (i.e., complete vs. partial correction). Given the broad infection susceptibility of DOCK8-deficient patients, cure would require that reversions occur in a diverse repertoire of T cells. Somatic repair failed to achieve this in our patients, as demonstrated by low numbers of corrected naïve phenotype CD4+ T cells.
Currently, a diagnosis of DOCK8 deficiency can often be made by commercially available deletion analysis, which detected ~60% of the families of patients in our cohort who had deletions in one or both alleles (data not shown). Full sequencing of the DOCK8 gene and confirmation of loss of protein expression by immunoblotting are available only through a few research laboratories. Our results now demonstrate that intracellular flow cytometric detection of DOCK8 protein could serve as a simple and rapid method for diagnosis. Because B cells show minimal reversion, their analysis is highly sensitive for detecting DOCK8 deficiency, and in fact identified all patients tested using this screening methodology. Monitoring the proportions of DOCK8-expressing lymphocytes over time with disease activity might be useful in selected patients. However, our data suggest that in most cases, reversions at best delay the progression of disease but do not abrogate the need for HCT. Thus, patients with homozygous large deletions or compound heterozygous overlapping large deletions, who are incapable of generating revertants, can be predicted to have more severe disease and earlier severe complications. In this patient subgroup especially, we advocate early HCT to minimize the development of infection-related disease pathology that might otherwise complicate delayed HCT.
Supplementary Material
Key Messages.
Somatic repair within lymphocytes occurs in some DOCK8 immunodeficient patients, especially when recombination is possible.
A rapid intracellular flow cytometry based assay can be used to screen for DOCK8 deficiency and identify reversions.
Reversions occur most often in T cells and NK cells, and are associated with improved disease phenotypes but are inadequate to eliminate disease.
Acknowledgments
We thank Brandon Newell and Rima Wakim for patient care; Dara Strauss-Albee for technical assistance; Kent Barbian, Steve Porcella, and Julie Niemela for DNA sequencing; Thomas Dimaggio, Angela Wang, Joie Davis, and Kim Montgomery-Recht for clinical support; Michael Lenardo for critically reading the manuscript and helpful discussions; and the patients and their families for participating in this research study.
Funding sources Supported by the Intramural Research Program and the Vaccine Research Center of the National Institutes of Health, National Institute of Allergy and Infectious Diseases
Abbreviations used
- CGH
comparative genomic hybridization
- EBV
Epstein-Barr virus
- HPV
human papillomavirus
- HCT
hematopoietic cell transplantation
- HSV
herpes simplex virus
- HVS
Herpesvirus saimiri
- MCV
molluscum contagiosum virus
- PBMC
peripheral blood mononuclear cells
- SNP
single nucleotide polymorphism
- TCR
T-cell receptor
- TEMRA
effector memory CD45RA+ CD8+ T cells
- TH2
T helper type 2 cells
- VZV
varicella-zoster virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–55. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–302.e4. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su HC, Jing H, Zhang Q. DOCK8 deficiency. Ann N Y Acad Sci. 2011;1246:26–33. doi: 10.1111/j.1749-6632.2011.06295.x. [DOI] [PubMed] [Google Scholar]
- 4.Chu EY, Freeman AF, Jing H, Cowen EW, Davis J, Su HC, et al. Cutaneous Manifestations of DOCK8 Deficiency Syndrome. Arch Dermatol. 2011;148:79–84. doi: 10.1001/archdermatol.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alsum Z, Hawwari A, Alsmadi O, Al-Hissi S, Borrero E, Abu-Staiteh A, et al. Clinical, immunological and molecular characterization of DOCK8 and DOCK8-like deficient patients: single center experience of twenty five patients. J Clin Immunol. 2013;33:55–67. doi: 10.1007/s10875-012-9769-x. [DOI] [PubMed] [Google Scholar]
- 6.Sanal O, Jing H, Ozgur T, Ayvaz D, Strauss-Albee DM, Ersoy-Evans S, et al. Additional diverse findings expand the clinical presentation of DOCK8 deficiency. J Clin Immunol. 2012;32:698–708. doi: 10.1007/s10875-012-9664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambe T, Crawford G, Johnson AL, Crockford TL, Bouriez-Jones T, Smyth AM, et al. DOCK8 is essential for T-cell survival and the maintenance of CD8(+) T-cell memory. Eur J Immunol. 2011;41:3423–35. doi: 10.1002/eji.201141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208:2305–20. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randall Kl, Lambe T, Johnson A, Treanor B, Kucharska E, Domaschenz H, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10:1283–91. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;13:612–20. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2013;131:840–8. doi: 10.1016/j.jaci.2012.12.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford G, Enders A, Gileadi U, Stankovic S, Zhang Q, Lambe T, et al. DOCK8 is critical for the survival and function of NKT cells. Blood. 2013;122:2052–61. doi: 10.1182/blood-2013-02-482331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bittner TC, Pannicke U, Renner ED, Notheis G, Hoffmann F, Belohradsky BH, et al. Successful long-term correction of autosomal recessive hyper-IgE syndrome due to DOCK8 deficiency by hematopoietic stem cell transplantation. Klin Padiatr. 2010;222:351–5. doi: 10.1055/s-0030-1265135. [DOI] [PubMed] [Google Scholar]
- 14.Gatz SA, Benninghoff U, Schutz C, Schulz A, Honig M, Pannicke U, et al. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:552–6. doi: 10.1038/bmt.2010.169. [DOI] [PubMed] [Google Scholar]
- 15.Metin A, Tavil B, Azik F, Azkur D, Ok-Bozkaya I, Kocabas C, et al. Successful bone marrow transplantation for DOCK8 deficient hyper IgE syndrome. LID - 10.1111/j.1399-3046.2011.01641.x [doi] Pediatr Transplant. 2012 doi: 10.1111/j.1399-3046.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 16.Boztug H, Karitnig-Weiss C, Ausserer B, Renner ED, Albert MH, Sawalle-Belohradsky J, et al. Clinical and immunological correction of DOCK8 deficiency by allogeneic hematopoietic stem cell transplantation following a reduced toxicity conditioning regimen. Pediatr Hematol Oncol. 2012;29:585–94. doi: 10.3109/08880018.2012.714844. [DOI] [PubMed] [Google Scholar]
- 17.Barlogis V, Galambrun C, Chambost H, Lamoureux-Toth S, Petit P, Stephan JL, et al. Successful allogeneic hematopoietic stem cell transplantation for DOCK8 deficiency. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Wada T, Candotti F. Somatic mosaicism in primary immune deficiencies. Curr Opin Allergy Clin Immunol. 2008;8:510–4. doi: 10.1097/ACI.0b013e328314b651. [DOI] [PubMed] [Google Scholar]
- 19.Palendira U, Low C, Bell AI, Ma CS, Abbott RJ, Phan TG, et al. Expansion of somatically reverted memory CD8+ T cells in patients with X-linked lymphoproliferative disease caused by selective pressure from Epstein-Barr virus. J Exp Med. 2012;209:913–24. doi: 10.1084/jem.20112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jongmans MC, Verwiel ET, Heijdra Y, Vulliamy T, Kamping EJ, Hehir-Kwa JY, et al. Revertant somatic mosaicism by mitotic recombination in dyskeratosis congenita. Am J Hum Genet. 2012;90:426–33. doi: 10.1016/j.ajhg.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dasouki M, Okonkwo KC, Ray A, Folmsbeel CK, Gozales D, Keles S, et al. Deficient T Cell Receptor Excision Circles (TRECs) in autosomal recessive hyper IgE syndrome caused by DOCK8 mutation: Implications for pathogenesis and potential detection by newborn screening. Clin Immunol. 2011;141:128–32. doi: 10.1016/j.clim.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai T, Saito M, Nishikomori R, Yasumi T, Izawa K, Murakami T, et al. Multiple reversions of an IL2RG mutation restore T cell function in an X-linked severe combined immunodeficiency patient. J Clin Immunol. 2012;32:690–7. doi: 10.1007/s10875-012-9684-1. [DOI] [PubMed] [Google Scholar]
- 23.Lai-Cheong JE, McGrath JA, Uitto J. Revertant mosaicism in skin: natural gene therapy. Trends Mol Med. 2011;17:140–8. doi: 10.1016/j.molmed.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purandare SM, Patel PI. Recombination hot spots and human disease. Genome Res. 1997;7:773–86. doi: 10.1101/gr.7.8.773. [DOI] [PubMed] [Google Scholar]
- 25.Trifari S, Scaramuzza S, Catucci M, Ponzoni M, Mollica L, Chiesa R, et al. Revertant T lymphocytes in a patient with Wiskott-Aldrich syndrome: analysis of function and distribution in lymphoid organs. J Allergy Clin Immunol. 2010;125:439–48.e8. doi: 10.1016/j.jaci.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 26.Macallan DC, Asquith B, Irvine AJ, Wallace DL, Worth A, Ghattas H, et al. Measurement and modeling of human T cell kinetics. Eur J Immunol. 2003;33:2316–26. doi: 10.1002/eji.200323763. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121:258–65. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macallan DC, Wallace DL, Zhang Y, Ghattas H, Asquith B, de Lara C, et al. B-cell kinetics in humans: rapid turnover of peripheral blood memory cells. Blood. 2005;105:3633–40. doi: 10.1182/blood-2004-09-3740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.