Abstract
Commensal bacterial communities residing within the intestinal lumen of mammals have evolved to flourish in this microenvironment. To preserve this niche, commensal bacteria act with the host to prevent colonization by invasive pathogens that induce inflammation and disrupt the intestinal niche commensal bacteria rely upon. Thus, it is mutually beneficial to the host and commensal bacteria to inhibit a pathogen's ability to establish an infection. Commensal bacteria express factors that support colonization, maximize nutrient uptake, and produce metabolites that confer a survival advantage over pathogens. Further, commensal bacteria stimulate the host's immune defenses and drive tonic expression of anti-microbial factors. In combination, these mechanisms preserve the niche for commensal bacteria and assist the host in preventing infection.
Keywords: commensal bacteria, intestinal microbiota, bacteria, infection, pathogens, intestinal immune response, colonization resistance
Introduction
The mammalian gastrointestinal tract harbors one of the densest microbial ecosystems found in nature, peaking in the proximal colon at 1011 -1012 cells/mL [1]. This microbial community, which is composed of all domains of life, exists in a mutually beneficial relationship with the host. The microbes thrive in relatively stable environmental conditions (pH, temperature, low oxygen tension) while the host exploits enzymes produced by the microbes to break down complex carbohydrates into absorbable small units, thereby increasing the efficiency of energy uptake [2]. Recent studies have started to decipher the complex host-microbe relationship, and these commensal bacterial communities have been linked to a wide range of human diseases including obesity, diabetes, cancer, atherosclerosis, atopic disorders, and inflammatory bowel disease [3-5]. The focus of this review is on the role of commensal bacteria in defense against infectious diseases.
The diverse microbiota within the intestine reduces the ability of invasive pathogens to establish an infection, cause damage to host cells and drive disease. Loss of microbiota diversity following antibiotic administration is postulated to increase susceptibility to intestinal pathogens such as Salmonella typhirmurium and Clostridum difficile [6-8]. These experimental studies support clinical experience linking antibiotic use with increased frequencies of opportunistic intestinal infections [9-11]. Reconstituting commensal bacterial communities of the gastrointestinal tract can facilitate the clearance of chronic C. difficile infection. A recent study demonstrated in a controlled clinical trial that fecal microbiota transplantation is highly effective at clearing recurrent C. difficile infection [12] and restoring the biodiversity of the microbiota [13]. Several studies using mouse models of enteric bacterial infection have demonstrated that transferring commensal bacterial populations can effectively displace antibiotic-resistant pathogens from the intestine [14-18]. This approach of transferring defined commensal bacterial populations into a host to re-establish a diverse microbiota offers an antibiotic-independent approach to combat infection. However, the therapeutic potential of repopulating patients with commensal bacterial communities is currently limited by incomplete characterization of commensal bacterial species and products that antagonize pathogens. The recently described mechanisms of commensal bacteria-driven protection against intestinal pathogens and the countermeasures pathogens employ to establish infection will be discussed below.
Commensal bacteria outcompete pathogens for nutrients
Evolutionary pressures have selected for bacterial communities best adapted at acquiring available nutrients and resources in the intestine. For example, the human commensal bacteria Bacteroides fragilis expresses a gene cluster, termed commensal colonization factors, that is essential for penetration of colonic mucus and colonization of intestinal crypts [19]. Other commensal species, such as Bacteroides thetaiotaomicron, carry genes that express multiple non-redundant corrinoid transporters, each conferring a survival advantage by enabling uptake of distinct corrinoids (vitamin B12 analogs) that are finitely available in the intestine [20]. Competition amongst the commensal bacterial species deprives potential invasive pathogens of the essential nutrients needed to replicate (Figure 1). This phenomenon, referred to as colonization resistance, is most clearly revealed by germ-free mice, which exhibit increased susceptibility to a wide range of bacterial infections, including Escherichia coli, C. difficile, Vibrio cholerae and Citrobacter rodentium [17,21-23]. The murine enteric pathogen C. rodentium colonizes the intestinal lumen of germ-free mice, but in the presence of non-pathogenic E. coli, C. rodentium is outcompeted for available carbohydrates and is eliminated from the intestinal lumen [23]. Only following upregulation of virulence factors that enable attachment to intestinal epithelial cells can C. rodentium create its own environmental niche and establish an infection [23]. Perturbation of the microbiota disrupts the intestinal ecosystem and enables pathogens to access resources that would otherwise be consumed by commensal bacteria. For example, antibiotic-mediated disruption of the microbiota results in elevated levels of free sialic acids, which two distinct intestinal pathogens, S. typhimurium and C. difficile, catabolize and use as an energy source to expand [24]. Understanding the minimally required biodiversity and bacterial-driven biochemical reactions needed to establish colonization resistance will continue to be an area of intense study as the field defines what comprises a healthy, intact microbiota.
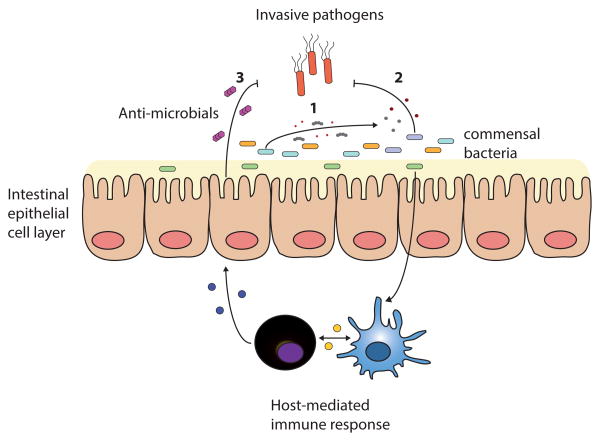
Figure 1.
Commensal bacteria-mediated mechanisms of protection against intestinal pathogens. (1) Commensal bacterial communities consume nutrients and energy sources, depriving pathogens of their niche. (2) Microbiota-derived metabolic byproducts directly inhibit pathogens. (3) Commensal bacteria stimulate host immune cells and drive basal expression of host defense factors.
Commensal bacterial-derived products directly inhibit pathogens
Commensal bacteria have evolved defense mechanisms that directly inhibit the ability of pathogens to survive. Most prominent among these mechanisms are a class of peptides called bacteriocins, which are anti-microbial factors produced by bacteria that target a narrow spectrum of competing bacteria. [25]. Bacteriocins, such as Thuricin CD derived from the Bacillus thurigiensis DPC 6431, hold the potential to target intestinal bacterial pathogens while minimally impacting the commensal microbial community [26,27].
Metabolic byproducts from commensal bacteria can also deter pathogen growth. Short chain fatty acids (SCFA) produced by commensal bacterial fermentation of ingested complex carbohydrates can inhibit the growth of enteropathogenic bacteria [28]. Commensal species that are high SCFA producers, such as Bifidobacteria spp., can mitigate the severity of S. typhimurium, enterohaemorrhagic E. coli or C. rodentium infection ([6,29,30]). Bifidobacteria-mediated protection is, in part, attributable to a gene encoding an ATP-binding cassette-type carbohydrate transporter that leads to increased production of acetate, a SCFA, and reduced gut permeability and bacterial translocation [31].
Commensal bacteria harbor a diverse assortment of bacteriophages, viruses that infect bacteria, which, in part, constitute the virobiota of the gut. [32]. The microvirome, the genes derived from the virobiota, encodes a broad range of functions and can influence the relative representation of different bacterial species within the intestine. One such example has been described with a bacteriophage isolated from the commensal species E. faecalis, which confers a competitive growth advantage over bacteria not harboring this bacteriophage [33]. The mechanisms employed by bacteriophages to support host bacteria remain largely undefined, yet it is clear from these initial studies that bacteriophages can help shape the bacterial communities in the intestine and in turn the ability of pathogens to establish infection.
Bacteria have also evolved defense mechanisms that involve direct attack against heterologous bacterial species. The bacterial type VI secretion system utilizes a multicomponent organelle to pierce the cell wall of neighboring cells and transport effector proteins into the targeted eukaryotic or prokaryotic cell [34] Pseudomonas aeruginosa directly inhibits V. chlorae or Acinetobacter baylyi growth using its Type VI secretion system, leaving sister cells or non-invasive adjacent bacteria unharmed [35]. V. chlorae can counter P. aeruginosa by expressing a gene that confers immunity against Type VI effector proteins [36]. Such mechanisms of direct bacterial interaction may contribute to the establishment of stable commensal populations in the competitive microenvironment of the intestine.
Commensal bacterial-derived signals indirectly inhibit pathogens
Commensal bacteria can also support pathogen clearance by activating host defense mechanisms that maintain the physical separation between the microbial world of the intestinal lumen and the host (Figure 1). To accomplish this goal, a complex network of immune and non-immune cells act in concert following detection of microbial molecules by innate immune receptors to upregulate host defenses that limit bacterial dissemination, repair the intestinal epithelial barrier, and maintain intestinal homeostasis [37]. Tonic stimulation of the host's defenses by commensal bacteria benefits the host and resident bacteria by inhibiting expansion of invasive pathogens and preserving the environmental niche for non-invasive commensal species.
Anti-microbial peptides, such as calprotectin, defensins and the RegIII family of proteins are produced by epithelial cells and have direct bactericidal properties [38]. Expression of anti-microbial peptides by intestinal epithelial cells is in driven by commensal bacteria [39], and is critical in limiting pathogen expansion [40]. The induction of these antimicrobial peptides by commensal bacteria is best understood with RegIIIγ and is a multi-step process that involves several cell types. A subset of intestinal dendritic cells (CD103+, CD11b+) can detect bacterial-derived signals, leading to secretion of IL-23 and production of IL-22 by neighboring immune cells [41]. IL-22 signals through intestinal epithelial cells and upregulates expression of host defense genes, including RegIII proteins [42]. RegIII proteins bind to peptidoglycans of the bacterial cell wall and form a hexameric pore that permeabilizes the bacterial membrane and kills the bacteria [43]. Resident Lactobacilli spp. can convert tryptophan available in the intestinal lumen into the aryl hydrocarbon receptor (AhR) ligand, indole-3 aldehyde (IAId). IAId stimulates innate lymphoid cells to produce IL-22 in an AhR-dependent manner [44]. This commensal bacteria-driven IL-22/anti-microbial peptide axis is critical in the defense against bacterial pathogens such as C. rodentium and vancomycin-resistant Enterococcus as well as fungi such as Candida albicans [40,44-46]. Genetic ablation of IL-22 or AhR alters the intestinal microbiota and predisposes the host to inflammatory colitis, demonstrating the importance of the commensal bacteria-driven IL-22 axis in promoting intestinal homeostasis [47,48]. Microbiota-mediated stimulation of host pathways and innate immune cells can activate host defenses prior to pathogen encounter, pre-emptively protecting against infection.
Commensal bacteria shape intestinal immune cell function
Commensal bacteria influence the activation and differentiation status of immune cells. This was first described with the commensal species segmented filamentous bacteria (SFB), which enhances CD4+ T cell differentiation into T helper 17 (TH17) cells in the small intestine [49]. Additional microbiota-derived signals induce IL-1β production in the intestine and further drive CD4+ TH17 cell differentiation as well as support IL-1R+ innate lymphoid cells [50,51]. The presence of these cell types in the intestine is important in host defenses against the enteric bacterial infections C. rodentium and S. typhimurium [45,46,51]. Following pathogen induced epithelial damage, n-formyl peptides from commensal bacteria such as γ-proteobacteria drive neutrophils and inflammatory monocytes into the lumen of the intestine. Here, these immune cells produce reactive oxygen species to aid in the containment of both pathogen and commensal bacteria while the epithelial layer is repaired [52]. Upon resolution of the infection commensal bacterial-derived signals help restore intestinal homeostasis by eliciting production of prostaglandin E2 from inflammatory monocytes, limiting neutrophil-induced pathology and the opportunity pathogens have to utilize inflammation-induced dysbiosis to expand [53]. Commensal bacteria also shape immune-mediated resistance to Leishmania major and influenza virus infection in the skin and lungs, respectively [54-56]. These studies demonstrate that microbiota-induced signals can act at distinct anatomic sites in addition to the intestine to support host defense against infection.
Recent studies indicate that microbiota-induced signals can play a role in driving epigenetic modifications of pathogen response genes. Loss of tonic microbiota-driven stimulation results in increased methylation at the promoter region of immune defense genes, reduced binding of key transcription factors and decreased basal expression of molecules important for defense against viral pathogens [56,57]. Thus microbiota-driven signaling maintains immune response genes in an open, transcriptionally active state that enables a rapid and robust response upon encountering a pathogen. Loss of epigenetic modifiers in intestinal epithelial cells leads to disruption of a healthy and diverse microbiota and diminished expression of immune defense genes [58]. Epigenetic modification of genes involved in host defense by the microbiota facilitates rapid host responses to pathogens.
Commensal bacteria regulate inflammation to limit pathogen expansion
Microbiota-driven activation of host defenses must be carefully regulated to prevent rampant inflammation that would alter the intestinal microenvironment and harm both commensal bacteria and their host. Therefore, many commensal bacteria elicit immunoregulatory activities within the host that also limit pathogen expansion. For example, commensal Bifidobacteria breve express an exopolysaccaride that elicits an immunoregulatory response, enabling their colonization of the intestine and impeding colonization of C. rodentium [29]. A class of commensal Clostridia species promotes expansion of colonic regulatory T (Treg) cells, which limit inflammation and maintain intestinal homeostasis, thereby preserving colonization resistance [59]. The anti-inflammatory properties of microbiota-derived SCFA are, in part, attributed to augmentation of the colonic Treg cell population [60-62]. SCFA inhibit histone deacetylase expression and increase histone acetylation in the promoter region of foxp3 locus, skewing differentiation of CD4+ T cells toward a regulatory T cell lineage. [60-62]. SCFA derived from commensal bacteria enhance Treg cell suppressive activities that help maintain a diverse commensal microbial community.
Mechanisms utilized by pathogens to outcompete the commensal bacteria
Pathogens can use microbiota or host-derived products to gain a competitive advantage in the intestine. Host-driven inflammation alters the intestinal microenvironment and creates conditions favorable for select bacterial species, and the resulting dysbiosis can be exploited by opportunistic pathogens [63][64]. Inflammatory by-products produced by the host, including reactive oxygen species, nitrates, elastases, and ethanolamines all can enhance the growth of pathogenic bacteria. [63,65-67]. Nutrients released by commensal bacteria can also trigger pathogen expansion. Enterohemorrhagic E. coli upregulates virulence genes that aid in establishing an infection following detection of fucose, which is released after enzymatic break down of mucins by Bacteroides thetaiotaomicron [68]. Samonella can use hydrogen, a byproduct of commensal bacteria metabolizing carbohydrates into SCFA, by expressing a hydrogenase enzyme. Hydrogen is used by Samonella as an electron source during respiration and enables the pathogen to colonize the intestine [69-71]. Different classes of intestinal pathogens, such as enteric viruses, utilize microbiota-derived signals to establish infection of the host [72,73]. Enteric viruses can establish infections by attaching to commensal bacteria surface polysaccharides, which increases the virion's stability and promotes viral transmission [74].
6. Concluding Remarks
Commensal bacteria of the intestine provide an important but often imperfect line of defense against infection. Marked differences in microbiota composition between healthy individuals, as demonstrated by the Human Microbiome Project [75], suggest that variation in the representation of distinct commensal bacterial taxa may, at least in part, explain variation in susceptibility to a wide range of intestinal pathogens. As the identities of protective commensal bacterial species are revealed and their mechanisms of protection defined, it is becoming increasingly realistic to speculate that the human microbiota can be modified to optimize resistance to pathogens while reducing the risk of deleterious inflammatory diseases. Progress in this area will depend on the meticulous characterization of the heretofore mysterious commensal intestinal microbes and extensive study of their impact on the innate and adaptive components of the mucosal immune system.
Highlights.
Commensal bacteria directly inhibit invasive pathogen colonization.
The microbiota indirectly inhibit pathogens by stimulating host defense mechanisms.
Pathogens exploit host and commensal bacteria-derived products to grow.
Manipulating the microbiota is an effective therapeutic option against infection.
Abbreviations used
- SCFA
short chain fatty acids
- SFB
Segmented Filamentous Bacteria
- VRE
vancomycin resistant enterococcus
- ILC
innate lymphoid cells
- AhR
aryl hydrocarbon receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography & References cited
- 1.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: The unseen majority. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(12):6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 3.McLoughlin RM, Mills KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. The Journal of allergy and clinical immunology. 2011;127(5):1097–1107. doi: 10.1016/j.jaci.2011.02.012. quiz 1108-1099. [DOI] [PubMed] [Google Scholar]
- 4.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nature reviews Immunology. 2013;13(5):321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 6.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a salmonella enterica serovar typhimurium colitis model that allows analysis of both pathogen and host. Infection and immunity. 2003;71(5):2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to clostridium difficile-induced colitis. Infection and immunity. 2012;80(1):62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C, Finlay BB. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infection and immunity. 2008;76(10):4726–4736. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donskey CJ, Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan JA, Hujer AM, Hutton-Thomas RA, Whalen CC, Bonomo RA, Rice LB. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. The New England journal of medicine. 2000;343(26):1925–1932. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donskey CJ. Antibiotic regimens and intestinal colonization with antibiotic-resistant gram-negative bacilli. Clin Infect Dis. 2006;43(Suppl 2):S62–69. doi: 10.1086/504481. [DOI] [PubMed] [Google Scholar]
- 11.Owens RC, Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S19–31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 12*.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, et al. Duodenal infusion of donor feces for recurrent clostridium difficile. The New England journal of medicine. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. The first controlled clinical trial to demonstrate enhanced efficacy of fecal matter transplant in clearing C. difficile infection compared to standard antibiotic treatment. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Garg S, Girotra M, Maddox C, von Rosenvinge EC, Dutta A, Dutta S, Fricke WF. Microbiota dynamics in patients treated with fecal microbiota transplantation for recurrent clostridium difficile infection. PloS one. 2013;8(11):e81330. doi: 10.1371/journal.pone.0081330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawley TD, Clare S, Walker AW, Stares MD, Connor TR, Raisen C, Goulding D, Rad R, Schreiber F, Brandt C, Deakin LJ, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing clostridium difficile disease in mice. PLoS pathogens. 2012;8(10):e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ubeda C, Bucci V, Caballero S, Djukovic A, Toussaint NC, Equinda M, Lipuma L, Ling L, Gobourne A, No D, Taur Y, et al. Intestinal microbiota containing barnesiella species cures vancomycin-resistant enterococcus faecium colonization. Infection and immunity. 2013;81(3):965–973. doi: 10.1128/IAI.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van Maele L, Sirard JC, Mueller AJ, Heikenwalder M, Macpherson AJ, et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal salmonella diarrhea. PLoS pathogens. 2010;6(9):e1001097. doi: 10.1371/journal.ppat.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves AE, Koenigsknecht MJ, Bergin IL, Young VB. Suppression of clostridium difficile in the gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family lachnospiraceae. Infection and immunity. 2012;80(11):3786–3794. doi: 10.1128/IAI.00647-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willing BP, Vacharaksa A, Croxen M, Thanachayanont T, Finlay BB. Altering host resistance to infections through microbial transplantation. PloS one. 2011;6(10):e26988. doi: 10.1371/journal.pone.0026988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013;501(7467):426–429. doi: 10.1038/nature12447. This study identifies a specific gene cluster in B. fragilis that confers the ability to colonize the colonic mucus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Degnan PH, Barry NA, Mok KC, Taga ME, Goodman AL. Human gut microbes use multiple transporters to distinguish vitamin b12 analogs and compete in the gut. Cell host & microbe. 2014;15(1):47–57. doi: 10.1016/j.chom.2013.12.007. This study shows that commensal bacteria encode multiple non-redundant corrinoid receptors that enable efficient uptake of vitamin B12 analogs and confer a competitive growth advantage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins FM, Carter PB. Growth of salmonellae in orally infected germfree mice. Infection and immunity. 1978;21(1):41–47. doi: 10.1128/iai.21.1.41-47.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butterton JR, Ryan ET, Shahin RA, Calderwood SB. Development of a germfree mouse model of vibrio cholerae infection. Infection and immunity. 1996;64(10):4373–4377. doi: 10.1128/iai.64.10.4373-4377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Kamada N, Kim YG, Sham HP, Vallance BA, Puente JL, Martens EC, Nunez G. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012;336(6086):1325–1329. doi: 10.1126/science.1222195. This study finds that comensal bacteria outcompete C. rodentium for available carbohydrates in the intesinal lumen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, Sonnenburg JL. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502(7469):96–99. doi: 10.1038/nature12503. This study demonstrates that disruption of the microbiota increases the availability of intesitnal carbohydrates, which enteric pathogens consume to drive expansion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammami R, Fernandez B, Lacroix C, Fliss I. Anti-infective properties of bacteriocins: An update. Cell Mol Life Sci. 2013;70(16):2947–2967. doi: 10.1007/s00018-012-1202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rea MC, Sit CS, Clayton E, O'Connor PM, Whittal RM, Zheng J, Vederas JC, Ross RP, Hill C. Thuricin cd, a posttranslationally modified bacteriocin with a narrow spectrum of activity against clostridium difficile. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(20):9352–9357. doi: 10.1073/pnas.0913554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rea MC, Alemayehu D, Casey PG, O'Connor PM, Lawlor PG, Walsh M, Shanahan F, Kiely B, Ross RP, Hill C. Bioavailability of the anti-clostridial bacteriocin thuricin cd in gastrointestinal tract. Microbiology. 2014;160(Pt 2):439–445. doi: 10.1099/mic.0.068767-0. [DOI] [PubMed] [Google Scholar]
- 28.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Hautefort I, Thompson A, Hinton JC, Van Immerseel F. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Applied and environmental microbiology. 2006;72(1):946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, Motherway MO, Shanahan F, Nally K, Dougan G, van Sinderen D. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(6):2108–2113. doi: 10.1073/pnas.1115621109. This study identifies a surface exopolysaccaride on Bifdobacteria that interacts with the host and is critical for Bifdobacteria-mediated protection against C. rodentium infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimura K, Matsui T, Itoh K. Prevention of escherichia coli o157:H7 infection in gnotobiotic mice associated with bifidobacterium strains. Antonie van Leeuwenhoek. 2010;97(2):107–117. doi: 10.1007/s10482-009-9391-y. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 32.Minot S, Sinha R, Chen J, Li H, Keilbaugh SA, Wu GD, Lewis JD, Bushman FD. The human gut virome: Inter-individual variation and dynamic response to diet. Genome Res. 2011;21(10):1616–1625. doi: 10.1101/gr.122705.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Duerkop BA, Clements CV, Rollins D, Rodrigues JL, Hooper LV. A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(43):17621–17626. doi: 10.1073/pnas.1206136109. The authors identify a bacteriophage in E. faecalis that confers a competitive survival advantage in the intestinal lumen over strains lacking the bacteriophage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basler M, Mekalanos JJ. Type 6 secretion dynamics within and between bacterial cells. Science. 2012;337(6096):815. doi: 10.1126/science.1222901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Basler M, Ho BT, Mekalanos JJ. Tit-for-tat: Type vi secretion system counterattack during bacterial cell-cell interactions. Cell. 2013;152(4):884–894. doi: 10.1016/j.cell.2013.01.042. This reference describes bacterial “dueling” where the type VI secretion system is used by competitive bacterial species to establish dominance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Fu Y, Waldor MK, Mekalanos JJ. Tn-seq analysis of vibrio cholerae intestinal colonization reveals a role for t6ss-mediated antibacterial activity in the host. Cell host & microbe. 2013;14(6):652–663. doi: 10.1016/j.chom.2013.11.001. The authors identify the importance of the type VI secretion system in supporting bacterial colonization of the intestine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nature reviews Immunology. 2013;13(11):790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nature reviews Immunology. 2012;12(7):503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455(7214):804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal cd103(+)cd11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36(2):276–287. doi: 10.1016/j.immuni.2011.12.011. This reference identifies the immune cell that produces IL-23 in response to flaggellin and drives the IL-22/RegIIIγ axis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonnenberg GF, Fouser LA, Artis D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by il-22. Nature immunology. 2011;12(5):383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 43*.Mukherjee S, Zheng H, Derebe MG, Callenberg KM, Partch CL, Rollins D, Propheter DC, Rizo J, Grabe M, Jiang QX, Hooper LV. Antibacterial membrane attack by a pore-forming intestinal c-type lectin. Nature. 2014;505(7481):103–107. doi: 10.1038/nature12729. This study identifies the molecular mechanism for RegIII proteins bactericidal activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–385. doi: 10.1016/j.immuni.2013.08.003. This study finds that commensal Lactobacilli can use trytophan as an enegy source and produce an aryl-hydrocarbon receptor ligand that stimulates IL-22 production and enhances protection against C. albicans infection. [DOI] [PubMed] [Google Scholar]
- 45.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. Cd4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34(1):122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, et al. Microbial flora drives interleukin 22 production in intestinal nkp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29(6):958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, Fu YX, Zhou L. Group 3 innate lymphoid cells inhibit t-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39(2):386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zenewicz LA, Yin X, Wang G, Elinav E, Hao L, Zhao L, Flavell RA. Il-22 deficiency alters colonic microbiota to be transmissible and colitogenic. Journal of immunology. 2013;190(10):5306–5312. doi: 10.4049/jimmunol.1300016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, et al. Induction of intestinal th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw MH, Kamada N, Kim YG, Nunez G. Microbiota-induced il-1beta, but not il-6, is critical for the development of steady-state th17 cells in the intestine. The Journal of experimental medicine. 2012;209(2):251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen VL, Surana NK, Duan J, Kasper DL. Role of murine intestinal interleukin-1 receptor 1-expressing lymphoid tissue inducer-like cells in salmonella infection. PloS one. 2013;8(6):e65405. doi: 10.1371/journal.pone.0065405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Molloy MJ, Grainger JR, Bouladoux N, Hand TW, Koo LY, Naik S, Quinones M, Dzutsev AK, Gao JL, Trinchieri G, Murphy PM, et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell host & microbe. 2013;14(3):318–328. doi: 10.1016/j.chom.2013.08.003. This study observed that following infection-induced epithelial damage, commensal bacteria derived n-formyl peptide promote neutrophil emigration into lumen to contain bacterial translocation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser ID, Belkaid Y. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nature medicine. 2013;19(6):713–721. doi: 10.1038/nm.3189. The authors demonstrate that following infection-induced epithelial damage, commensal bacteria promote prostaglandin E2 production by inflammatory monocytes to limit the inflammatory response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012 doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza a virus infection. Proc Natl Acad Sci U S A. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, Wherry EJ, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37(1):158–170. doi: 10.1016/j.immuni.2012.04.011. This reference demonstrates that tonic commensal bacterial-derived signals modulate expression of antiviral defense genes in the steady state and alter susceptibility to viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, Diefenbach A. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37(1):171–186. doi: 10.1016/j.immuni.2012.05.020. The authors demonstrate that commensal bacterial-derived signals imprint epigenetic modification in interferon response genes maintaining these genes in an open, transcriptionally active state. [DOI] [PubMed] [Google Scholar]
- 58.Alenghat T, Osborne LC, Saenz SA, Kobuley D, Ziegler CG, Mullican SE, Choi I, Grunberg S, Sinha R, Wynosky-Dolfi M, Snyder A, et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature. 2013;504(7478):153–157. doi: 10.1038/nature12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, et al. Treg induction by a rationally selected mixture of clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 60*.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory t-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. This reference along with Ref 61 and 62 demonstrate that SCFA produced by commensal bacteria promote chromatin remodeling and drive Treg cell differentiation in the intestine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory t cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. See annotation to Ref 60. [DOI] [PubMed] [Google Scholar]
- 62*.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. See annotation to Ref 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of enterobacteriaceae. Cell host & microbe. 2007;2(3):204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Haag LM, Fischer A, Otto B, Plickert R, Kuhl AA, Gobel UB, Bereswill S, Heimesaat MM. Intestinal microbiota shifts towards elevated commensal escherichia coli loads abrogate colonization resistance against campylobacter jejuni in mice. PloS one. 2012;7(5):e35988. doi: 10.1371/journal.pone.0035988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65**.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, et al. Host-derived nitrate boosts growth of e. Coli in the inflamed gut. Science. 2013;339(6120):708–711. doi: 10.1126/science.1232467. This references shows that nitrates released into the lumen by the host during inflammation leads to outgrowth of E. coli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ. Intestinal inflammation allows salmonella to use ethanolamine to compete with the microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(42):17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gill N, Ferreira RB, Antunes LC, Willing BP, Sekirov I, Al-Zahrani F, Hartmann M, Finlay BB. Neutrophil elastase alters the murine gut microbiota resulting in enhanced salmonella colonization. PloS one. 2012;7(11):e49646. doi: 10.1371/journal.pone.0049646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. Fucose sensing regulates bacterial intestinal colonization. Nature. 2012;492(7427):113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lamichhane-Khadka R, Kwiatkowski A, Maier RJ. The hyb hydrogenase permits hydrogen-dependent respiratory growth of salmonella enterica serovar typhimurium. MBio. 2010;1(5) doi: 10.1128/mBio.00284-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70**.Maier L, Vyas R, Cordova CD, Lindsay H, Schmidt TS, Brugiroux S, Periaswamy B, Bauer R, Sturm A, Schreiber F, von Mering C, et al. Microbiota-derived hydrogen fuels salmonella typhimurium invasion of the gut ecosystem. Cell host & microbe. 2013;14(6):641–651. doi: 10.1016/j.chom.2013.11.002. This study demonstrates that Samonella consume hydrogen in the lumen to powers respirtation and early replication. [DOI] [PubMed] [Google Scholar]
- 71.Maier RJ, Olczak A, Maier S, Soni S, Gunn J. Respiratory hydrogen use by salmonella enterica serovar typhimurium is essential for virulence. Infection and immunity. 2004;72(11):6294–6299. doi: 10.1128/IAI.72.11.6294-6299.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334(6053):245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334(6053):249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson CM, Jesudhasan PR, Pfeiffer JK. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell host & microbe. 2014;15(1):36–46. doi: 10.1016/j.chom.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]