Summary
Background
Sorafenib was tested for neoadjuvant treatment with an anthracycline/taxane-based chemotherapy in the open-label, multicentre, single-arm phase II study, ‘SOFIA’.
Patients and Methods
Inclusion criteria were: HER2 negative, cT3, cT4 or cT2 cN+, M0 primary breast cancer. Patients received 4 × epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 (EC) intravenously (i.v.) in 3-weekly cycles followed or preceded by 12 weeks of paclitaxel (Pw) 80 mg/m2. In cohort 1, sorafenib started at 800 mg daily with chemotherapy. An initial daily sorafenib dose of 200 mg was escalated, based on individual toxicities, every 3 weeks in cohort 2 (starting with EC) and every 2 weeks in cohort 3 (starting with Pw). The primary objective was to identify the most feasible regimen; secondary objectives were safety, pathological complete response (pCR) at surgery and pharmacokinetics.
Results
Of the 36 recruited patients, 7/12 patients completed the study in cohort 1 and 24/24 patients in cohorts 2 and 3. The median cumulative sorafenib dose per patient was 37%, 65% and 46% in cohorts 1, 2 and 3, respectively. The main grade 3–4 toxicities were neutropenia and hand-foot syndrome. The pCR (ypT0/is) rate was 27.7%. No pharmacokinetic interaction was observed between sorafenib and epirubicin.
Conclusion
Sorafenib EC-Pw is feasible if the starting dose is 200 mg, escalated every 3 weeks based on the patients’ individual toxicities.
Keywords: Breast cancer, Sorafenib, Pharmacokinetics, Anthracycline, Taxane
Introduction
The vascular endothelial growth factor (VEGF) receptor plays an important role in tumour vascularisation and growth. Bevacizumab, an anti-VEGF antibody, has improved progression-free survival of metastatic breast cancer when added to various chemotherapy regimens [1]. In combination with anthracycline/taxane-based chemotherapy given preoperatively, bevacizumab significantly increased the pathological complete response (pCR) rate in early breast cancer [2, 3].
Sorafenib, a potent multikinase inhibitor, targets the VEGF-2 receptor, the platelet-derived growth factor receptor-β (PDGFR-β), but also the Raf kinase, c-Kit and Flt [4]. Preclinical studies demonstrated antitumor activity independent of Ras mutations and suggested additive anti-tumour effects [5]. 2 phase II studies including 79 heavily pretreated breast cancer patients revealed only modest single-agent activity but good tolerability [6, 7]. A subsequent randomised phase II study in 229 human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer patients investigated the combination of sorafenib and capecitabine. The addition of sorafenib to capecitabine resulted in a significant improvement in progression-free survival versus placebo (median, 6.4 vs. 4.1 months; hazard ratio (HR), 0.58; 95% confidence interval (CI), 0.41–0.81; P = 0.001), favouring sorafenib across subgroups, but was associated with higher toxicity and treatment discontinuation [8]. A randomised phase III study (RESILIENCE; NCT01234337) currently assesses this concept [9].
The neoadjuvant systemic treatment of breast cancer yields disease-free and overall survival rates comparable to those with adjuvant systemic therapy but allows for treatment monitoring in previously untreated patients [10]. Before any other combination therapy was explored, the non-randomised phase II SOFIA study (NCT00548899) was started to investigate the addition of sorafenib to standard epirubicin and cyclophosphamide (EC)-paclitaxel weekly chemotherapy as neoadjuvant treatment in medium-to-high-risk primary HER2-negative breast cancer [11].
Methods and Patients
Objectives
The primary objective of this open-label, single-arm, multicentre phase II study was to find the most feasible regimen of sorafenib (i.e. the regimen with the highest cumulative dose of sorafenib) in combination with epirubicin, cyclophosphamide and paclitaxel in patients with HER2-negative primary breast cancer. Secondary end points were tolerability, pCR rate after neoadjuvant chemotherapy with sorafenib, the response rates of breast tumours and axillary nodes as assessed by palpation or ultrasonography before surgery, the rates of pathological stage ypN0 after neoadjuvant therapy and the rate of breast conservation (for methods, see supplementary material). Pharmacokinetic interaction of sorafenib and epirubicin was assessed additionally.
Patients
Women with histologically confirmed uni- or bilateral primary untreated breast cancer who provided written informed consent were eligible. The HER2 status of the tumour had to be negative. The tumour had to be at least 2 cm in size by palpation and measurable preferably by ultrasound. In case of inflammatory breast cancer, the extension of the inflammation could be used as measurable lesion. Patients should have a disease stage in which adjuvant chemotherapy would be considered; therefore, the following stages were eligible: locally advanced disease with cT3-T4 or cT2, cN+. Further relevant criteria were a normal cardiac function measured by echocardiography (left ventricular ejection fraction ≥ 55%), no known thromboembolic events or ischemic attacks in the previous 6 months, no haemorrhagic diathesis or coagulopathy, no pre-existing sensory neuropathy grade ≥ 2 (National Cancer Institute Common Toxicity Criteria v. 3.0 (NCI-CTC)), no major surgery within the past 4 weeks, no other serious illnesses or medical conditions. The study adhered to the Declaration of Helsinki. The protocol was reviewed by the responsible ethics committee at each participating site. The conduct of the trial was supervised by an independent data and safety monitoring committee.
Therapy
All patients received neoadjuvant EC (epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 body surface area) once every 3 weeks for 4 cycles, followed or preceded by paclitaxel 80 mg/m2 every week for 12 weeks. In cohort 1, patients received a fixed oral daily dose of 800 mg sorafenib on days 2–19 during EC and continuously during paclitaxel. Sorafenib was stopped after 23 weeks of treatment. This would result in a cumulative dose of 119.2 g sorafenib. After an amendment due to increased skin toxicities (any type) resulting in therapy discontinuation, patients in cohorts 2 and 3 started with sorafenib 200 mg daily and increased the dose during EC every cycle and during paclitaxel every 2 weeks if no skin toxicity occurred. In case of skin toxicity grade 1, the same dose was continued. The maximum achieved dose was maintained throughout the treatment. In cohort 3, patients started with paclitaxel followed by EC using the same dose-escalating schedule for sorafenib (supplementary fig. S1). All patients were then operated on and received adjuvant radiotherapy and endocrine treatment as standard of care if indicated.
Pharmakokinetics
During EC treatment, pharmacokinetic samples were taken during cycles 1 and 2 (alternatively 3) on day 1 prior to the start of epirubicin infusion, at the termination of epirubicin infusion and at 5, 20, 180 min and 24 h after the end of epirubicin infusion. Population pharmacokinetic analyses on total epirubicin concentrations quantified by a previously published high-performance liquid chromatography (HPLC) method were carried out using NONMEM 7.2 (ICON Development Solutions, Dublin, Ireland) [12].
Statistics
All patients who started therapy were included in the efficacy and safety analyses. Patients with missing data regarding response were counted as having no response.
The initial sample size calculation was based on the GeparDuo study [13]. An exact binominal test with an alpha of 10% would have had an 80% power to detect the difference between the null hypothesis proportion (pCR of 25% (22.3%)) and the alternative proportion (pCR of 40%) when the sample size was 62 patients. The original primary end point pCR was modified by an amendment to find the most feasible regimen for sorafenib (i.e. the regimen with the highest cumulative dose of sorafenib), as an early safety assessment revealed that tolerability of the initial schedule was not given, and 3 equal-sized cohorts with 12 patients each were recruited to establish the best regimen.
Results
Patients
From November 2007 until December 2010, 36 patients (12 in each cohort) entered the study in 10 sites in Germany. The baseline characteristics overall and per treatment cohort are outlined in table 1.
Table 1.
Baseline characteristics
| Cohort 1 (N = 12) | Cohort 2 (N = 12) | Cohort 3 (N = 12) | Total (N = 36) | |
|---|---|---|---|---|
| Age, years: median (range) | 44 (31–67) | 44 (28–53) | 47 (28–56) | 45 (28–67) |
| Menopausal status | ||||
| Pre | 9 (75.0%) | 12 (100%) | 8 (66.7%) | 29 (80.6%) |
| Post | 3 (25%) | 0 (0%) | 4 (33.3%) | 7 (19.4%) |
| Karnofsky score | ||||
| 100% | 11 (91.7%) | 12 (100.0%) | 10 (83.3%) | 33 (91.7%) |
| 90% | 1 (8.3%) | 0 (0.0%) | 2 (16.7%) | 3 (8.3%) |
| Clinical T stage | ||||
| I | 0 (0%) | 2 (16.7%) | 4 (33.3%) | 6 (16.7%) |
| II | 11 (91.7%) | 10 (83.3%) | 6 (50.0%) | 27 (75.0%) |
| III | 1 (8.3%) | 0 (0%) | 2 (16.7%) | 3 (8.3%) |
| Clinical N stage | ||||
| 0 | 3 (25.0%) | 8 (66.7%) | 6 (50.0%) | 17 (47.2%) |
| 1 | 9 (75.0%) | 4 (33.3%) | 6 (50.0%) | 19 (52.8%) |
| 2 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Sentinel node biopsy | ||||
| Yes | 2 (16.7%) | 4 (33.3%) | 5 (41.7%) | 11 (30.5%) |
| No | 10 (83.3%) | 8 (66.7%) | 7 (58.3%) | 25 (69.5%) |
| Histological tumour type | ||||
| Ductal or ductal/lobular invasive | 9 (75.0%) | 10 (83.3%) | 11 (91.7%) | 30 (83.3%) |
| Lobular invasive | 0 (0.0%) | 1 (8.3%) | 1 (8.3%) | 2 (5.6%) |
| Other | 3 (25.0%) | 1 (8.3%) | 0 (0.0%) | 4 (11.1%) |
| Tumour grade | ||||
| I | 1 (8.3%) | 0 (0.0%) | 0 (0.0%) | 1 (2.8%) |
| II | 4 (33.3%) | 6 (50.0%) | 6 (50.0%) | 16 (44.4%) |
| III | 7 (58.3%) | 6 (50.0%) | 6 (50.0%) | 19 (52.8%) |
| Hormone receptor status | ||||
| Positive | 8 (66.7%) | 6 (50.0%) | 7 (58.3%) | 21 (58.3%) |
| Negative | 4 (33.3%) | 6 (50.0%) | 5 (41.7%) | 15 (41.7%) |
| HER2 status | ||||
| Negative | 12 (100%) | 12 (100%) | 12 (100%) | 36 (100%) |
Compliance and Toxicity
The 36 patients were distributed into 3 equal-sized cohorts. All patients received 4 cycles of EC, and 33 patients received 12 weeks of paclitaxel. 1 patient never started paclitaxel, 1 patient stopped after 6 weeks and 1 after 9 weeks. 18 patients received at least 23 weeks of sorafenib as planned (supplementary table S1). In cohort 1, 7 of 12 patients completed the study and 6 of them received sorafenib at a reduced dose; 5 patients discontinued sorafenib prematurely, 3 due to adverse events, 1 due to progression and 1 on the patient's request. In cohort 1, the maximum tolerated dose of sorafenib was 400 mg per day. Only 1 patient could be treated with 800 mg sorafenib daily. In cohorts 2 and 3, all 12 patients completed the study. In cohort 2, the sorafenib dose could be escalated to 800 mg in 6 of the 12 patients, to 600 mg in 4 patients and to 200 mg daily in 2 patients. In cohort 3, 1 patient was escalated to 800 mg, 3 to 600 mg, 6 to 400 mg and 2 patients remained on 200 mg sorafenib daily. The median cumulative dose per patient was 44.5 g in cohort 1, 77.4 g in cohort 2 and 55.2 g in cohort 3, which corresponds to 37%, 65% and 46% relative to the maximum pre-planned cumulative dose of 800 mg, respectively.
The main toxicities were neutropenia grade 3–4 in all patients and hand-foot syndrome (HFS) of any grade in 30/36 patients, which was severe in 5 patients (table 2). Sensory neuropathy grade 1–2 was reported in 26 patients. Nausea and vomiting grade 1–2 were observed in 32 and 12 patients, respectively. 1 patient had severe nausea. No patient died while on treatment.
Table 2.
Adverse events per patient
| Cohort 1 (N = 12) | Cohort 2 (N = 12) | Cohort 3 (N = 12) | Overall (N = 36) | |||||
|---|---|---|---|---|---|---|---|---|
| Toxicity, grade | 1–4 | 3–4 | 1–4 | 3–4 | 1–4 | 3–4 | 1–4 | 3–4 |
| Hematologic toxicity, n | ||||||||
| Leukopenia | 12 | 10 | 12 | 10 | 11 | 8 | 35 | 28 |
| Neutropenia | 12 | 11 | 12 | 12 | 12 | 12 | 36 | 35 |
| Febrile neutropenia | 0 | 0 | 1 | 1 | 2 | 2 | 3 | 3 |
| Anaemia | 12 | 0 | 12 | 0 | 12 | 1 | 36 | 1 |
| Thrombopenia | 2 | 0 | 5 | 0 | 5 | 1 | 12 | 1 |
| Non-haematologic toxicity, n | ||||||||
| Alopecia | 12 | n.a. | 12 | n.a. | 12 | n.a. | 36 | n.a. |
| Allergic reaction | 5 | 1 | 2 | 1 | 4 | 1 | 11 | 3 |
| Conjunctivitis | 2 | 0 | 0 | 0 | 2 | 0 | 4 | 0 |
| Diarrhoea | 9 | 0 | 12 | 0 | 9 | 0 | 30 | 0 |
| Dyspnoea | 1 | 0 | 1 | 0 | 4 | 0 | 6 | 0 |
| Fatigue | 9 | 0 | 9 | 0 | 12 | 1 | 30 | 1 |
| Fever without neutropenia | 0 | 0 | 2 | 1 | 5++ | 1 | 7 | 2 |
| Flu and flu-like symptoms | 5 | 1 | 4 | 0 | 6 | 2 | 15 | 3 |
| Haemorrhage | 6 | 0 | 11 | 0 | 10 | 0 | 27 | 0 |
| Hypertension | 4 | 0 | 4 | 0 | 3 | 0 | 11 | 0 |
| Infection without neutropeniaa | 4 | n.a. | 0 | n.a. | 3 | n.a. | 7 | n.a. |
| Mucositis | 8 | 0 | 11 | 0 | 10 | 1 | 29 | 1 |
| Nail changes | 6 | 0 | 3 | 0 | 7 | 0 | 16 | 0 |
| Nausea | 10 | 1 | 11 | 0 | 11 | 0 | 32 | 1 |
| Oedema | 3 | 0 | 2 | 0 | 5 | 0 | 10 | 0 |
| Pain | 10 | 1 | 8 | 0 | 10 | 0 | 28 | 1 |
| Thromboembolic event | 3 | 2 | 2 | 0 | 2 | 2 | 7 | 4 |
| Skin toxicity (all events)b | 31 | 8 | 26 | 1 | 34 | 1 | 92 | 11 |
| Hand-foot syndrome | 11 | 4 | 9 | 1 | 10 | 0 | 30 | 5 |
| Skin rash/acne | 9 | 2 | 8 | 0 | 12 | 1 | 29 | 3 |
| Pruritus | 3 | 0 | 3 | 0 | 5 | 0 | 11 | 0 |
| Erythema | 5 | 2 | 1 | 0 | 4 | 0 | 10 | 2 |
| Dry skin | 3 | 0 | 5 | 0 | 3 | 0 | 11 | 0 |
| Sensory neuropathy | 5 | 0 | 10 | 0 | 11 | 0 | 26 | 0 |
| Vomiting | 7 | 0 | 4 | 0 | 1 | 0 | 12 | 0 |
Included local and systemic infections.
1 patient could have experienced more than 1 event. n.a. = not applicable.
Efficacy
A total of 10 patients had a pCR (ypT0/is) (27.8%, 95% CI 13.1–42.3%). If nodal involvement was considered for pCR definition, 9 patients (25.0%) had no invasive residuals in the breast and no involved lymph nodes (ypT0/Tis, ypN0) and 8 patients (22.2%) had no invasive and no non-invasive residuals in breast and nodes (ypT0/ypN0). Overall, 15 patients had triple-negative breast cancer (TNBC), 6 of whom achieved a pCR (40.0%, 95% CI 15.2–64.8%). From the total of 36 patients, 14 (38.9%) were treated with mastectomy.
After a median follow-up of 3.9 years (range 3.1–4.5 years), five relapses and 2 deaths were observed.
Pharmacokinetics Results
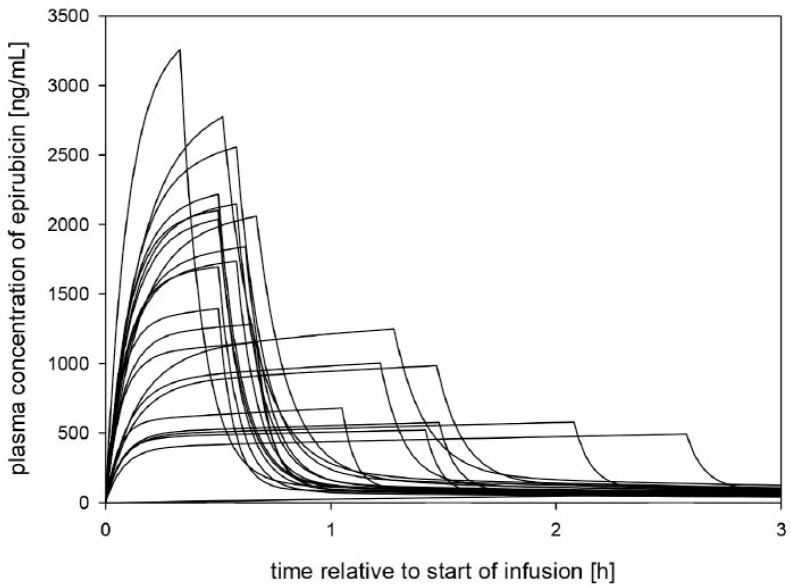
Samples from 23 patients were available. Absolute epirubicin doses of 160 ± 16 mg (mean ± standard deviation (SD)) were infused over 1.1 ± 0.8 h. The pharmacokinetics of epirubicin was best described by a 3-compartment model with linear elimination (supplementary fig. S2), with a terminal half-life of 23.9 h. The pharmacokinetic parameter estimates and their CIs of a bootstrap analysis (1000 runs) are summarised in table 3. The average area under the curve (AUC) as derived from the population pharmacokinetic evaluation was 2,880 ± 870 ng/ml/h. Cmax was calculated as 1,400 ± 800 ng/ml. Individual estimates of the concentration-versus-time profile of the population are shown in figure 1. More detailed information on model development and validation is shown in the supplementary data (supplementary figs. S3 and S4). It could be demonstrated that sorafenib has no major effect on the epirubicin pharmacokinetics for the treatment schedule used.
Table 3.
Parameter estimates and their bootstrap CIs from the pharmacokinetic model
| Parameter | Estimate (95% CI) | Literaturea |
|---|---|---|
| V1, l | 15.2 (11.2–21.5) | 10.3 |
| V2, l | 51.8 (26.8–99.5) | 35.7 |
| V3, l | 1070 (826–1990) | 772 |
| CL1, l/h | 55.9 (35.2–65.5) | 72.9 |
| CL2, l/h | 26.9 (17.5–42.0) | 30.2 |
| CL3, l/h | 73.8 (58.8–103) | 61.5 |
Values taken from [25]. V = Volume, CL = clearance.
Fig. 1.
Individual estimates of epirubicin concentrations (ng/ml) versus time (h) of the first period, based on the population pharmacokinetic model.
Discussion
SOFIA is the first study evaluating sorafenib in the neoadjuvant setting in patients with primary breast cancer using a standard anthracycline/taxane-based chemotherapy regimen. The starting dose of sorafenib was 400 mg twice daily (800 mg) [14]. After the first 12 patients had been recruited, the starting dose of 800 mg sorafenib daily was reduced to 200 mg and an individual dose escalation scheme was implemented. This approach of individual dose escalation of sorafenib within a standard sequential chemotherapy of EC and weekly paclitaxel was necessary to reduce treatment discontinuation of sorafenib from 42% in cohort 1 to 0% in cohorts 2 and 3. This resulted in a higher cumulative sorafenib dose. It seems that the dose escalation was more feasible when starting with EC than with weekly paclitaxel, leading to a higher median dose of sorafenib and more patients achieving 800 mg. This could be due to the fact that in cohort 2 the dose escalation was performed every 3 weeks and in cohort 3 every 2 weeks. Non-haematologic side effects were mainly of low grade; 83% of the patients reported HFS, which was severe in 5 patients. HFS was less frequent in cohorts 2 and 3, despite achieving a higher cumulative dose of sorafenib, which was reported to be associated with increased hand-foot skin toxicity [15].
In an adjuvant phase II study for primary breast cancer, patients received doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 (AC) every 3 weeks, followed by paclitaxel 175 mg/m2 intravenously on day 1 every 3 weeks for 4 cycles or 80 mg/m2 for 12 weeks (physician's discretion), combined with sorafenib 400 mg orally twice daily. Sorafenib was continued for a total of 12 months and in combination with adjuvant hormonal therapy where indicated [16]. Of the 45 patients recruited, only 14 (31%) completed the chemotherapy-plus-sorafenib treatment phase, entered the maintenance phase and continued up to 15 weeks; 2 patients completed paclitaxel plus sorafenib but did not enter the maintenance phase. 60% of the patients stopped treatment prematurely either due to toxicity or due to the physician's/patient's decision. Toxicities were mainly of low grade and included neutropenia, anorexia, arthralgia, diarrhoea, and dyspnoea. In the metastatic setting, the addition of sorafenib to either capecitabine or paclitaxel resulted in higher rates of adverse events for the combination therapy compared to chemotherapy alone. Main grade 3–4 toxicities with paclitaxel plus sorafenib versus placebo were neutropenia (13% vs. 7%), anaemia (11% vs. 6%) and HFS (31% vs. 3%) [17]. In the capecitabine study, grade 3–4 toxicities were higher only for HFS (44% vs. 14%) when sorafenib was added, and any-grade toxicities were significantly higher mainly for HFS (90% vs. 14%), rash (22% vs. 8%), diarrhoea (58% vs. 30%), neutropenia (13% vs. 4%) and hypertension (18% vs. 12%). 20% compared to 9% of the patients in the control arm stopped the treatment prematurely due to toxicity.
This demonstrated that the 800-mg daily dose used for sorafenib as monotherapy is not feasible in combination with chemotherapy and therefore treatment modifications that are associated with a higher compliance rate have to be explored. One of these actions to increase compliance with the oral therapy with sorafenib can be a dose escalation based on the individual patient's toxicities, as explored here. The phase III RESILIENCE study [9] investigating the combination of sorafenib and capecitabine starts sorafenib at 600 mg daily and capecitabine at 2000 mg/m2 on days 1–14 of a 21-day cycle, but allows both drugs to be escalated based on individual toxicities related to the treatment. The dose of sorafenib was based on the preceding phase II study with a mean daily dose per patient of 584 mg [8]. Capecitabine 2000 mg/m2 is a well-tolerated and highly effective therapy [18]. In summary, dermatologic toxicities – especially hand-foot (skin) syndrome or reactions – are among the main reasons to stop or reduce multikinase targeting agents such as sorafenib, especially when combined with a chemotherapy inducing similar toxicities [19]. This has, for example, been shown in the case of sunitinib [20, 21], which was consequently stopped for further development in breast cancer.
Based on early reports of possible drug-drug interactions of anthracyclines with sorafenib and taking into account the long mean half-life of sorafenib, which is about 24–48 h [22], pharmacokinetic data for epirubicin were collected [23]. The epirubicin concentration time profile was successfully described by a 3-compartment pharmacokinetic model, whereas a 2-compartment model, which has also been reported [24], was not sufficient to describe the data. The parameter estimates for the final model are very similar to those published in the literature for another 3-compartment model of epirubicin, suggesting that sorafenib has no major effect on the epirubicin pharmacokinetics in the treatment schedule used [25].
The pCR rate, defined as no invasive residuals in the breast, was 27.8%, which is comparable to 24.5% (21.927.4%) reached with bevacizumab in addition to EC followed by docetaxel in the GeparQuinto study, but slightly lower than 34.5% (30.7–38.3%) in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B40 study [2, 3]. The pCR rates, irrespective of the definitions, were slightly above those seen with bevacizumab [2, 3]. However, the sample size was smaller than initially planned in order to demonstrate an added effect. The failure of bevacizumab to prolong disease-free and overall survival has tempered expectations for the success of anti-angiogenic tyrosine kinase inhibitors [26, 27]. Nevertheless, these anti-VEGF compounds certainly have activity, and translational work to identify the subgroups of responsive patients will be critical [28]. The lack of success of anti-angiogenic therapies in breast cancer to date may in part be explained by activation of additional pro-angiogenic switches upon blockade with bevacizumab, as has been shown in experimental systems [29].
The main limitation of our study is that the initial protocol was not feasible, resulting in a low recruitment probably due to low acceptance of the combination and more attractive competing trials. The 3 different treatment approaches generated feasibility data of the combination of EC and paclitaxel in combination with sorafenib. Paclitaxel weekly as single agent was thought to allow for a more rapid dose escalation of sorafenib every 2 weeks, which could not be proven.
In conclusion, a dose of 800 mg sorafenib as it is given as single agent in metastatic breast cancer and renal cell cancer was not tolerated as a starting dose in combination with standard anthracycline/taxane-based chemotherapy in early breast cancer. Sorafenib can be combined with epirubicin without drug-drug interactions, and an individual dose escalation model starting from 200 mg was feasible, resulting in significantly higher cumulative doses of sorafenib compared to a fixed starting dose and in no treatment discontinuations.
Online Supplemental Material
Table S1. Summary of maximum treatment cycles per drug and cohort
Figure S1. Study Design and Dose escalation model.
Figure S2. Pharmacokinetic model for the prediction of epirubicin plasma concentrations after infusion.
Figure S3. Population predicted epirubicin concentrations versus observed concentrations
Figure S4. Individual predicted epirubicin concentrations versus observed concentrations.
Supplementary Material and Methods
To access the online supplemental material please refer to www.karger.com/? DOI = 000363430.
Disclosure Statement
The trial received funding support from Bayer Health Care, Germany, and Roche, Germany. The funders had no access to the study database and were not involved in the analysis and interpretation of the results. No grant number applicable.
G.v.M. has received speaker honoraria from Roche and research grants from Bayer. All other authors have declared no conflicts of interest.
Supplementary Material
Supplementary data
References
- 1.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 2.von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C, Kreienberg R, Solbach C, Gerber B, Jackisch C, Kunz G, Blohmer JU, Huober J, Hauschild M, Fehm T, Müller BM, Denkert C, Loibl S, Nekljudova V, Untch M German Breast Group; Arbeitsgemeinschaft Gynäkologische Onkologie – Breast Study Groups. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 3.Bear HD, Tang G, Rastogi P, Geyer CE, Jr, Robidoux A, Atkins JN, Baez-Diaz L, Brufsky AM, Mehta RS, Fehrenbacher L, Young JA, Senecal FM, Gaur R, Margolese RG, Adams PT, Gross HM, Costantino JP, Swain SM, Mamounas EP, Wolmark N. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366:310–320. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merz M, Komljenovic D, Zwick S, Semmler W, Bauerle T. Sorafenib tosylate and paclitaxel induce anti-angiogenic, anti-tumour and anti-resorptive effects in experimental breast cancer bone metastases. Eur J Cancer. 2011;47:277–286. doi: 10.1016/j.ejca.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi G, Loibl S, Zamagni C, Salvagni S, Raab G, Siena S, Laferriere N, Peña C, Lathia C, Bergamini L, Gianni L. Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anticancer Drugs. 2009;20:616–624. [PubMed] [Google Scholar]
- 7.Moreno-Aspitia A, Morton RF, Hillman DW, Lingle WL, Rowland KM, Jr, Wiesenfeld M, Flynn PJ, Fitch TR, Perez EA. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27:11–15. doi: 10.1200/JCO.2007.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baselga J, Segalla JG, Roché H, Del Giglio A, Pinczowski H, Ciruelos EM, Filho SC, Gómez P, Van Eyll B, Bermejo B, Llombart A, Garicochea B, Durán MÁ, Hoff PM, Espié M, de Moraes AA, Ribeiro RA, Mathias C, Gil Gil M, Ojeda B, Morales J, Kwon Ro S, Li S, Costa F. Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol. 2012;30:1484–1491. doi: 10.1200/JCO.2011.36.7771. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Costa F, Gomez H, Hudis CA, Rapoport B, Roche H, Schwartzberg LS, Petrenciuc O, Shan M, Gradishar WJ. A phase 3 tRial comparing capecitabinE in combination with SorafenIb or pLacebo for treatment of locally advanced or metastatIc HER2-Negative breast CancEr (the RESILIENCE study): study protocol for a randomized controlled trial. Trials. 2013;14:228. doi: 10.1186/1745-6215-14-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, Blohmer JU, Eiermann W, Jackesz R, Jonat W, Lebeau A, Loibl S, Miller W, Seeber S, Semiglazov V, Smith R, Souchon R, Stearns V, Untch M, Minckwitz von G. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24:1940–1949. doi: 10.1200/JCO.2005.02.6187. [DOI] [PubMed] [Google Scholar]
- 11.Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, Cristofanilli M, Booser DJ, Pusztai L, Rivera E, Theriault RL, Carter C, Frye D, Hunt KK, Symmans WF, Strom EA, Sahin AA, Sikov W, Hortobagyi GN. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–5992. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 12.Hunz M, Jetter A, Warm M, Pantke E, Tuscher M, Hempel G, Jaehde U, Untch M, Kurbacher C, Fuhr U. Plasma and tissue pharmacokinetics of epirubicin and paclitaxel in patients receiving neoadjuvant chemotherapy for locally advanced primary breast cancer. Clin Pharmacol Ther. 2007;81:659–668. doi: 10.1038/sj.clpt.6100067. [DOI] [PubMed] [Google Scholar]
- 13.von Minckwitz G, Raab G, Caputo A, Schütte M, Hilfrich J, Blohmer JU, Gerber B, Costa SD, Merkle E, Eidtmann H, Lampe D, Jackisch C, du Bois A, Kaufmann M. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005;23:2676–2685. doi: 10.1200/JCO.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 14.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, Schwartz B, Awada A, Voigtmann R, Scheulen ME, Seeber S. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 15.Azad NS, Aragon-Ching JB, Dahut WL, Gutierrez M, Figg WD, Jain L, Steinberg SM, Turner ML, Kohn EC, Kong HH. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:1411–1416. doi: 10.1158/1078-0432.CCR-08-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spigel DR, Hainsworth JD, Burris HA, 3rd, Molthrop DC, Peacock N, Kommor M, Vazquez ER, Greco FA, Yardley DA. A pilot study of adjuvant doxorubicin and cyclophosphamide followed by paclitaxel and sorafenib in women with node-positive or high-risk early-stage breast cancer. Clin Adv Hematol Oncol. 2011;9:280–286. [PubMed] [Google Scholar]
- 17.Gradishar WJ, Kaklamani V, Sahoo TP, Lokanatha D, Raina V, Bondarde S, Jain M, Ro SK, Lokker NA, Schwartzberg L. A double-blind, randomised, placebo-controlled, phase 2b study evaluating sorafenib in combination with paclitaxel as a first-line therapy in patients with HER2-negative advanced breast cancer. Eur J Cancer. 2013;49:312–322. doi: 10.1016/j.ejca.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann M, Maass N, Costa SD, Schneeweiss A, Loibl S, Sütterlin MW, Schrader I, Gerber B, Bauer W, Wiest W, Tomé O, Distelrath A, Hagen V, Kleine-Tebbe A, Ruckhaeberle E, Mehta K, von Minckwitz G GBG-39 Trialists. First-line therapy with moderate dose capecitabine in metastatic breast cancer is safe and active: results of the MONICA trial. Eur J Cancer. 2010;46:3184–3191. doi: 10.1016/j.ejca.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, Anderson RT, Wood L, Dutcher JP. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 20.Robert NJ, Saleh MN, Paul D, Generali D, Gressot L, Copur MS, Brufsky AM, Minton SE, Giguere JK, Smith JW, 2nd, Richards PD, Gernhardt D, Huang X, Liau KF, Kern KA, Davis J. Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer. 2011;11:82–92. doi: 10.1016/j.clbc.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergh J, Bondarenko IM, Lichinitser MR, Liljegren A, Greil R, Voytko NL, Makhson AN, Cortes J, Lortholary A, Bischoff J, Chan A, Delaloge S, Huang X, Kern KA, Giorgetti C. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J Clin Oncol. 2012;30:921–929. doi: 10.1200/JCO.2011.35.7376. [DOI] [PubMed] [Google Scholar]
- 22.Grandinetti CA, Goldspiel BR. Sorafenib and sunitinib: novel targeted therapies for renal cell cancer. Pharmacotherapy. 2007;27:1125–1144. doi: 10.1592/phco.27.8.1125. [DOI] [PubMed] [Google Scholar]
- 23.Richly H, Henning BF, Kupsch P, Passarge K, Grubert M, Hilger RA, Christensen O, Brendel E, Schwartz B, Ludwig M, Flashar C, Voigtmann R, Scheulen ME, Seeber S, Strumberg D. Results of a phase I trial of sorafenib (BAY 43-9006) in combination with doxorubicin in patients with refractory solid tumors. Ann Oncol. 2006;17:866–873. doi: 10.1093/annonc/mdl017. [DOI] [PubMed] [Google Scholar]
- 24.Wade JR, Kelman AW, Kerr DJ, Robert J, Whiting B. Variability in the pharmacokinetics of epirubicin: a population analysis. Cancer Chemother Pharmacol. 1992;29:391–395. doi: 10.1007/BF00686009. [DOI] [PubMed] [Google Scholar]
- 25.Ralph LD, Thomson AH, Dobbs NA, Twelves C. A population model of epirubicin pharmacokinetics and application to dosage guidelines. Cancer Chemother Pharmacol. 2003;52:34–40. doi: 10.1007/s00280-003-0608-x. [DOI] [PubMed] [Google Scholar]
- 26.Miles DW, Diéras V, Cortés J, Duenne AA, Yi J, O'Shaughnessy J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013;24:2773–2780. doi: 10.1093/annonc/mdt276. [DOI] [PubMed] [Google Scholar]
- 27.Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X, Steger GG, Suter TM, Toi M, Parmar M, Laeufle R, Im YH, Romieu G, Harvey V, Lipatov O, Pienkowski T, Cottu P, Chan A, Im SA, Hall PS, Bubuteishvili-Pacaud L, Henschel V, Deurloo RJ, Pallaud C, Bell R. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14:933–942. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 28.Loibl S, De la Haba J, von Minckwitz F, Morales S, Crespo C, Antón A, Carrasco E, Aktas B, Mehta K, Martin M. on behalf of GEICAM and GBG: Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer – final analysis LEA study. Eur J Cancer Suppl. 2013;49 abstr E17-2128. [Google Scholar]
- 29.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data