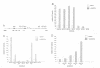
Abstract
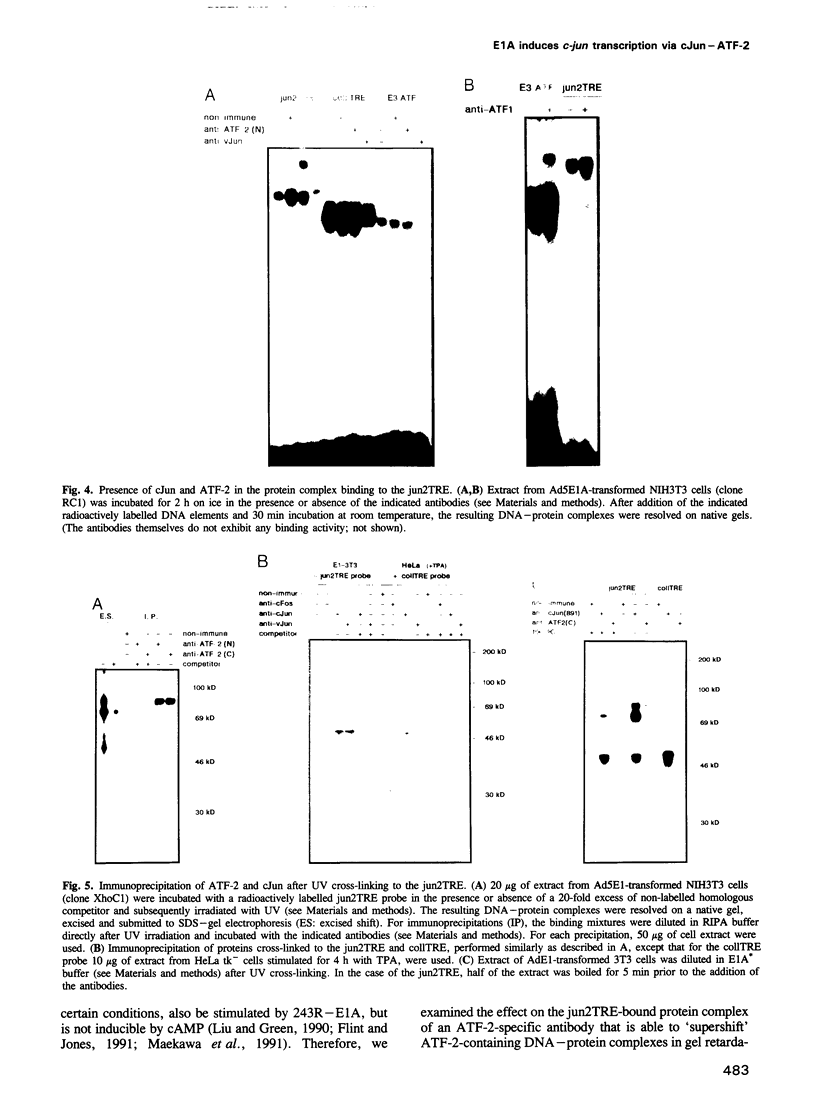
The adenovirus E1A proteins differentially regulate AP-1-responsive genes. Collagenase and stromelysin are repressed by E1A, whereas the expression of c-jun is elevated. Inhibition of collagenase has been found to be exerted through the consensus AP-1 binding site TGAGTCA. Here we show that the distal AP-1 binding site in the c-jun promoter, the jun2TRE (TTACCTCA), is the decisive element of this promoter in mediating the positive response to the 243 amino acid E1A product. In vitro binding studies revealed that, in contrast to the consensus AP-1 site which is preferentially targeted by dimers composed of the Jun and Fos families, the jun2TRE binds heterodimers composed of cJun and ATF-2(-like) proteins. Since stimulation of c-jun transcription is a function of the transforming domain of E1A encoded by conserved region 1, cJun--ATF-2 may be one of the effector factors involved in transformation. The data further suggest that E1A can distinguish between cJun--cJun and cJun--ATF-2 in imposing opposite states of activity.
Full text
PDF
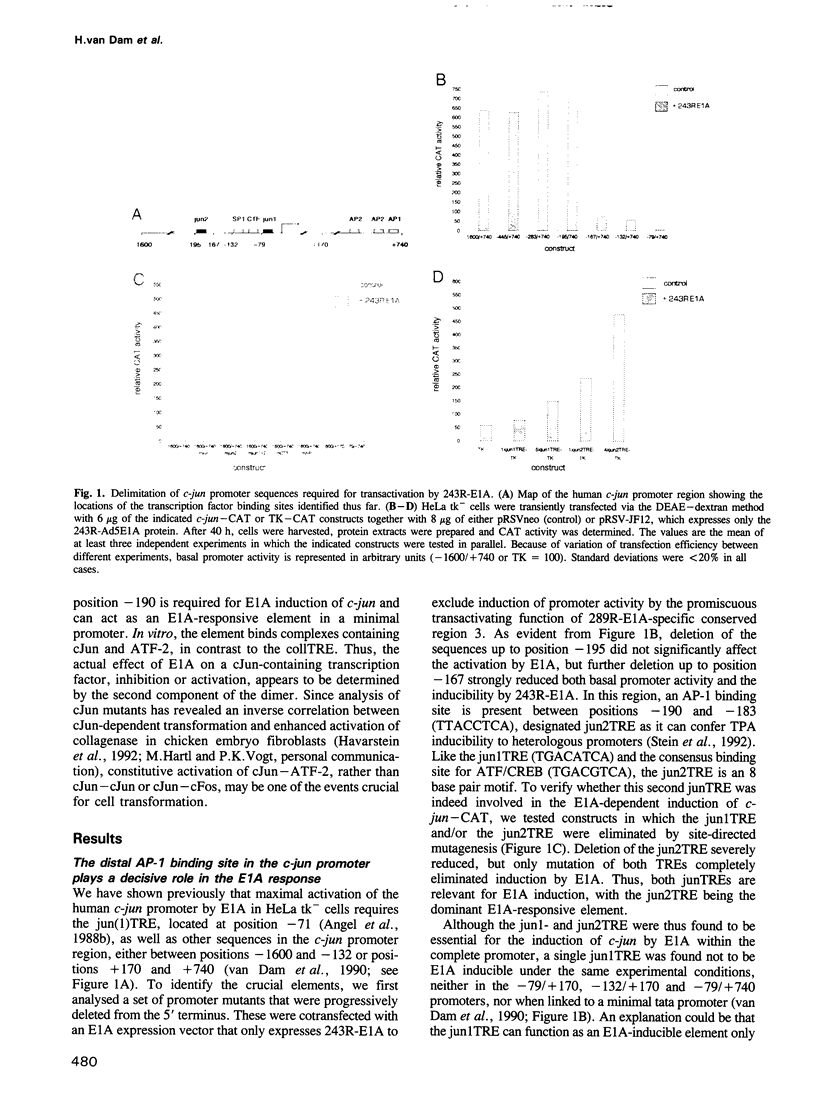
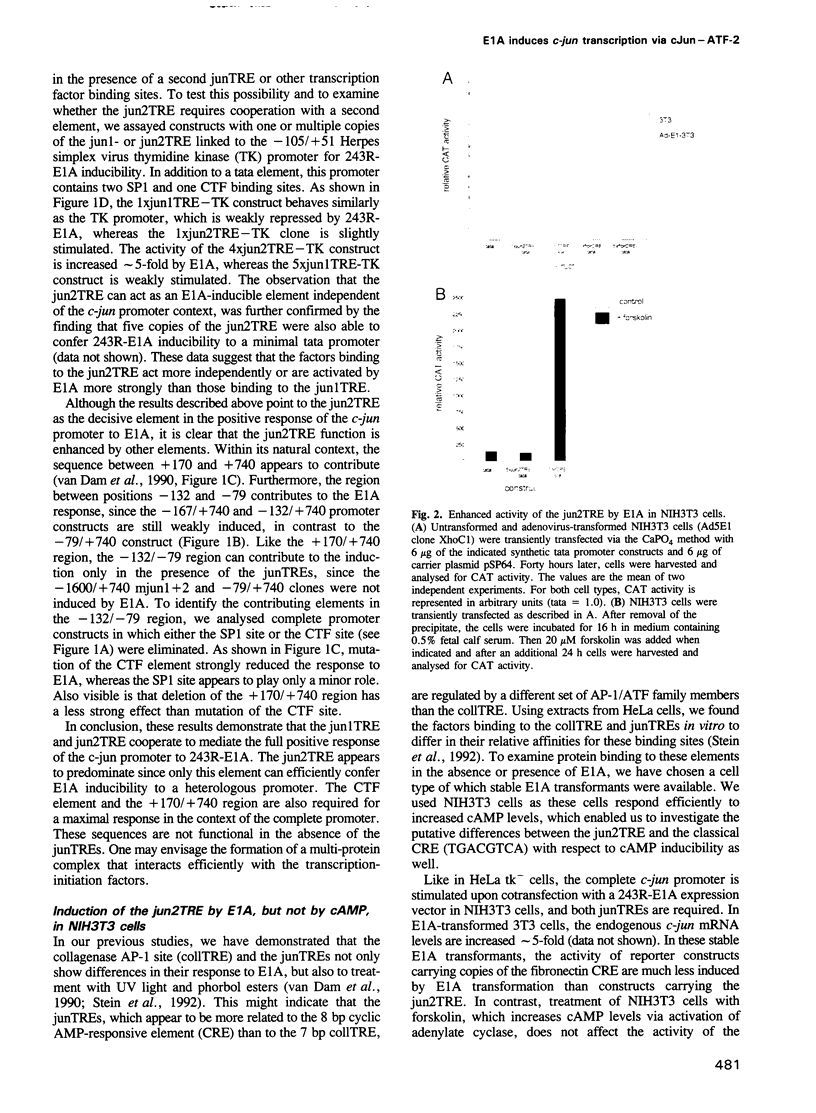





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani R., Brown P., Binétruy B., Dosaka H., Rosenberg R. K., Angel P., Karin M., Birrer M. J. The transactivating domain of the c-Jun proto-oncoprotein is required for cotransformation of rat embryo cells. Mol Cell Biol. 1991 Dec;11(12):6286–6295. doi: 10.1128/mcb.11.12.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Bagchi S., Raychaudhuri P., Nevins J. R. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell. 1990 Aug 24;62(4):659–669. doi: 10.1016/0092-8674(90)90112-r. [DOI] [PubMed] [Google Scholar]
- Bandara L. R., La Thangue N. B. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991 Jun 6;351(6326):494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- Benbrook D. M., Jones N. C. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990 Mar;5(3):295–302. [PubMed] [Google Scholar]
- Bernards R., Houweling A., Schrier P. I., Bos J. L., Van der Eb A. J. Characterization of cells transformed by Ad5/Ad12 hybrid early region I plasmids. Virology. 1982 Jul 30;120(2):422–432. doi: 10.1016/0042-6822(82)90042-3. [DOI] [PubMed] [Google Scholar]
- Bos T. J., Monteclaro F. S., Mitsunobu F., Ball A. R., Jr, Chang C. H., Nishimura T., Vogt P. K. Efficient transformation of chicken embryo fibroblasts by c-Jun requires structural modification in coding and noncoding sequences. Genes Dev. 1990 Oct;4(10):1677–1687. doi: 10.1101/gad.4.10.1677. [DOI] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Guarente L., Sharp P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989 Feb 24;56(4):549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., Dangy J. P., Mechta F., Hirai S., Yaniv M., Samarut J., Lassailly A., Brun G. Overexpression of avian or mouse c-jun in primary chick embryo fibroblasts confers a partially transformed phenotype. Oncogene. 1990 Oct;5(10):1541–1547. [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J., Shenk T. Adenovirus E1A protein paradigm viral transactivator. Annu Rev Genet. 1989;23:141–161. doi: 10.1146/annurev.ge.23.120189.001041. [DOI] [PubMed] [Google Scholar]
- Flint K. J., Jones N. C. Differential regulation of three members of the ATF/CREB family of DNA-binding proteins. Oncogene. 1991 Nov;6(11):2019–2026. [PubMed] [Google Scholar]
- Gaire M., Chatton B., Kedinger C. Isolation and characterization of two novel, closely related ATF cDNA clones from HeLa cells. Nucleic Acids Res. 1990 Jun 25;18(12):3467–3473. doi: 10.1093/nar/18.12.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos K., Morgan B. A., Moore D. D. Functionally distinct isoforms of the CRE-BP DNA-binding protein mediate activity of a T-cell-specific enhancer. Mol Cell Biol. 1992 Feb;12(2):747–757. doi: 10.1128/mcb.12.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A., McCall C., Whyte P., Franza B. R., Jr Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene. 1991 Mar;6(3):481–485. [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. A., Mymryk J. S., Egan C., Branton P. E., Bayley S. T. Retinoblastoma growth suppressor and a 300-kDa protein appear to regulate cellular DNA synthesis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5883–5887. doi: 10.1073/pnas.87.15.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst H. C., Masson N., Jones N. C., Lee K. A. The cellular transcription factor CREB corresponds to activating transcription factor 47 (ATF-47) and forms complexes with a group of polypeptides related to ATF-43. Mol Cell Biol. 1990 Dec;10(12):6192–6203. doi: 10.1128/mcb.10.12.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst H. C., Totty N. F., Jones N. C. Identification and functional characterisation of the cellular activating transcription factor 43 (ATF-43) protein. Nucleic Acids Res. 1991 Sep 11;19(17):4601–4609. doi: 10.1093/nar/19.17.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håvarstein L. S., Morgan I. M., Wong W. Y., Vogt P. K. Mutations in the Jun delta region suggest an inverse correlation between transformation and transcriptional activation. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):618–622. doi: 10.1073/pnas.89.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv L. B., Liou H. C., Kara C. J., Lamph W. W., Verma I. M., Glimcher L. H. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol Cell Biol. 1990 Apr;10(4):1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kitabayashi I., Chiu R., Gachelin G., Yokoyama K. E1A dependent up-regulation of c-jun/AP-1 activity. Nucleic Acids Res. 1991 Feb 11;19(3):649–655. doi: 10.1093/nar/19.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabayashi I., Kawakami Z., Chiu R., Ozawa K., Matsuoka T., Toyoshima S., Umesono K., Evans R. M., Gachelin G., Yokoyama K. Transcriptional regulation of the c-jun gene by retinoic acid and E1A during differentiation of F9 cells. EMBO J. 1992 Jan;11(1):167–175. doi: 10.1002/j.1460-2075.1992.tb05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Liu F., Green M. R. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1a protein. Cell. 1990 Jun 29;61(7):1217–1224. doi: 10.1016/0092-8674(90)90686-9. [DOI] [PubMed] [Google Scholar]
- Lloyd A., Yancheva N., Wasylyk B. Transformation suppressor activity of a Jun transcription factor lacking its activation domain. Nature. 1991 Aug 15;352(6336):635–638. doi: 10.1038/352635a0. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Matsuda S., Fujisawa J., Yoshida M., Ishii S. Cyclic AMP response element-binding protein, CRE-BP1, mediates the E1A-induced but not the Tax-induced trans-activation. Oncogene. 1991 Apr;6(4):627–632. [PubMed] [Google Scholar]
- Maekawa T., Sakura H., Kanei-Ishii C., Sudo T., Yoshimura T., Fujisawa J., Yoshida M., Ishii S. Leucine zipper structure of the protein CRE-BP1 binding to the cyclic AMP response element in brain. EMBO J. 1989 Jul;8(7):2023–2028. doi: 10.1002/j.1460-2075.1989.tb03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens I., Nilsson M., Magnusson G., Linder S. Glucocorticoids facilitate the stable transformation of embryonal rat fibroblasts by a polyomavirus large tumor antigen-deficient mutant. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5571–5575. doi: 10.1073/pnas.85.15.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offringa R., Gebel S., van Dam H., Timmers M., Smits A., Zwart R., Stein B., Bos J. L., van der Eb A., Herrlich P. A novel function of the transforming domain of E1a: repression of AP-1 activity. Cell. 1990 Aug 10;62(3):527–538. doi: 10.1016/0092-8674(90)90017-9. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Stabel S., Argos P., Philipson L. The release of growth arrest by microinjection of adenovirus E1A DNA. EMBO J. 1985 Sep;4(9):2329–2336. doi: 10.1002/j.1460-2075.1985.tb03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Angel P., van Dam H., Ponta H., Herrlich P., van der Eb A., Rahmsdorf H. J. Ultraviolet-radiation induced c-jun gene transcription: two AP-1 like binding sites mediate the response. Photochem Photobiol. 1992 Mar;55(3):409–415. doi: 10.1111/j.1751-1097.1992.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R., Foulkes N., Mulder M., Kruijer W., Sassone-Corsi P. Positive regulation of jun/AP-1 by E1A. Mol Cell Biol. 1991 Jan;11(1):192–201. doi: 10.1128/mcb.11.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam H., Offringa R., Meijer I., Stein B., Smits A. M., Herrlich P., Bos J. L., van der Eb A. J. Differential effects of the adenovirus E1A oncogene on members of the AP-1 transcription factor family. Mol Cell Biol. 1990 Nov;10(11):5857–5864. doi: 10.1128/mcb.10.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam H., Offringa R., Smits A. M., Bos J. L., Jones N. C., van der Eb A. J. The repression of the growth factor-inducible genes JE, c-myc and stromelysin by adenovirus E1A is mediated by conserved region 1. Oncogene. 1989 Oct;4(10):1207–1212. [PubMed] [Google Scholar]