Abstract
The purpose was to investigate the protective effects of Vitamin C (Vit C) and the regulatory mechanism between Vit C and sirtuin 1 (SIRT1) in PREs during oxidative stress as Vit C and SIRT1 exerted famous effects as antioxidants. We found that moderate Vit C (100 µM) prevented ARPE-19 cells from damages induced by H2O2, including increasing viability, reducing apoptosis, and attenuating intracellular ROS levels. But lower and higher concentration of Vit C had no effects. Further results indicated that Vit C caused the dysregulation of some stress responses factors (SIRT1, p53 and FOXO3) in ARPE-19 cells response to H2O2. Moreover we found that SIRT1 activator resveratrol (SRV) stimulated significantly the protective effects of moderate Vit C, provided the property of antioxidative stress for the lower and higher concentration of Vit C in ARPE-19 cells as well. Consistently, nicotinamide (NA) relieved the protective functions of moderate Vit C. Interestingly, data also revealed the dysregulation of p53 and FOXO3 was dependent on the regulation of SIRT1 rather than Vit C. Summarily, the protective effect of Vit C against oxidative stress was involved in regulation of SIRT1. It suggested that combined application of Vit C and RSV might be a promising therapeutic method for AMD.
1. Introduction
Age-related macular degeneration (AMD) is the most common cause of vision loss in the elderly [1, 2]. AMD patients have extensive free radicals, the injury of the proteins, lipids, DNA, and mitochondria, in their retinal pigment epithelial cells (RPEs) by postmortem [3]. Proteomic analysis also found numerous proteins which were caused by oxidative damage in drusen [4]. Some reports have found that antioxidants could slow the process of AMD [5]. Thereby oxidative stress plays an important role in AMD pathogenesis and oxidative injury contributed to the pathogenesis of AMD [1, 6]. RPEs are essential for vision by providing nutrients as transporters and maintenance functions for photoreceptors [7]. One of the potential contributors to AMD pathology is dysfunction of RPEs [8]. So, finding effective therapeutics that protect RPEs from damage is promising treatments for AMD.
Oxidative stress promoted the development of age-related RPEs degeneration, dysfunction, and loss [9, 10]. Cells have some protective strategies to minimize oxidative damage. The most famous defense system is to develop the endogenous antioxidants, including superoxide dismutases (SODs), carotenoids, and vitamins [6]. Vitamin C (Vit C), known for its role in development and maintenance of connective tissues, has effects on bone formation, wound healing, and the maintenance of healthy gums. The famous roles of Vit C are protection of immune system, reduction of allergic reactions, and combating infections [11]. It is also an antioxidant that protects the body against oxidative stress. Vit C has been regarded as the most important one which provides protection against atherogenesis and Alzheimer's disease [11, 12]. In recent studies, Vit C attenuated the oxidative stress in diabetic aged rats [13, 14]. Regarding the role of Vit C in retinopathy, Vit C and Vit E provided protection for retinal glutathione reductase, glutathione peroxidase, and superoxide dismutase activities. So, Vit C and Vit E relieve retinopathy induced by oxidative stress [14]. Nevertheless, Vit C did not mitigate the effects of oxidative stress on RPEs [15]. In the study of Zeitz et al., Vit C, potent hydroxyl radical quenchers in vitro, failed to protect cultured ARPE-19 cells from oxidative stress induced cell death [16]. Interestingly, Yin et al. reported that Vit C supplementation of 100–200 μM appeared to strongly inhibit oxidative stress, but no additional advantage was found as the concentration of Vit C was higher [17]. So, Vit C, the wildly used antioxidant, which exerted performances in protection of RPEs against oxidative stress continued to merit further investigation.
SIRT1, a class III protein deacetylase, played regulatory roles in cellular stressors, genotoxic, oxidative, and proteotoxic stress [18, 19]. It was found that moderate overexpression of SIRT1 provided protection for mouse cardiac muscle against oxidative stress [20]. SIRT1 could also prohibit some transcription factors, which regulated cellular redox balance such as inhibition of the transactivation capacity of NRF2 in HepG2 cells [21]. However we kept enhancing understanding on detailed mechanism of the SIRT1 against oxidative stress, especially in RPEs.
In this study, we focused on the antioxidant effects of Vit C and the regulatory mechanism between Vit C and SIRT1 in PREs during oxidative stress.
2. Materials and Methods
2.1. Cell Culture
ARPE-19 cell line (human RPEs) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco's modified Eagle medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Invitrogen, Carlsbad, USA), 100 μg/mL of streptomycin, and 100 U/mL of penicillin in 5% CO2 at 37°C. The cells were subcultured when reaching about 90% confluence.
2.2. Cells Viability and Apoptosis Assay
ARPE-19 cells were grown at 80% confluence. Then cells were treated with H2O2 (100 μM) for 12 h or 24 h after supplementation of Vit C as indicated concentration. Cell viability was assayed by a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay (Sigma, St. Louis, MO, USA) after being treated with indicated experiments. Briefly MTT was added to the cells at 37°C for 4 h. After incubation, MTT-containing medium was discarded and dimethyl sulfoxide (DMSO) was performed to dissolve formazan crystals. Optical densities (OD) were measured at 450 nm by VersaMax microplate reader (Molecular Devices, Sunnyvale, CA, USA). Viability was normalized by OD value/cell number and ARPE-19 cells without treatment (control) were denoted as 100%. Apoptosis was assessed by annexin V-FITC apoptosis detection kit (Sigma-Aldrich, St. Louis, Missouri) following the manufacturer's protocol.
2.3. Measurement of Intracellular ROS Levels
Intracellular ROS levels were measured by using intracellular ROS assay kit (Cell Biolabs, San Diego, CA). Briefly, ARPE-19 cells were incubated with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) in the culture medium for 30 min at 37°C. The cells were assessed by using flow cytometry at 488 and 525 nm wavelengths. DCFH fluorescence of the cell lysate was captured and quantified by ImageJ software (NIH). The average fluorescence intensity was analyzed from five fields for each treatment.
2.4. Cell Treatments
Cells were incubated with Vit C (SIGMA-Aldrich) at indicated concentration; then H2O2 (100 μM) was added for 12 h or 24 h. Then, H2O2 was detoxified by adding catalase (100,000 μ/L; Worthington Biochemical) at the end of the incubation [22]. 10 mM resveratrol (RSV) and 5 mM nicotinamide (NA) (Sigma, St. Louis, USA) were performed to incubate cells before treatments of oxidants [23]. SIRT1 knockdown was performed by using siRNA for SIRT1 and shRNAi MISSION nontarget shRNA Control/Puro was performed as negative control (SIRT1si and C-SIRT1si) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). pRC/CMV-SRIT1 and pcRC/CMV (Promega, Madison, WI) expression vectors (SIRT1up and C-SIRT1up) were acquired from Promega (Promega, Madison, WI). Cell transfection was conducted by using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions.
2.5. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted by using RNeasy kits (Qiagen, Valencia, CA). First-strand cDNA was synthesized using SuperScript III (Invitrogen). qRT-PCR was performed on ABI 7500 with SYBR Premix Ex Taq kit (Takara, Japan). GAPDH was denoted as the internal control. Data were normalized by using 2−ΔΔCt method as relative quantification. The used primers were p53: F agt cac agc aca tga cgg agg t, R tac aca tgt act tgt agt gga t; FOXO3: F aac aga cca gcc acc ttc tct t, R gct gac aga att tga caa ggc a; SirT1: F tgt ggt aga gct tgc att gat ctt, R ggc ctg ttg ctc tcc tca t; GAPDH: F gga gtc aac gga ttt ggt c, R gga atc att gga aca tgt aaa c. The reactions were started at 95°C for 5 min followed by 40 cycles of 95°C for 15 sec and 60°C for 30 sec and extension for 1 min at 72°C.
2.6. Western Blot
Proteins were extracted from cells. Then, the protein samples were subjected to 10% SDSPAGE. Proteins were transferred onto nitrocellulose membranes after electrophoresis. The membrane was blocked with TBST (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.05% Tween 20) containing 5% nonfat milk or 5% BSA for 30 min. The membrane was incubated with primary antibodies overnight at 4°C. Then, goat anti-mouse IgG or goat anti-rabbit IgG (Pierce) was utilized to visualize the results, which ECL detection systems (Super Signal West Femto, Pierce) were conducted to assay. Human monoclonal primary antibodies were used as follows: anti-FOXO3 (Cell Signaling, Beverly, MA), anti-p53 (Cell Signaling, Beverly, MA), SIRT1 primary antibody (Sigma-Aldrich), and β-actin antibody (Sigma-Aldrich).
2.7. Statistical Analysis
Data were denoted as means ± standard deviation (SD). Student's t-test was used to analyze differences of two groups. The experimental results were analyzed with one-way ANOVA to compare the differences of three groups (or more than three). The criterion for significance was P < 0.05. Statistical analyses were conducted by SPSS 17.0 software (SPSS, Chicago, IL, USA). The criterion for significance was expressed as *P < 0.05 and **P < 0.01. All the experiments were performed in triplicate.
3. Results
3.1. Moderate Vit C Prevented RPEs from Oxidant Injury Induced by H2O2
To investigate the effect of Vit C on oxidant injury induced by H2O2, the ARPE-19 cells were pretreated with Vit C at 0 μM, 20 μM, 100 μM, or 500 μM. Then, the RPEs were incubated with H2O2 at 100 μM for 12 h and 24 h. We first detected the effect of the Vit C on viability during oxidative stress in ARPE-19 cells. There was significant difference in viability when the concentration of Vit C was increased to 100 μM (P < 0.05 at 12 h and 24 h). Higher concentration (500 μM) and lower concentration (20 μM) of Vit C could not significantly influence the cell viability in the presence of H2O2 (Figure 1(a)). Corresponding to the effect of Vit C on viability, Vit C reduced the number of apoptotic RPEs significantly at 100 μM concentration (P < 0.05) after 12 h exposure to H2O2 Figure 1(b). Because of accumulation of intracellular ROS in AMD [24], we also tested the intracellular ROS. Figure 1(c) displayed the fact that treatment with Vit C caused the changes of intracellular production of ROS also, especially when the concentration of Vit C reached 100 μM after 24 h exposure to H2O2 (P < 0.05). These findings illustrated that it was moderate Vit C (100 μM) that had protective effect against oxidant injury induced by H2O2 in RPEs.
Figure 1.
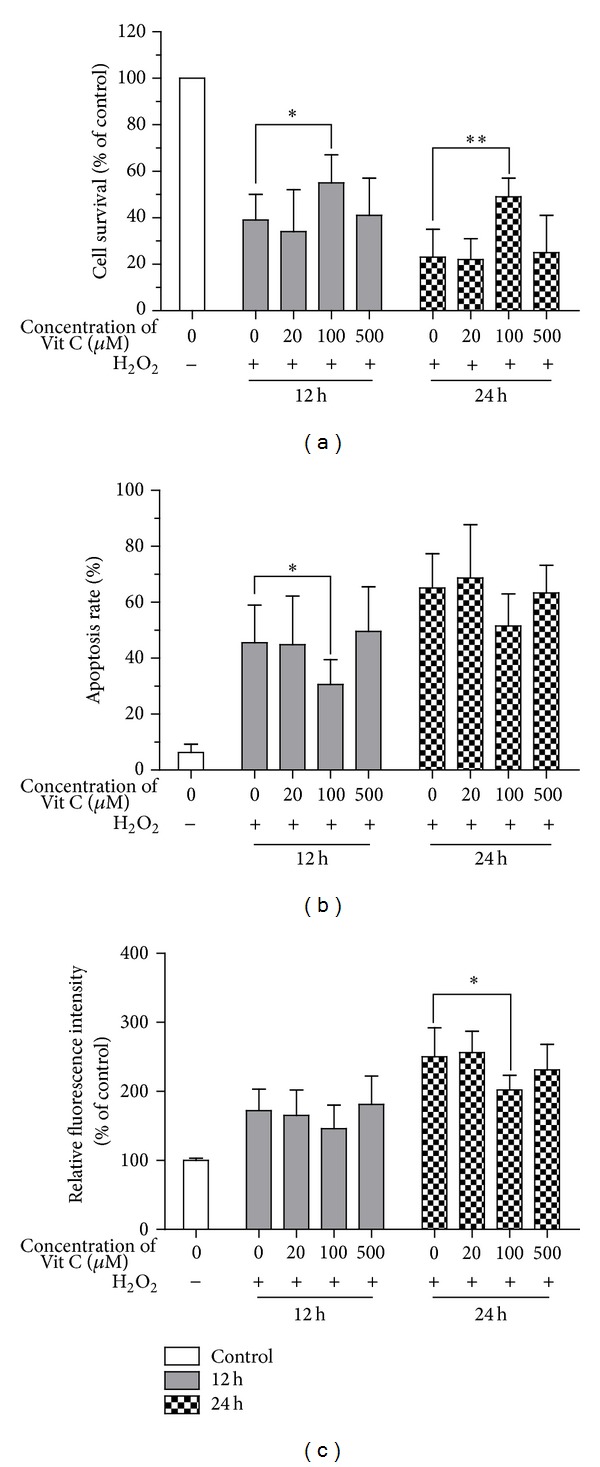
Effects of Vit C on viability, apoptosis, and intracellular ROS of ARPE-19 cells induced by H2O2. ARPE-19 cells were incubated for 24 h with 0 μM, 20 μM, 100 μM, or 500 μM of Vit C and then exposed to 100 μM of H2O2 for 12 h or 24 h. Viability, apoptosis, and intracellular ROS were measured by using MTT assays, TUNEL assays, and DCFH-DA, respectively, as shown in Section 2. Normal ARPE-19 cells which did not undergo treatment were expressed as “control.” Data were expressed as a percentage of cell survival (a), apoptosis (b), and relative fluorescence intensity (c). Bars represented mean ± SD of three independent experiments. ANOVA was performed to analyze the differences statistically. Statistical significance was expressed as *P < 0.05 and **P < 0.01.
3.2. Vit C Affected the Expression of the SIRT1 Transcription Factor and Stress Responses Factors (p53 and FOXO3)
As the key and ubiquitous roles of SIRT1 in inflammaging and senescence, especially in antioxidative stress, we suspected if SIRT1 referred to the mechanism of protection of Vit C against oxidative stress [25]. To confirm the hypothesis, we assayed the expression of SIRT1 when RPEs were treated with Vit C after 12 h or 24 h exposure to H2O2. The relative expression of SIRT1 was shown in Figure 2(a). The results showed that H2O2 reduced the expression of SIRT1 and Vit C affected the expression of SIRT1. The expression of SIRT1 reached significant difference when the level of Vit C concentration was 100 μM (P = 0.021 and P = 0.039 at 12 h and 24 h, resp.). We also detected the expression of stress responses factors (p53 and FOXO3) to further support our hypothesis. Figure 2(b) displayed the fact that the expression of p53 was upregulated significantly when the RPEs were pretreated with moderate Vit C (100 μM) after exposure to H2O2 for 12 h (P = 0.046). The expression of FOXO3 gene was increased in accordance with SIRT1, significantly at 12 h with 100 μM Vit C (P = 0.032) (Figure 2(c)). These findings revealed that only the moderate Vit C (100 μM) regulated the SIRT1 transcription factor and stress responses factors (p53 and FOXO3). It is also suggested that if the protective effect of Vit C against oxidative stress was involved in a SIRT1 signaling pathway because of the regulatory effects of SIRT1 on p53 and FOXO3.
Figure 2.
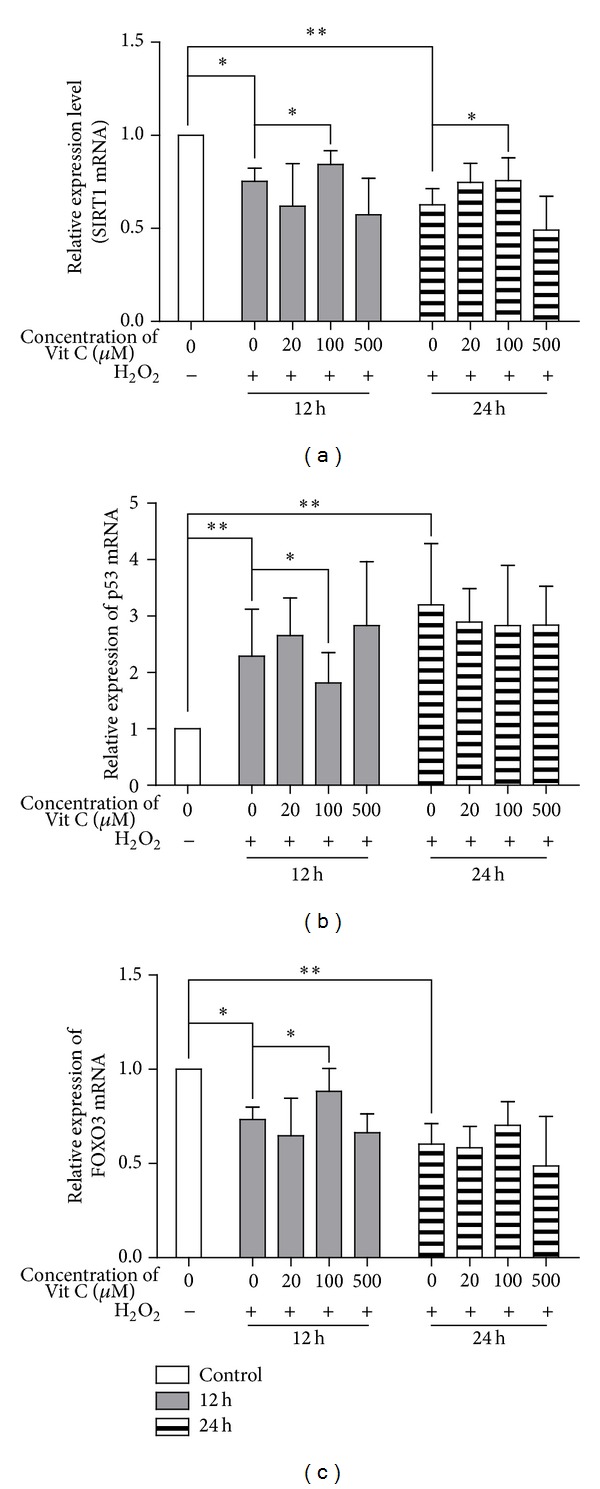
Vit C resulted in the dysregulation of SIRT1, p53, and FOXO3 during oxidative stress in ARPE-19 cells. ARPE-19 cells were treated with indicated methods. The expression of SIRT1 (a), p53 (b), and FOXO3 (c) mRNA was detected by qRT-PCR. Ct values were normalized by 2−ΔΔCt method as relative quantification. Bars represented mean ± SD of three independent experiments. ANOVA was performed to analyze the differences statistically. Statistical significance was expressed as *P < 0.05 and **P < 0.01.
3.3. The Protective Effect of Vit C against Oxidative Stress Was Involved in Regulation of SIRT1
To reveal the crosstalk between Vit C and SIRT1 during oxidative stress in ARPE-19 cells, we analyzed viability, apoptosis, and intracellular ROS after treatments of Vit C at indicated concentration and treatments of SIRT1 activator RSV or SIRT1 inhibitor NA upon 12 h exposure to H2O2 as shown in Figure 3. The results of the MTT assays showed that the RSV could significantly augment the promoting effects of Vit C on the cells viability, compared to the N group (N1, N2, N3, and N4). Importantly, RSV increased the effects of lower Vit C (20 μM) and higher Vit C (500 μM) on cells viability in the presence of H2O2 also, whereas inhibitor NA could attenuate the viability dramatically when the concentration of Vit C was 100 μM (P < 0.05) as shown in Figure 3(a). TUNEL assays displayed the fact that RSV not only stimulated the effects of moderate Vit C (100 μM) on reducing the number of apoptotic cells but also provided the antiapoptotic ability for the lower Vit C (20 μM) and higher Vit C (500 μM) significantly (P < 0.05). NA increased the number of apoptotic cells which were pretreated with moderate Vit C (100 μM) significantly, compared with the N group (N1, N2, N3, and N4) (Figure 3(b)). It meant that RSV could promote the antiapoptotic effects of Vit C, and NA attenuated the antiapoptotic effects of Vit C during oxidative stress. Treatment with RSV and NA resulted in inhibition and promotion of intracellular production of ROS also as shown in (Figure 3(c)). All findings illustrated that SIRT1 played key roles in the protective effect of Vit C against oxidative stress.
Figure 3.
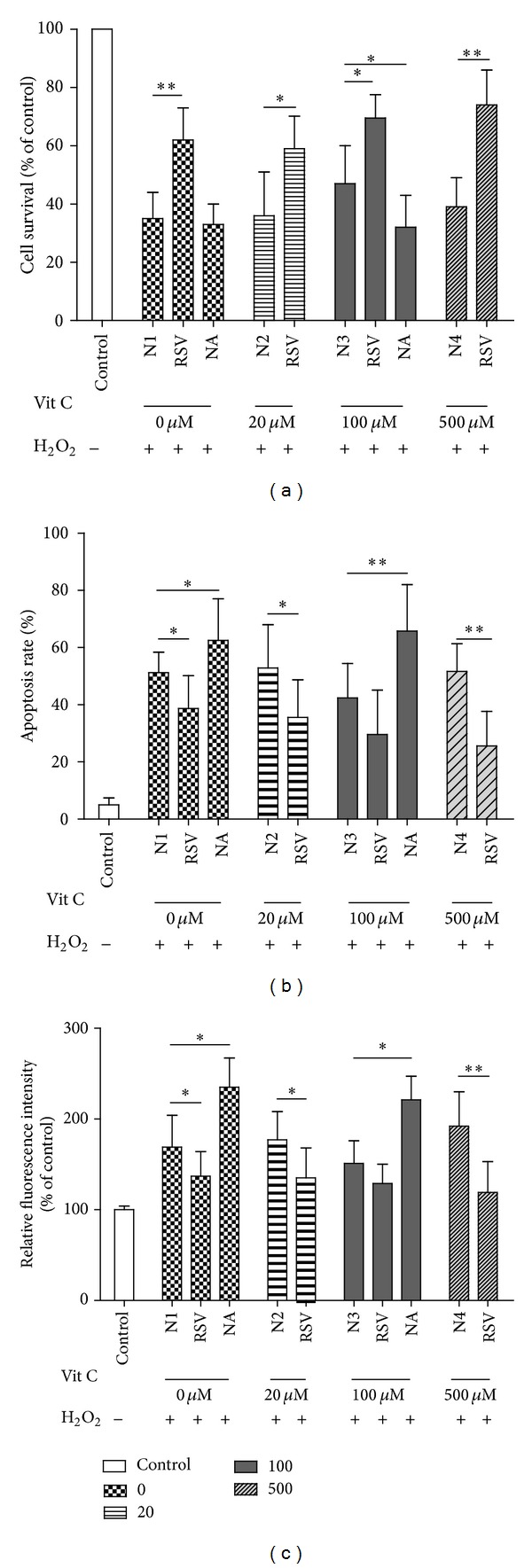
SIRT1 changed the effects of Vit C on viability, apoptosis, and intracellular ROS of ARPE-19 cells induced by H2O2. ARPE-19 cells were preincubated with RSV (10 mM) or NA (5 mM) for 4 h. Then, cells were exposed to H2O2 for 12 h after treatments of indicated concentration of Vit C. Viability (a), apoptosis (b), and intracellular ROS (c) were measured and compared among different groups. N1, N2, N3, and N4 were standing for the cells treated with H2O2 and without Vit C preincubation, cells treated with H2O2 and 20 μM Vit C, cells treated with H2O2 and 100 μM Vit C, and cells treated with H2O2 and 500 μM Vit C, respectively. Single treatment was performed in triplicate. Statistical significance was expressed as *P < 0.05 and **P < 0.01.
To exclude the possibility that Vit C contributed to the expression of stress responses factors (p53 and FOXO3) which have protective effects on apoptosis, viability, and intracellular ROS, we examined the expression of p53 and FOXO3 after knockdown or overexpression of SIRT1 genes in RPEs with supplementation of 100 μM Vit C during oxidative stress. The comparisons of p53 expression among indicated groups found SIRT1 knockdown and upregulated SIRT1 significantly increased and inhibited the expression of p53, respectively (Figure 4(a)). It was found that FOXO3 was decreased slightly in SIRT1 silenced RPEs and the upregulated SIRT1 promoted the expression of FOXO3 significantly as shown in Figure 4(b). Figure 4(c) displayed the expression of SIRT1, p53, and FOXO3 proteins in indicated groups. It further supported the fact that SIRT1 was the key regulator to modulate the p53 and FOXO3. These findings indicated that the regulatory effects of moderate Vit C on the expression of FOXO3 and p53 were closely related to the SIRT1. In other words, the protective effect of Vit C against oxidative stress was involved in regulation of SIRT1.
Figure 4.
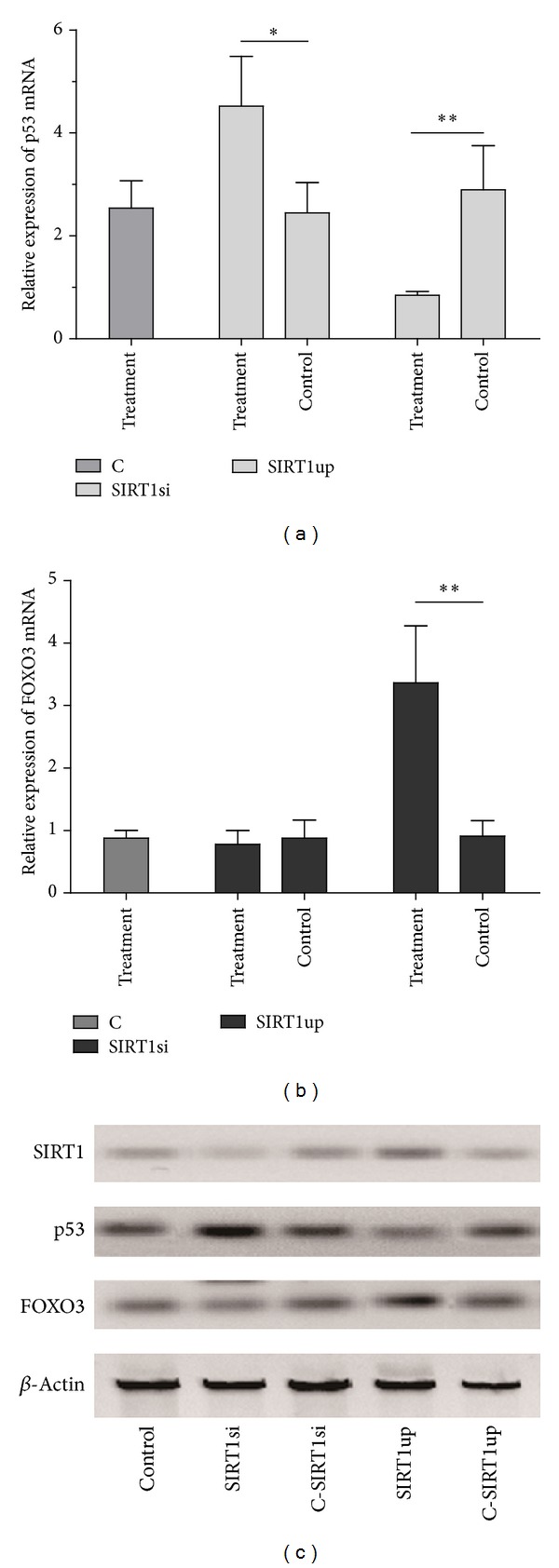
Regulation of FoxO3 and p53 was involved in the expression of SIRT1 rather than Vit C. ARPE-19 cells underwent SIRT1 knockdown and overexpression of SIRT1 by using siRNA (SIRT1si group), pRC/CMV-SRIT1 (SIRT1up group), and its control (C-SIRT1si and C-SIRT1up groups, resp.). Then, cells were exposed to 100 μM of H2O2 for 12 h after being incubated for 24 h with 100 μM of Vit C. The expression of p53 (a) and FOXO3 (b) mRNA was detected by qRT-PCR and was normalized by 2−ΔΔCt method as relative quantification. The expression of SIRT1, p53, and FOXO3 proteins was assayed by western blot in ARPE-19 cells with indicated treatments (c). Bars represented mean ± SD of three independent experiments. ANOVA was performed to analyze the differences statistically. Statisticalsignificance was expressed as *P < 0.05 and **P < 0.01.
4. Discussion
Oxidative stress has been considered as a major factor to contribute to aging associated diseases, including Alzheimer's disease, diabetes mellitus, cardiovascular disorders, and AMD [6, 12, 14]. Vitamins have primary effects on oxidative stress as antioxidants. Vit C provided protection against atherogenesis and Alzheimer's disease as antioxidants, except for protection of immune system, reduction of allergic reactions and combating infections [11]. In terms of protection of retina, Vit C could relieve retinopathy induced by oxidative stress in rats and played controversial roles in preventing human RPEs from oxidative stress [13]. Owing to extensive clinical application of Vit C, the investigation of effects of Vit C on RPEs in response to oxidative stress is a challenging and promising therapeutic method for protection of RPEs to delay the process of AMD [26].
In this paper, we illustrated that the injuries of RPEs induced by H2O2 were not reduced dramatically by Vit C treatment at lower and higher concentration. It was Vit C supplementation of 100 μM that appeared to significantly alleviate oxidative stress, concluding viability, apoptosis, and intracellular ROS (Figure 1). The data were similar to the results of Yin et al. about the functions of Vit C on RPEs [17]. Our findings further illustrated the effects of Vit C on viability, apoptosis, and intracellular ROS of RPEs response to oxidative stress. Even though the beneficial action of Vit C against oxidative stress was well reported in the literature, the advantage of Vit C was too obvious to exert antioxidative effects on RPEs as a matter of fact.
Some authors had reported that Vit C modulated the level of SIRT1 and played an important roles in lung development through affecting oxidant-antioxidant balance in rats [27]. Vit C also stimulated the activity of SIRT1 which deacetylated 7-amino-4-methylcoumarin-labeled acetylated peptide [28]. Conversely, recent reports had documented pretreatment human dermal fibroblasts cells with 2-O-α-glucopyranosyl-l-ascorbic acid (2A-Vit C) before H2O2 exposure significantly inhibited this decrease in SIRT1 expression, whereas ascorbic acid (Vit C) had no effect [29]. So we made a hypothesis that crosstalk between Vit C and SIRT1 might be highly related to microenvironment. And the mechanism needs to merit further investigation. For this reason, we surveyed to characterize the crosstalk between Vit C and SIRT1 in RPEs during oxidative stress. In this paper, we found that Vit C treatments affected the level of SIRT1 in RPEs under H2O2-mediated stress. Interestingly, the increase of SIRT1 level with consequent enhancement of protection PREs from damage induced by H2O2 occurred when concentration of Vit C was 100 μM (Figure 2(a)). The results implied that the benefit of Vit C action might closely be related to the expression level of SIRT1. SIRT1 modulates lots of signaling pathways concerned with cellular senescence, proliferation, and apoptosis, because of its ability to deacetylate some cytokines such as forkhead box class O (FOXO)3, NF-κB, and p53 [30, 31]. In this paper, levels of SIRT1 associated cytokines (p53 and FOXO3) displayed similar trend to the SIRT1 levels after Vit C treatments during oxidative stress (Figures 2(b) and 2(c)). These data revealed that Vit C could affect the expression of the SIRT1 transcription factors and SIRT1 associated cytokines.
In order to identify the relationship between SIRT1 and Vit C, we investigated if SIRT1 activator or inhibitor (RSV or NA) could affect the effect of Vit C on RPEs in the presence of oxidative stress. The results showed that RSV treatment significantly provided the ability against oxidative stress for the lower concentration of Vit C and higher concentration of Vit C. RSV also dramatically augmented the ability of moderate Vit C to prevent damage induced by H2O2. Furthermore, NA counteracted the protective roles of moderate level of Vit C (Figure 3).
Since p53 expression, which is regulated by SIRT1, could be modulated by Vit C in cancer and in alcoholic liver fibrosis also [28, 29], we investigated whether the SIRT1 or Vit C took the primary regulatory effects on stress responses factors (FOXO3 and p53) in PREs exposure to H2O2. Here, data displayed the fact that Vit C could not influence the expression of FoxO3 and p53 in the absence of SIRT1 (Figure 4). It confirmed that the SIRT1 caused the dysregulation of FoxO3 and p53 rather than Vit C in RPEs.
In conclusion, Vit C protected RPEs from oxidant injury depending on regulating SIRT1. This mechanism further suggested that combined application of Vit C and RSV, which exerted effective resistance to the damage of RPEs induced by oxidative stress, might be a promising therapeutic method for AMD.
Acknowledgments
This study was supported by Renmin Hospital of Wuhan University and Inner Mongolia People's Hospital.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Survey of Ophthalmology. 2000;45(2):115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 2.Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. Journal of the American Medical Association. 2003;290(15):2057–2060. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- 3.Plafker SM, O’Mealey GB, Szweda LI. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. International Review of Cell and Molecular Biology. 2012;298:135–177. doi: 10.1016/B978-0-12-394309-5.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Archives of Ophthalmology. 2001;119:1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Molecular Aspects of Medicine. 2012;33(4):399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauss O. The retinal pigment epithelium in visual function. Physiological Reviews. 2005;85(3):845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZY, Shen LJ, Tu L, et al. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radical Biology & Medicine. 2009;46(8):1032–1041. doi: 10.1016/j.freeradbiomed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Cai J, Cai J, Nelson KC, et al. Oxidative damage and protection of the RPE. Progress in Retinal and Eye Research. 2000;19(2):205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Dong X, Liu H, et al. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/AkT. Molecular Vision. 2013;19:1656–1666. [PMC free article] [PubMed] [Google Scholar]
- 11.Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian Journal of Clinical Biochemistry. 2013;28:314–328. doi: 10.1007/s12291-013-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heo JH, Hyon-Lee, Lee KM. The possible role of antioxidant vitamin C in Alzheimer's disease treatment and prevention. American Journal of Alzheimer's Disease and other Dementias. 2013;28(2):120–125. doi: 10.1177/1533317512473193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Özkaya D, Naziroğlu M, Armağan A, et al. Dietary vitamin C and E modulates oxidative stress induced-kidney and lens injury in diabetic aged male rats through modulating glucose homeostasis and antioxidant systems. Cell Biochemistry and Function. 2011;29(4):287–293. doi: 10.1002/cbf.1749. [DOI] [PubMed] [Google Scholar]
- 14.Naziroğlu M, Butterworth PJ, Sonmez TT. Dietary vitamin C and E modulates antioxidant levels in blood, brain, liver, muscle, and testes in diabetic aged rats. Internationale Zeitschrift für Vitamin- und Ernährungsforschung. 2011;81(6):347–357. doi: 10.1024/0300-9831/a000083. [DOI] [PubMed] [Google Scholar]
- 15.Kagan DB, Liu H, Hutnik CML. Efficacy of various antioxidants in the protection of the retinal pigment epithelium from oxidative stress. Clinical Ophthalmology. 2012;6(1):1471–1476. doi: 10.2147/OPTH.S35139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeitz O, Schlichting L, Richard G, Strauß O. Lack of antioxidative properties of vitamin C and pyruvate in cultured retinal pigment epithelial cells. Graefe's Archive for Clinical and Experimental Ophthalmology. 2007;245(2):276–281. doi: 10.1007/s00417-006-0384-5. [DOI] [PubMed] [Google Scholar]
- 17.Yin J, Thomas F, Lang JC, Chaum E. Modulation of oxidative stress responses in the human retinal pigment epithelium following treatment with vitamin C. Journal of Cellular Physiology. 2011;226(8):2025–2032. doi: 10.1002/jcp.22532. [DOI] [PubMed] [Google Scholar]
- 18.Horio Y, Hayashi T, Kuno A, Kunimoto R. Cellular and molecular effects of sirtuins in health and disease. Clinical Science. 2011;121(5):191–203. doi: 10.1042/CS20100587. [DOI] [PubMed] [Google Scholar]
- 19.Raynes R, Brunquell J, Westerheide SD. Stress inducibility of SIRT1 and its role in cytoprotection and cancer. Genes & Cancer. 2013;4:172–182. doi: 10.1177/1947601913484497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circulation Research. 2007;100(10):1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 21.Kawai Y, Garduño L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. The Journal of Biological Chemistry. 2011;286(9):7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polidoro L, Properzi G, Marampon F, et al. Vitamin D protects human endothelial cells from H2O2 oxidant injury through the Mek/Erk-Sirt1 axis activation. Journal of Cardiovascular Translational Research. 2013;6(2):221–231. doi: 10.1007/s12265-012-9436-x. [DOI] [PubMed] [Google Scholar]
- 23.Hori YS, Kuno A, Hosoda R, Horio Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0073875.e73875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada Y, Tian J, Yang Y, et al. Oxidized low density lipoproteins induce a pathologic response by retinal pigmented epithelial cells. Journal of Neurochemistry. 2008;105(4):1187–1197. doi: 10.1111/j.1471-4159.2008.05211.x. [DOI] [PubMed] [Google Scholar]
- 25.Salminen A, Kaarniranta K, Kauppinen A. Crosstalk between oxidative stress and SIRT1: impact on the aging process. International Journal of Molecular Sciences. 2013;14(2):3834–3859. doi: 10.3390/ijms14023834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kook D, Wolf AH, Yu AL, et al. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Investigative Ophthalmology and Visual Science. 2008;49(4):1712–1720. doi: 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- 27.Koike K, Kondo Y, Sekiya M, et al. Complete lack of vitamin C intake generates pulmonary emphysema in senescence marker protein-30 knockout mice. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2010;298(6):L784–L792. doi: 10.1152/ajplung.00256.2009. [DOI] [PubMed] [Google Scholar]
- 28.Feng Y, Wu J, Chen L, et al. A fluorometric assay of SIRT1 deacetylation activity through quantification of nicotinamide adenine dinucleotide. Analytical Biochemistry. 2009;395(2):205–210. doi: 10.1016/j.ab.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Taniguchi M, Arai N, Kohno K, Ushio S, Fukuda S. Anti-oxidative and anti-aging activities of 2-O-α-glucopyranosyl-L- ascorbic acid on human dermal fibroblasts. European Journal of Pharmacology. 2012;674(2-3):126–131. doi: 10.1016/j.ejphar.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Finkel T, Deng C, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins: novel therapeutic targets to treat age-associated diseases. Nature Reviews Drug Discovery. 2008;7(10):841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]


