Abstract
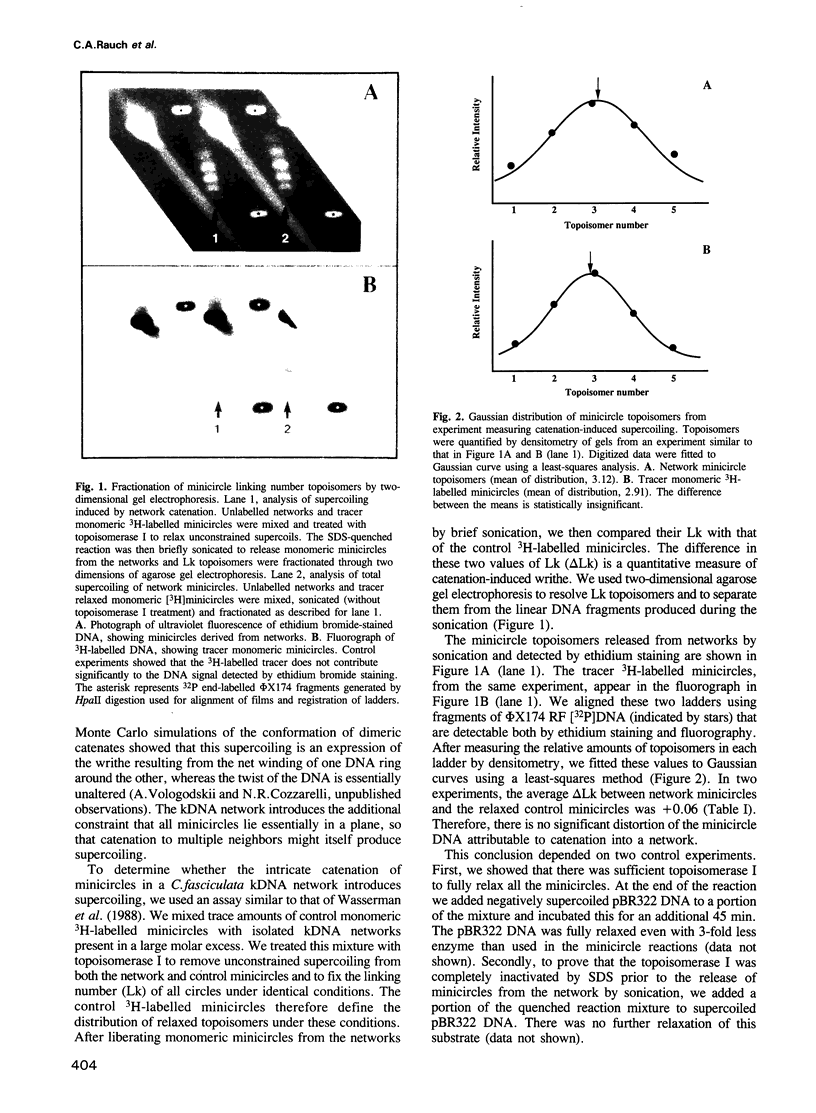
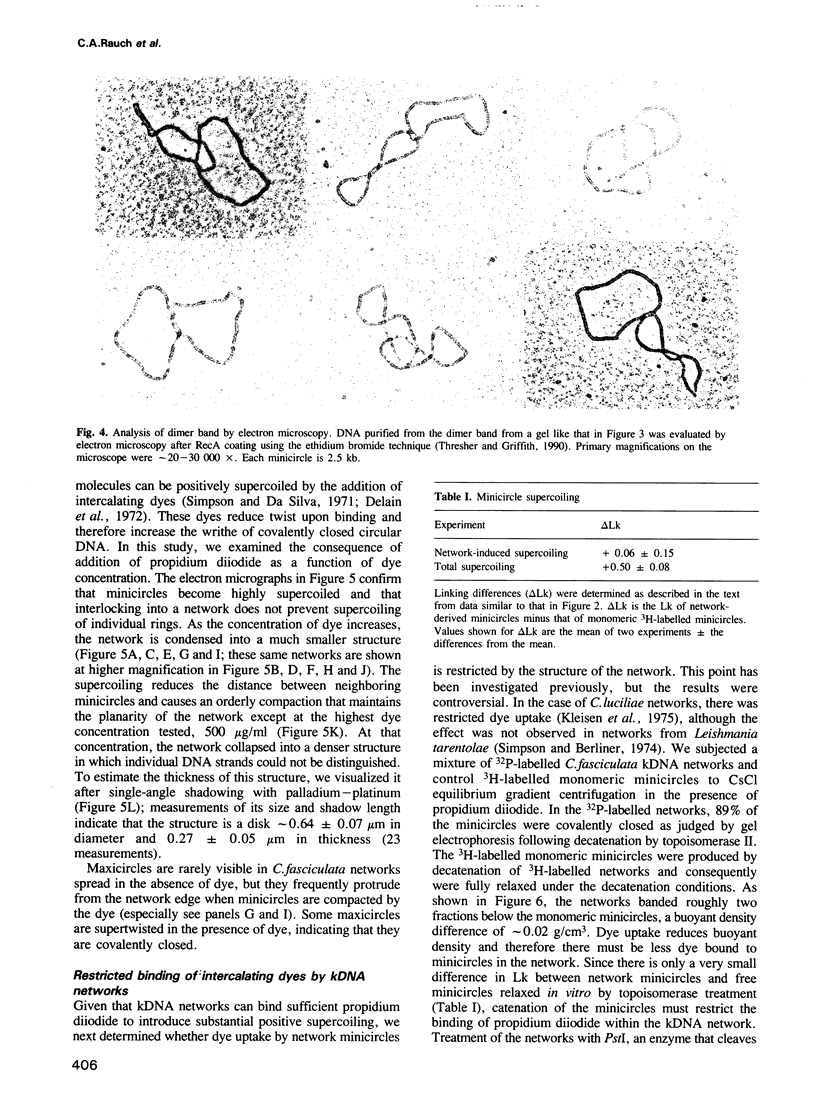
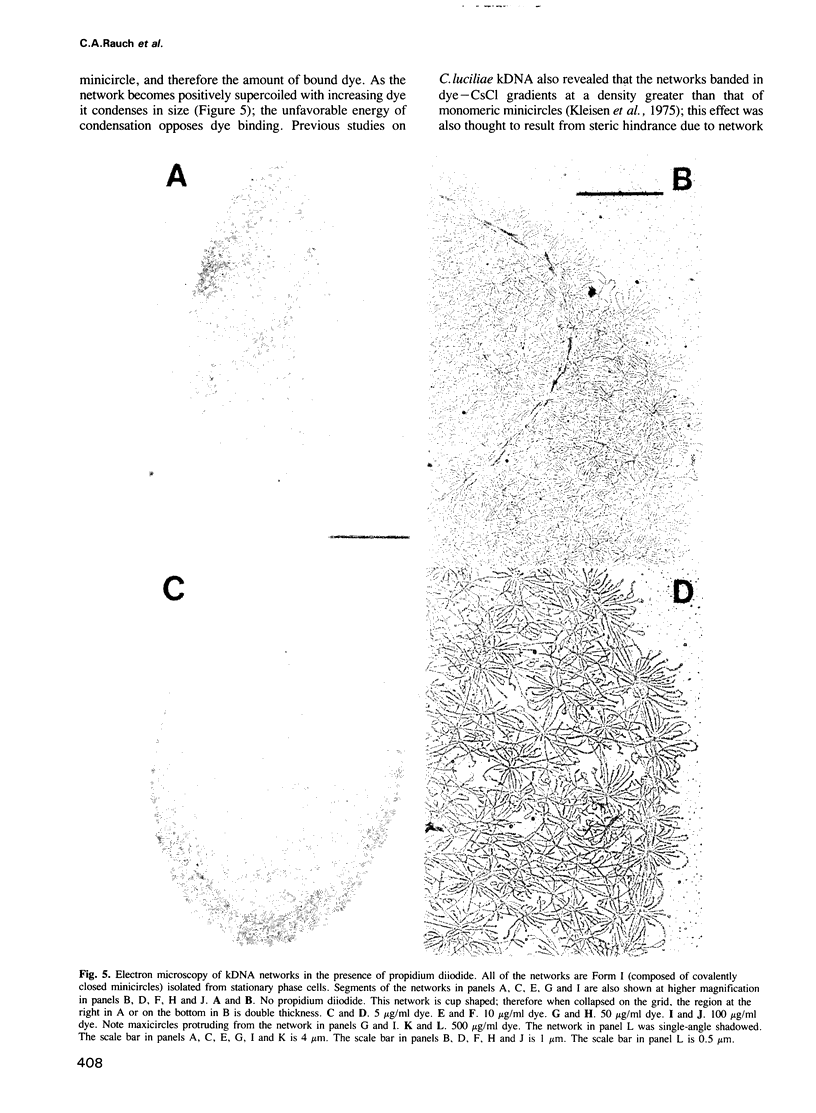
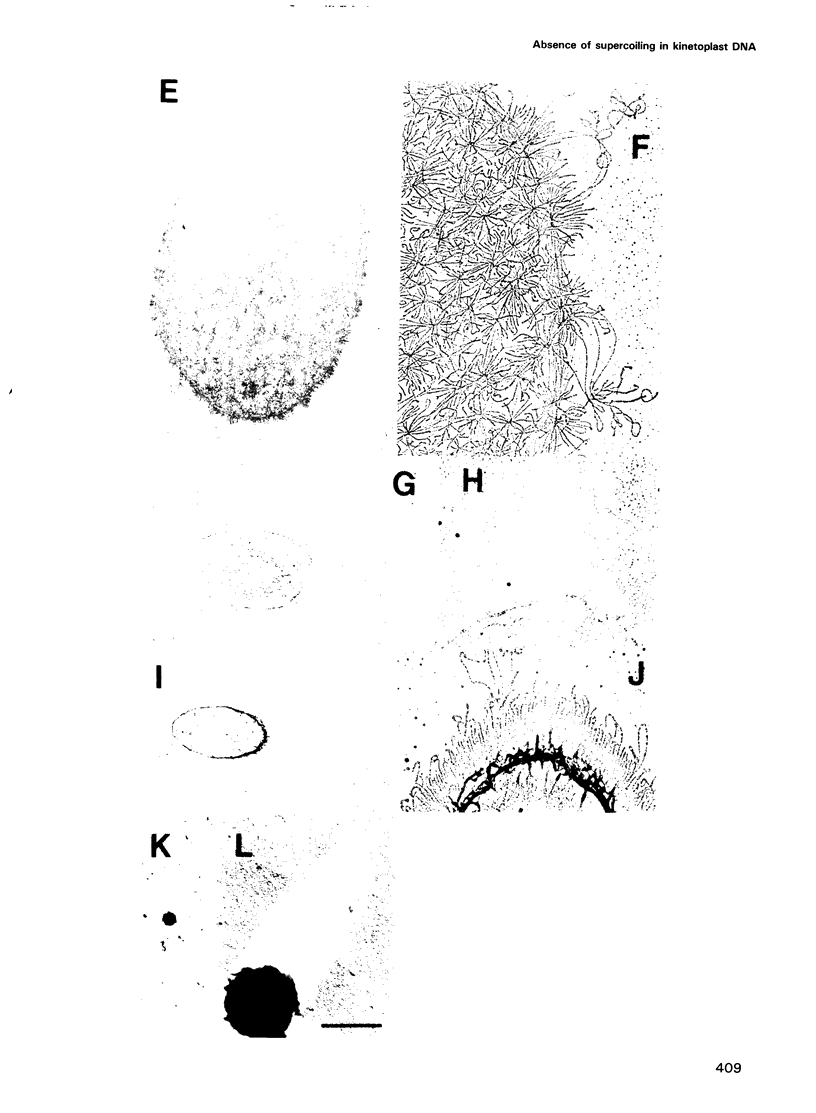
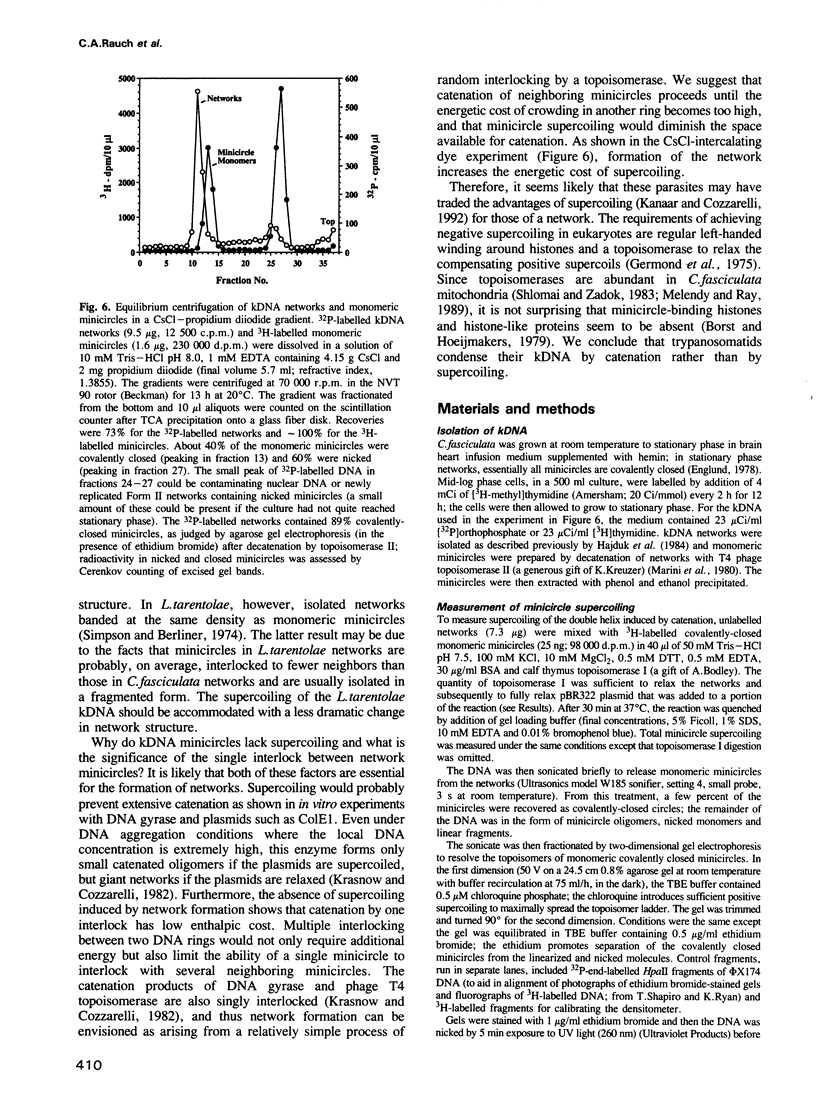
Crithidia fasciculata kinetoplast DNA is a mitochondrial DNA composed of 5000 minicircles and approximately 25 maxicircles, all catenated into a giant network. By comparing the linking number of minicircles released from the network by limited sonication with that of control minicircles, we demonstrate that not only does the elaborate catenation of the network not cause supercoiling, but that there is no minicircle supercoiling at all. The absence of catenation-induced supercoiling is explained by our finding [using electron microscopy (EM) and gel electrophoresis] that network minicircles are joined by only one interlock; single interlocking can be accommodated without helix distortion. EM revealed that propidium diiodide supertwists all the network minicircles and thereby condenses the network into a much smaller size while maintaining its planarity. At high dye concentration the network is condensed to a size comparable to that found in vivo. Nevertheless, network minicircles bind less propidium than free minicircles, indicating that catenation into a network restricts the supercoiling of individual rings. These studies show that the mitochondrion of trypanosomatids may be a unique niche in nature where a covalently-closed circular DNA is not supercoiled. This absence of supercoiling may be a major factor in promoting the formation of the network.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W., Hill G. C. Division and DNA synthesis in the kinetoplast ofCrithidia fasciculata. J Cell Sci. 1969 May;4(3):611–620. doi: 10.1242/jcs.4.3.611. [DOI] [PubMed] [Google Scholar]
- Bauer W. R. Structure and reactions of closed duplex DNA. Annu Rev Biophys Bioeng. 1978;7:287–313. doi: 10.1146/annurev.bb.07.060178.001443. [DOI] [PubMed] [Google Scholar]
- Benne R., Van den Burg J., Brakenhoff J. P., Sloof P., Van Boom J. H., Tromp M. C. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986 Sep 12;46(6):819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- Blum B., Bakalara N., Simpson L. A model for RNA editing in kinetoplastid mitochondria: "guide" RNA molecules transcribed from maxicircle DNA provide the edited information. Cell. 1990 Jan 26;60(2):189–198. doi: 10.1016/0092-8674(90)90735-w. [DOI] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Borst P. Why kinetoplast DNA networks? Trends Genet. 1991 May;7(5):139–141. doi: 10.1016/0168-9525(91)90374-y. [DOI] [PubMed] [Google Scholar]
- Cheng D., Simpson L. Isolation and characterization of kinetoplast DNA and RNA of Phytomonas davidi. Plasmid. 1978 Jun;1(3):297–315. doi: 10.1016/0147-619x(78)90047-1. [DOI] [PubMed] [Google Scholar]
- Englund P. T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978 May;14(1):157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Abraham J. M., Stuart K. Extensive editing of the cytochrome c oxidase III transcript in Trypanosoma brucei. Cell. 1988 May 6;53(3):413–422. doi: 10.1016/0092-8674(88)90161-4. [DOI] [PubMed] [Google Scholar]
- Feagin J. E. RNA editing in kinetoplastid mitochondria. J Biol Chem. 1990 Nov 15;265(32):19373–19376. [PubMed] [Google Scholar]
- Ferguson M., Torri A. F., Ward D. C., Englund P. T. In situ hybridization to the Crithidia fasciculata kinetoplast reveals two antipodal sites involved in kinetoplast DNA replication. Cell. 1992 Aug 21;70(4):621–629. doi: 10.1016/0092-8674(92)90431-b. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduk S. L., Klein V. A., Englund P. T. Replication of kinetoplast DNA maxicircles. Cell. 1984 Feb;36(2):483–492. doi: 10.1016/0092-8674(84)90241-1. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Weijers P. J. The segregation of kinetoplast DNA networks in Trypanosoma brucei. Plasmid. 1980 Jul;4(1):97–116. doi: 10.1016/0147-619x(80)90086-4. [DOI] [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Englund P. T. Intermediates in the replication of kinetoplast DNA minicircles. J Biol Chem. 1985 Mar 25;260(6):3844–3851. [PubMed] [Google Scholar]
- Kleisen C. M., Borst P., Weijers P. J. The structure of kinetoplast DNA. I. Properties of the intact multi-circular complex from Crithidia luciliae. Biochim Biophys Acta. 1975 May 1;390(2):155–167. [PubMed] [Google Scholar]
- Krasnow M. A., Cozzarelli N. R. Catenation of DNA rings by topoisomerases. Mechanism of control by spermidine. J Biol Chem. 1982 Mar 10;257(5):2687–2693. [PubMed] [Google Scholar]
- Krasnow M. A., Stasiak A., Spengler S. J., Dean F., Koller T., Cozzarelli N. R. Determination of the absolute handedness of knots and catenanes of DNA. Nature. 1983 Aug 11;304(5926):559–560. doi: 10.1038/304559a0. [DOI] [PubMed] [Google Scholar]
- Kusel J. P., Moore K. E., Weber M. M. The ultrastructure of Crithidia fasciculata and morphological changes induced by growth in acriflavin. J Protozool. 1967 May;14(2):283–296. doi: 10.1111/j.1550-7408.1967.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Marini J. C., Miller K. G., Englund P. T. Decatenation of kinetoplast DNA by topoisomerases. J Biol Chem. 1980 Jun 10;255(11):4976–4979. [PubMed] [Google Scholar]
- Melendy T., Ray D. S. Novobiocin affinity purification of a mitochondrial type II topoisomerase from the trypanosomatid Crithidia fasciculata. J Biol Chem. 1989 Jan 25;264(3):1870–1876. [PubMed] [Google Scholar]
- Pollard V. W., Rohrer S. P., Michelotti E. F., Hancock K., Hajduk S. L. Organization of minicircle genes for guide RNAs in Trypanosoma brucei. Cell. 1990 Nov 16;63(4):783–790. doi: 10.1016/0092-8674(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Ray D. S. Kinetoplast DNA minicircles: high-copy-number mitochondrial plasmids. Plasmid. 1987 May;17(3):177–190. doi: 10.1016/0147-619x(87)90026-6. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Englund P. T. Replication of kinetoplast DNA in trypanosomes. Annu Rev Microbiol. 1988;42:339–358. doi: 10.1146/annurev.mi.42.100188.002011. [DOI] [PubMed] [Google Scholar]
- Shlomai J., Zadok A. Reversible decatenation of kinetoplast DNA by a DNA topoisomerase from trypanosomatids. Nucleic Acids Res. 1983 Jun 25;11(12):4019–4034. doi: 10.1093/nar/11.12.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L. Isolation and characterization of kinetoplast DNA networks and minicircles from Crithidia fasciculata. J Protozool. 1974 Nov;21(5):774–781. doi: 10.1111/j.1550-7408.1974.tb03751.x. [DOI] [PubMed] [Google Scholar]
- Simpson L., Berliner J. Isolation of the kinetoplast DNA of Leishmania tarentolae in the form of a network. J Protozool. 1974 May;21(2):382–393. doi: 10.1111/j.1550-7408.1974.tb03675.x. [DOI] [PubMed] [Google Scholar]
- Simpson L., Da Silva A. Isolation and characterization of kinetoplast DNA from Leishmania tarentolae. J Mol Biol. 1971 Mar 28;56(3):443–473. doi: 10.1016/0022-2836(71)90394-9. [DOI] [PubMed] [Google Scholar]
- Simpson L. RNA editing--a novel genetic phenomenon? Science. 1990 Oct 26;250(4980):512–513. doi: 10.1126/science.1700474. [DOI] [PubMed] [Google Scholar]
- Simpson L., Shaw J. RNA editing and the mitochondrial cryptogenes of kinetoplastid protozoa. Cell. 1989 May 5;57(3):355–366. doi: 10.1016/0092-8674(89)90911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Sturm N. R., Simpson L. Kinetoplast DNA minicircles encode guide RNAs for editing of cytochrome oxidase subunit III mRNA. Cell. 1990 Jun 1;61(5):879–884. doi: 10.1016/0092-8674(90)90198-n. [DOI] [PubMed] [Google Scholar]
- Sugisaki H., Ray D. S. DNA sequence of Crithidia fasciculata kinetoplast minicircles. Mol Biochem Parasitol. 1987 Apr;23(3):253–263. doi: 10.1016/0166-6851(87)90032-6. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Thresher R. J., Griffith J. D. Intercalators promote the binding of RecA protein to double-stranded DNA. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5056–5060. doi: 10.1073/pnas.87.13.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. A., White J. H., Cozzarelli N. R. The helical repeat of double-stranded DNA varies as a function of catenation and supercoiling. Nature. 1988 Aug 4;334(6181):448–450. doi: 10.1038/334448a0. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Simpson L. Studies on kinetoplast DNA. II. Biophysical properties of minicircular DNA from Leishmania tarentolae. Biochim Biophys Acta. 1973 Sep 7;319(3):254–266. doi: 10.1016/0005-2787(73)90164-0. [DOI] [PubMed] [Google Scholar]