Abstract
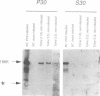
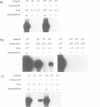
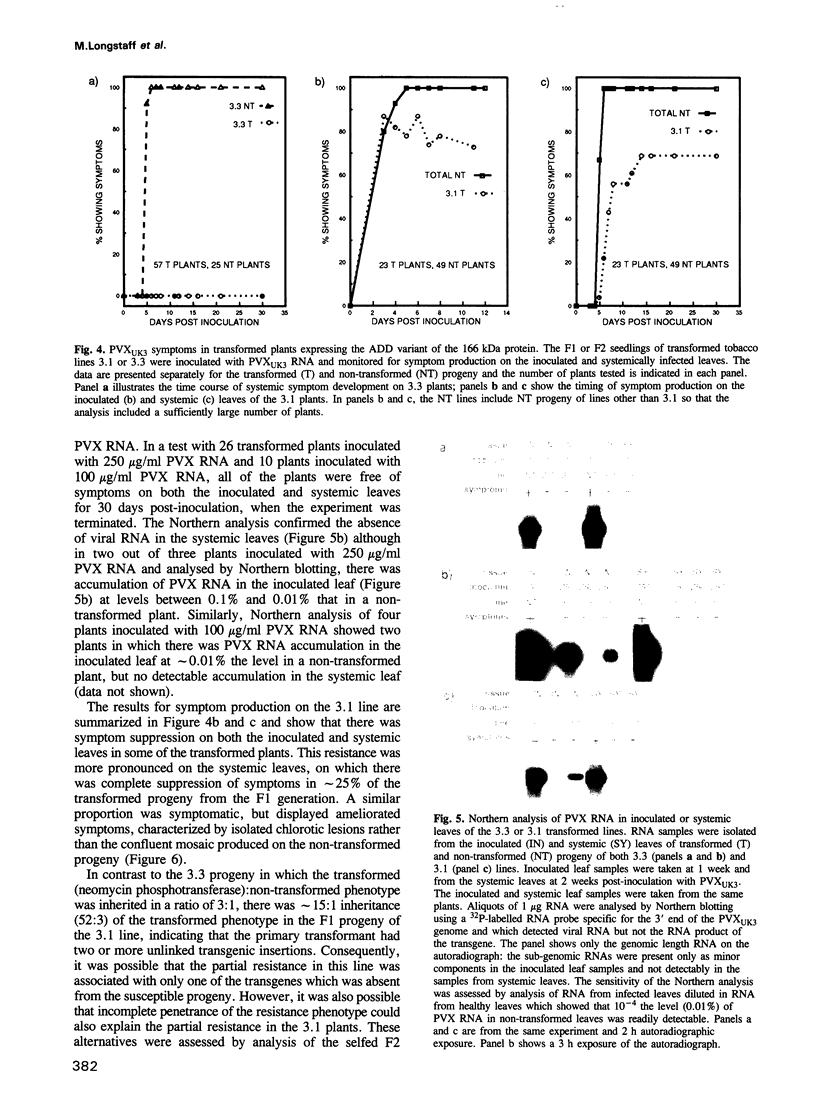
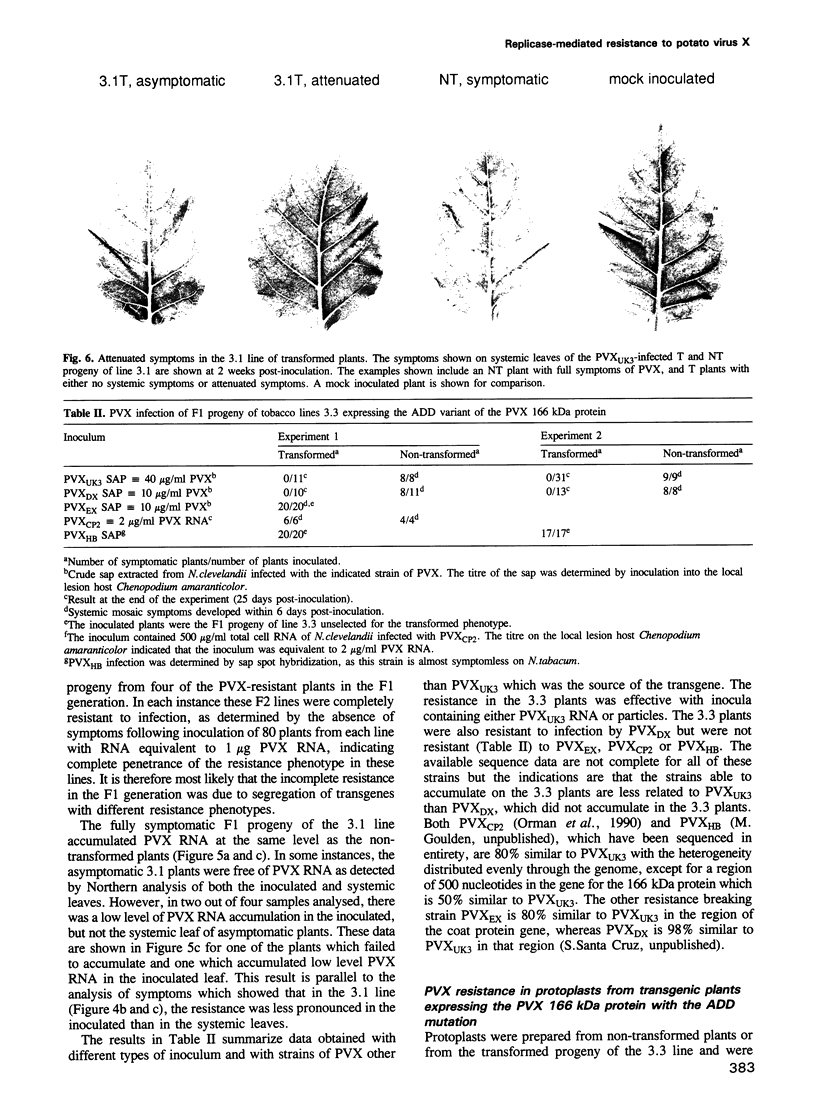
Three types of mutation were introduced into the sequence encoding the GDD motif of the putative replicase component of potato virus X (PVX). All three mutations rendered the viral genome completely noninfectious when inoculated into Nicotiana clevelandii or into protoplasts of Nicotiana tabacum (cv. Samsun NN). In order to test whether these negative mutations could inactivate the viral genome in trans, the mutant genes were expressed in transformed N.tabacum (cv. Samsun NN) under control of the 35S RNA promoter of cauliflower mosaic virus and the transformed lines were inoculated with PVX. In 10 lines tested in which the GDD motif was expressed as GAD or GED there was no effect on susceptibility to PVX. In two of four lines transformed to express the ADD form of the conserved motif, the F1 and F2 progeny plants were highly resistant to infection by PVX, although only to strains closely related to the source of the transgene. The resistance was associated with suppression of PVX accumulation in the inoculated and systemic leaves and in protoplasts of the transformed plants, although some low level viral RNA production was observed in the inoculated but not the systemic leaves when the inoculum was as high as 100 or 250 micrograms/ml PVX RNA. These results suggest for a plant virus, as reported previously for Q beta phage, that virus resistance may be engineered by expression of dominant negative mutant forms of viral genes in transformed cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Palukaitis P., Zaitlin M. A defective replicase gene induces resistance to cucumber mosaic virus in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8759–8763. doi: 10.1073/pnas.89.18.8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P. A sequence motif in many polymerases. Nucleic Acids Res. 1988 Nov 11;16(21):9909–9916. doi: 10.1093/nar/16.21.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C. J., Hemenway C. L. Expression of Amino-Terminal Portions or Full-Length Viral Replicase Genes in Transgenic Plants Confers Resistance to Potato Virus X Infection. Plant Cell. 1992 Jun;4(6):735–744. doi: 10.1105/tpc.4.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S., Kavanagh T., Baulcombe D. Potato virus X as a vector for gene expression in plants. Plant J. 1992 Jul;2(4):549–557. doi: 10.1046/j.1365-313x.1992.t01-24-00999.x. [DOI] [PubMed] [Google Scholar]
- David C., Gargouri-Bouzid R., Haenni A. L. RNA replication of plant viruses containing an RNA genome. Prog Nucleic Acid Res Mol Biol. 1992;42:157–227. doi: 10.1016/s0079-6603(08)60576-0. [DOI] [PubMed] [Google Scholar]
- Golemboski D. B., Lomonossoff G. P., Zaitlin M. Plants transformed with a tobacco mosaic virus nonstructural gene sequence are resistant to the virus. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6311–6315. doi: 10.1073/pnas.87.16.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habili N., Symons R. H. Evolutionary relationship between luteoviruses and other RNA plant viruses based on sequence motifs in their putative RNA polymerases and nucleic acid helicases. Nucleic Acids Res. 1989 Dec 11;17(23):9543–9555. doi: 10.1093/nar/17.23.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R. J., Buck K. W. Complete replication of a eukaryotic virus RNA in vitro by a purified RNA-dependent RNA polymerase. Cell. 1990 Oct 19;63(2):363–368. doi: 10.1016/0092-8674(90)90169-f. [DOI] [PubMed] [Google Scholar]
- Huisman M. J., Linthorst H. J., Bol J. F., Cornelissen J. C. The complete nucleotide sequence of potato virus X and its homologies at the amino acid level with various plus-stranded RNA viruses. J Gen Virol. 1988 Aug;69(Pt 8):1789–1798. doi: 10.1099/0022-1317-69-8-1789. [DOI] [PubMed] [Google Scholar]
- Inokuchi Y., Hirashima A. Interference with viral infection by RNA replicase deleted at the carboxy-terminal region. J Biochem. 1990 Jul;108(1):53–58. doi: 10.1093/oxfordjournals.jbchem.a123162. [DOI] [PubMed] [Google Scholar]
- Inokuchi Y., Hirashima A. Interference with viral infection by defective RNA replicase. J Virol. 1987 Dec;61(12):3946–3949. doi: 10.1128/jvi.61.12.3946-3949.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski S. A., Luo M., Morrow C. D. Enzymatic activity of poliovirus RNA polymerase mutants with single amino acid changes in the conserved YGDD amino acid motif. J Virol. 1991 Sep;65(9):4565–4572. doi: 10.1128/jvi.65.9.4565-4572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammann M., Laufs J., Schell J., Gronenborn B. Rapid insertional mutagenesis of DNA by polymerase chain reaction (PCR). Nucleic Acids Res. 1989 Jul 11;17(13):5404–5404. doi: 10.1093/nar/17.13.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh T., Goulden M., Santa Cruz S., Chapman S., Barker I., Baulcombe D. Molecular analysis of a resistance-breaking strain of potato virus X. Virology. 1992 Aug;189(2):609–617. doi: 10.1016/0042-6822(92)90584-c. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson C., Kaniewski W., Haley L., Rozman R., Newell C., Sanders P., Tumer N. E. Engineering resistance to mixed virus infection in a commercial potato cultivar: resistance to potato virus X and potato virus Y in transgenic Russet Burbank. Biotechnology (N Y) 1990 Feb;8(2):127–134. doi: 10.1038/nbt0290-127. [DOI] [PubMed] [Google Scholar]
- Lowe D. M., Parmar V., Kemp S. D., Larder B. A. Mutational analysis of two conserved sequence motifs in HIV-1 reverse transcriptase. FEBS Lett. 1991 May 6;282(2):231–234. doi: 10.1016/0014-5793(91)80484-k. [DOI] [PubMed] [Google Scholar]
- MacFarlane S. A., Davies J. W. Plants transformed with a region of the 201-kilodalton replicase gene from pea early browning virus RNA1 are resistant to virus infection. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5829–5833. doi: 10.1073/pnas.89.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Mise K., Okuno T., Furusawa I. Expression of brome mosaic virus-encoded replicase genes in transgenic tobacco plants. J Gen Virol. 1992 Jan;73(Pt 1):169–172. doi: 10.1099/0022-1317-73-1-169. [DOI] [PubMed] [Google Scholar]
- Orman B. E., Celnik R. M., Mandel A. M., Torres H. N., Mentaberry A. N. Complete cDNA sequence of a South American isolate of potato virus X. Virus Res. 1990 Jul;16(3):293–305. doi: 10.1016/0168-1702(90)90054-f. [DOI] [PubMed] [Google Scholar]
- Poch O., Sauvaget I., Delarue M., Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989 Dec 1;8(12):3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas J. C., Wickner R. B. RNA-dependent RNA polymerase consensus sequence of the L-A double-stranded RNA virus: definition of essential domains. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2185–2189. doi: 10.1073/pnas.89.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skryabin K. G., Kraev A. S., Morozov SYu, Rozanov M. N., Chernov B. K., Lukasheva L. I., Atabekov J. G. The nucleotide sequence of potato virus X RNA. Nucleic Acids Res. 1988 Nov 25;16(22):10929–10930. doi: 10.1093/nar/16.22.10929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner P. E., van der Kuyl A. C., Neeleman L., Bol J. F. Replication of an incomplete alfalfa mosaic virus genome in plants transformed with viral replicase genes. Virology. 1991 Apr;181(2):445–450. doi: 10.1016/0042-6822(91)90876-d. [DOI] [PubMed] [Google Scholar]