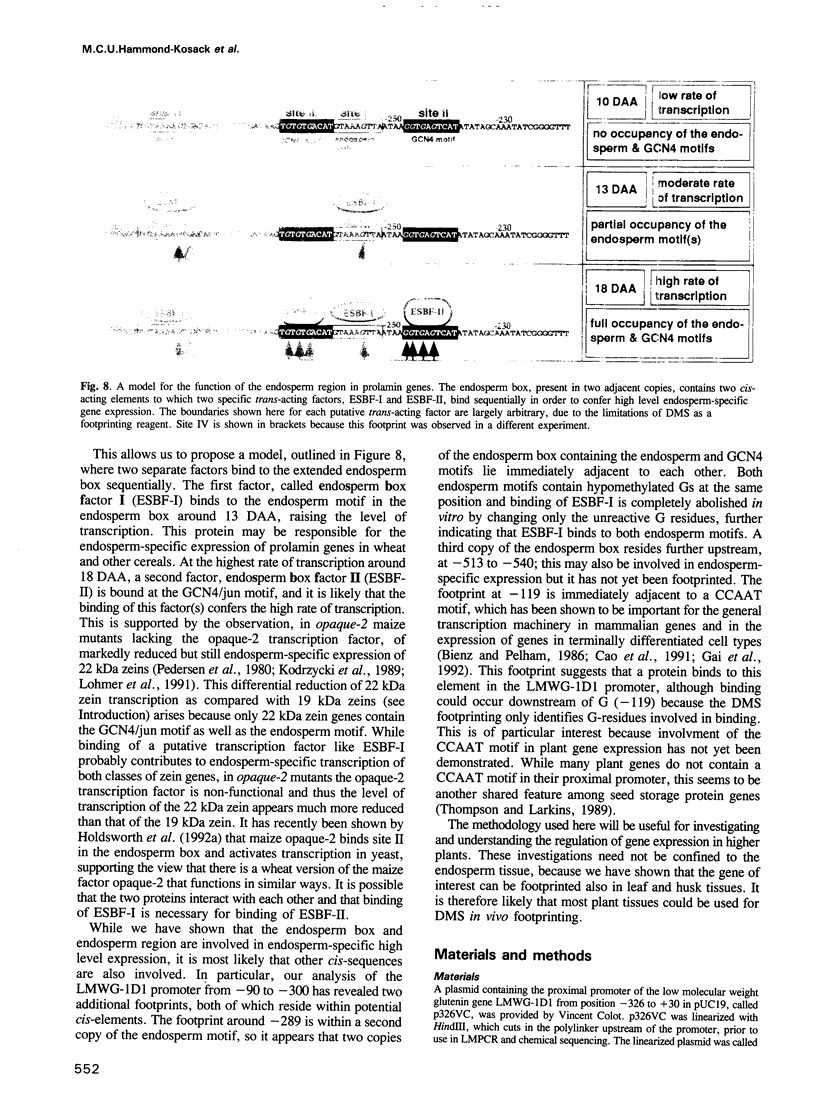
Abstract
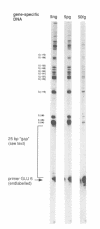
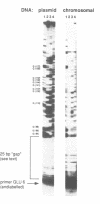
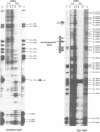
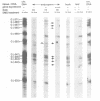
The quality of the wheat grain is determined by the quantity and composition of storage proteins (prolamins) which are synthesized exclusively in endosperm tissue. We are investigating the mechanisms underlying the regulation of expression of a prolamin gene, the low molecular weight glutenin gene LMWG-1D1. The LMWG-1D1 promoter contains the endosperm box, a sequence motif highly conserved in the promoter region of a large number of storage protein genes, which is thought to confer endosperm-specific expression of prolamin genes. Here we show by in vivo DMS footprinting of wheat endosperm tissue that the endosperm box becomes occupied by putative trans-acting factors during grain ripening. During early stages of development the endosperm motif within the 5' half of the endosperm box becomes occupied first, followed by binding of a second activity to a GCN4/jun-like motif in the 3' half just prior to the stage of maximum gene expression. Occupancy of the endosperm box is highly tissue-specific: no protection was observed in husk and leaf tissues. Several binding activities were identified in vitro from nuclear protein extracts of wheat endosperm which bind specifically to the endosperm and GCN4/jun motifs identified by in vivo footprinting.
Full text
PDF

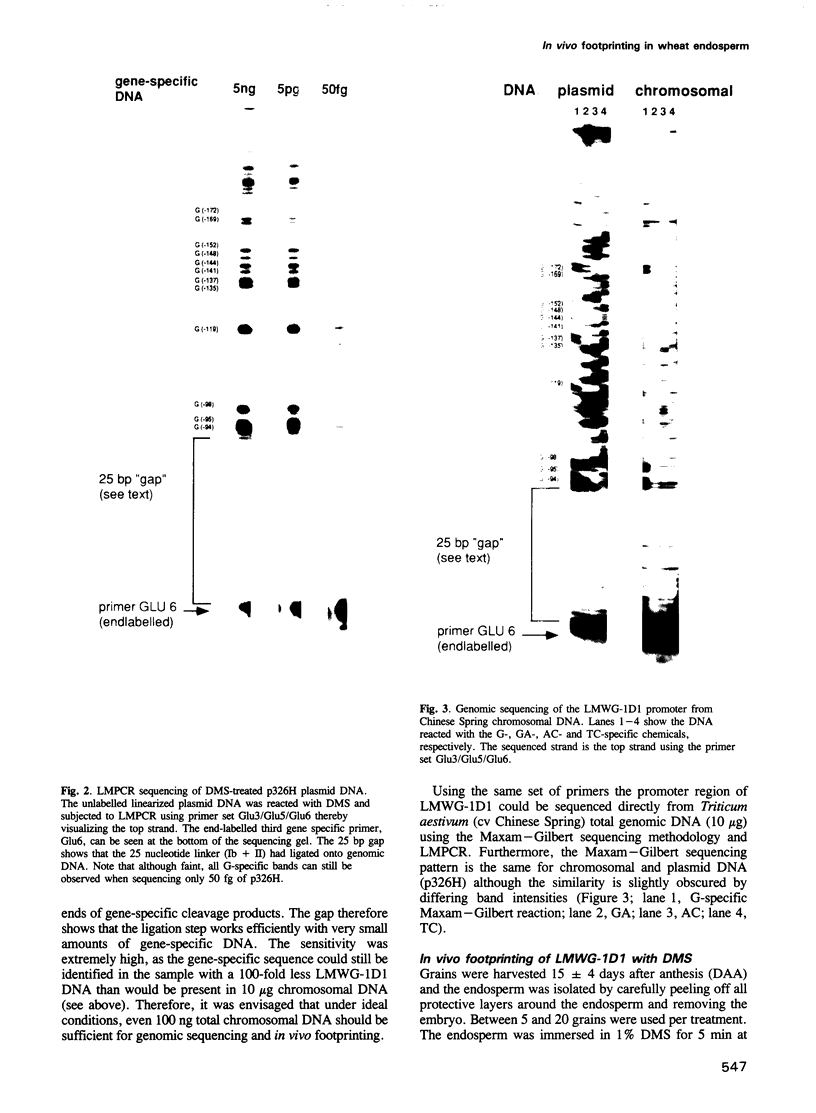
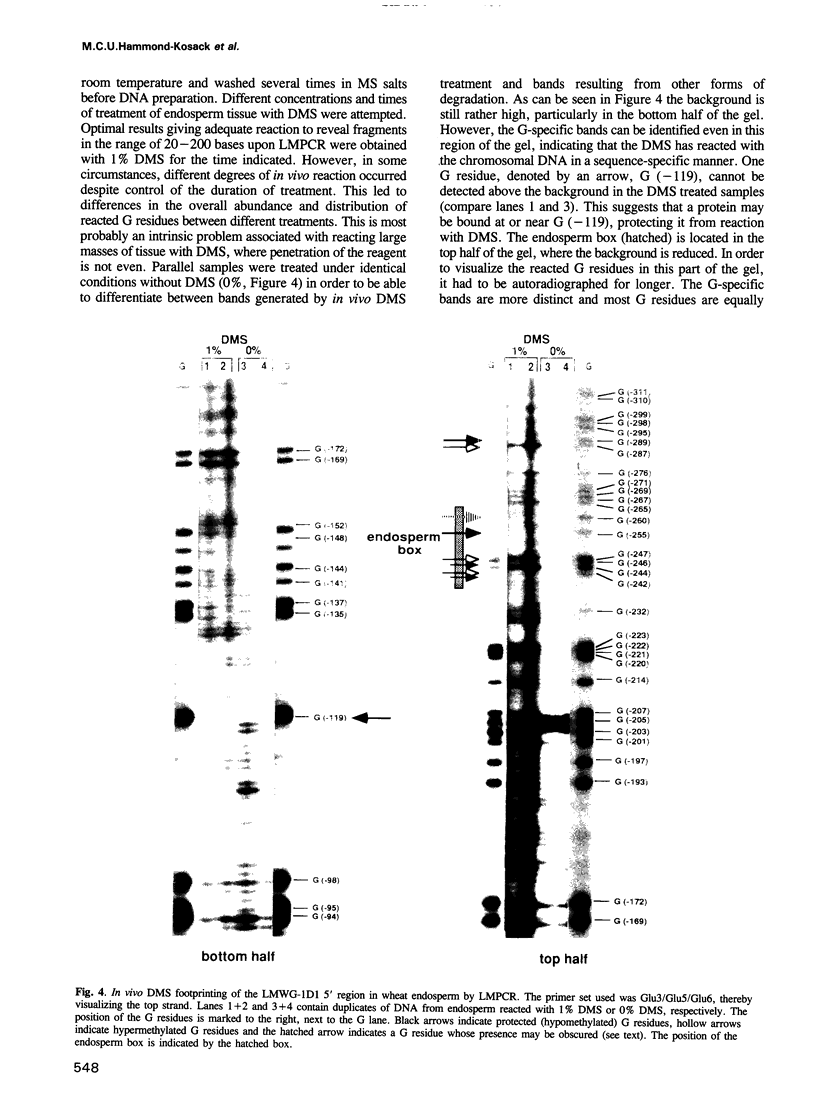

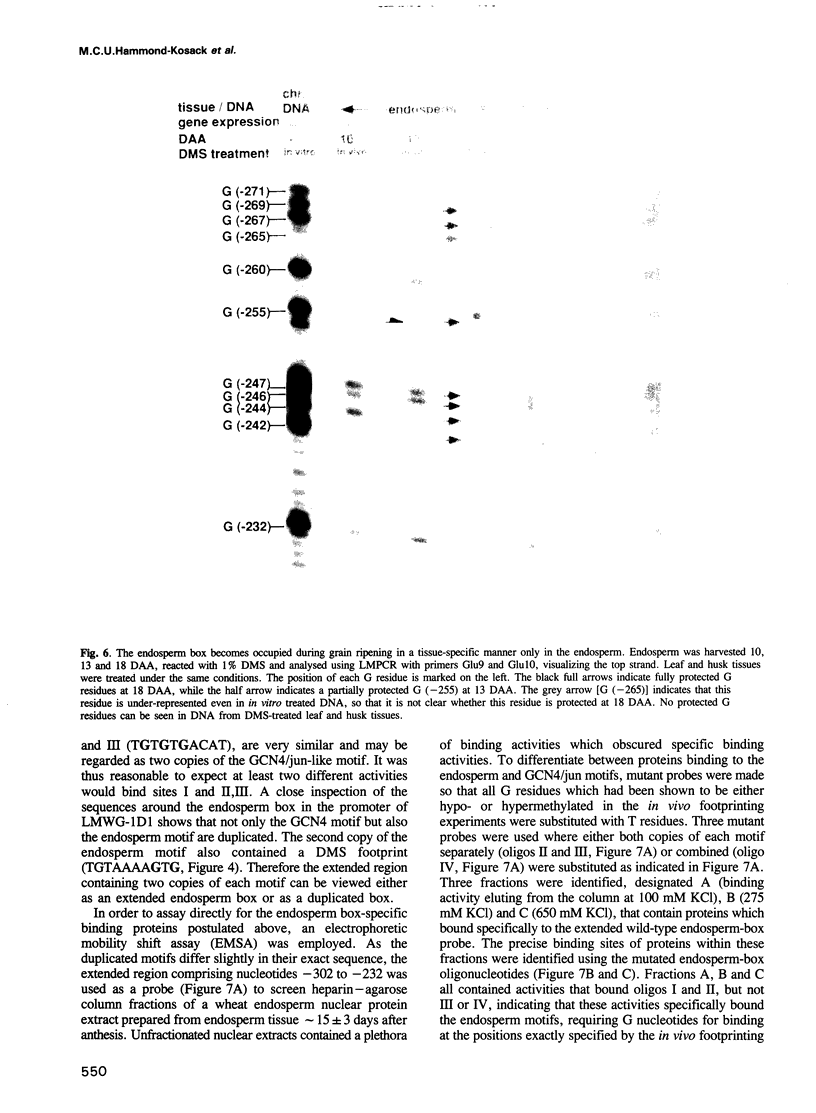
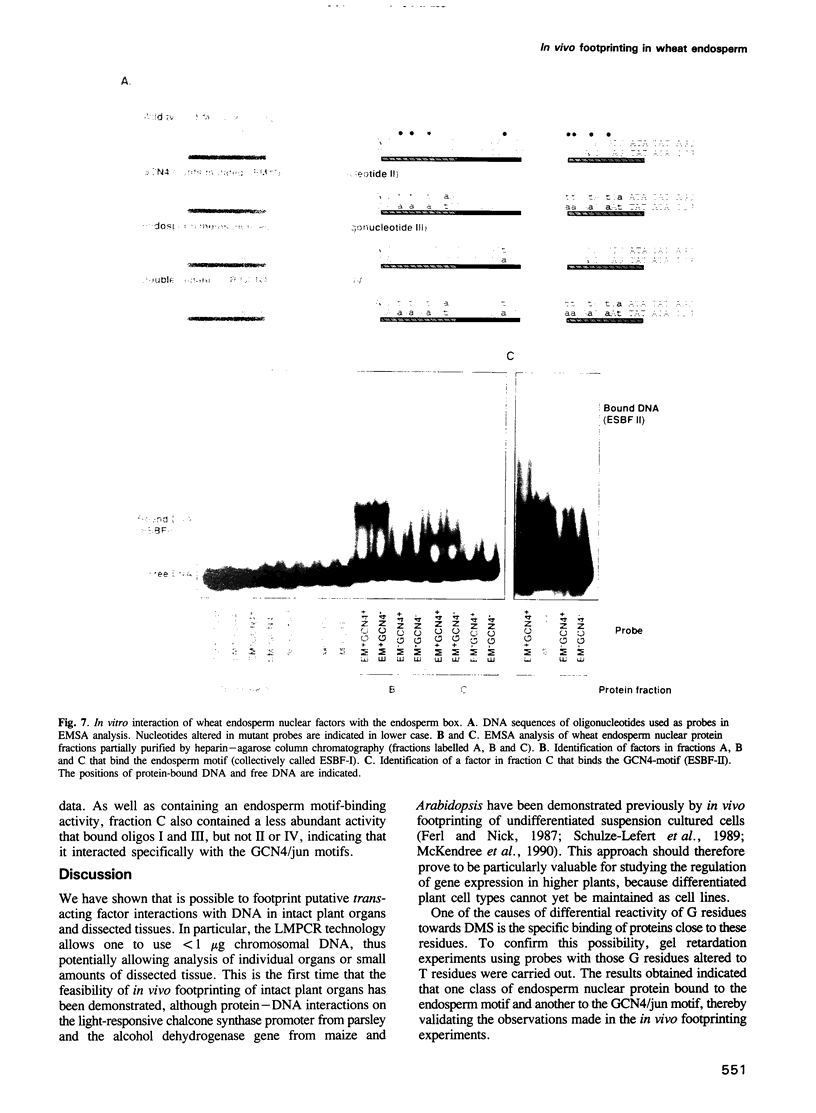


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienz M., Pelham H. R. Heat shock regulatory elements function as an inducible enhancer in the Xenopus hsp70 gene and when linked to a heterologous promoter. Cell. 1986 Jun 6;45(5):753–760. doi: 10.1016/0092-8674(86)90789-0. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Colot V., Bartels D., Thompson R., Flavell R. Molecular characterization of an active wheat LMW glutenin gene and its relation to other wheat and barley prolamin genes. Mol Gen Genet. 1989 Mar;216(1):81–90. doi: 10.1007/BF00332234. [DOI] [PubMed] [Google Scholar]
- Colot V., Robert L. S., Kavanagh T. A., Bevan M. W., Thompson R. D. Localization of sequences in wheat endosperm protein genes which confer tissue-specific expression in tobacco. EMBO J. 1987;6(12):3559–3564. doi: 10.1002/j.1460-2075.1987.tb02685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot V. The genes encoding wheat storage proteins: towards a molecular understanding of bread-making quality and its genetic manipulation. Genet Eng (N Y) 1990;12:225–241. doi: 10.1007/978-1-4613-0641-2_12. [DOI] [PubMed] [Google Scholar]
- Ferl R. J., Nick H. S. In vivo detection of regulatory factor binding sites in the 5' flanking region of maize Adh1. J Biol Chem. 1987 Jun 15;262(17):7947–7950. [PubMed] [Google Scholar]
- Forde B. G., Heyworth A., Pywell J., Kreis M. Nucleotide sequence of a B1 hordein gene and the identification of possible upstream regulatory elements in endosperm storage protein genes from barley, wheat and maize. Nucleic Acids Res. 1985 Oct 25;13(20):7327–7339. doi: 10.1093/nar/13.20.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Kreis M., Bahramian M. B., Matthews J. A., Miflin B. J., Thompson R. D., Bartels D., Flavell R. B. Molecular cloning and analysis of cDNA sequences derived from poly A+ RNA from barley endosperm: identification of B hordein related clones. Nucleic Acids Res. 1981 Dec 21;9(24):6689–6707. doi: 10.1093/nar/9.24.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai X. X., Lipson K. E., Prystowsky M. B. Unusual DNA binding characteristics of an in vitro translation product of the CCAAT binding protein mYB-1. Nucleic Acids Res. 1992 Feb 11;20(3):601–606. doi: 10.1093/nar/20.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack M. C., Dobrinski B., Lurz R., Docherty K., Kilpatrick M. W. The human insulin gene linked polymorphic region exhibits an altered DNA structure. Nucleic Acids Res. 1992 Jan 25;20(2):231–236. doi: 10.1093/nar/20.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings H., Lazzaroni N., Marsan P. A., Aragay A., Thompson R., Salamini F., Di Fonzo N., Palau J., Motto M. The b-32 protein from maize endosperm: characterization of genomic sequences encoding two alternative central domains. Plant Mol Biol. 1990 Jun;14(6):1031–1040. doi: 10.1007/BF00019399. [DOI] [PubMed] [Google Scholar]
- Hartings H., Maddaloni M., Lazzaroni N., Di Fonzo N., Motto M., Salamini F., Thompson R. The O2 gene which regulates zein deposition in maize endosperm encodes a protein with structural homologies to transcriptional activators. EMBO J. 1989 Oct;8(10):2795–2801. doi: 10.1002/j.1460-2075.1989.tb08425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. E., Hope I. A., Macke J. P., Struhl K. Saturation mutagenesis of the yeast his3 regulatory site: requirements for transcriptional induction and for binding by GCN4 activator protein. Science. 1986 Oct 24;234(4775):451–457. doi: 10.1126/science.3532321. [DOI] [PubMed] [Google Scholar]
- Holdsworth M. J., Grierson C., Schuch W., Bevan M. DNA-binding properties of cloned TATA-binding protein from potato tubers. Plant Mol Biol. 1992 Jun;19(3):455–464. doi: 10.1007/BF00023393. [DOI] [PubMed] [Google Scholar]
- Kodrzycki R., Boston R. S., Larkins B. A. The opaque-2 mutation of maize differentially reduces zein gene transcription. Plant Cell. 1989 Jan;1(1):105–114. doi: 10.1105/tpc.1.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis M., Shewry P. R., Forde B. G., Rahman S., Bahramian M. B., Miflin B. J. Molecular analysis of the effects of the lys 3a gene on the expression of Hor loci in developing endosperms of barley (Hordeum vulgare L.). Biochem Genet. 1984 Apr;22(3-4):231–255. doi: 10.1007/BF00484227. [DOI] [PubMed] [Google Scholar]
- Kridl J. C., Vieira J., Rubenstein I., Messing J. Nucleotide sequence analysis of a zein genomic clone with a short open reading frame. Gene. 1984 Apr;28(1):113–118. doi: 10.1016/0378-1119(84)90093-3. [DOI] [PubMed] [Google Scholar]
- Lohmer S., Maddaloni M., Motto M., Di Fonzo N., Hartings H., Salamini F., Thompson R. D. The maize regulatory locus Opaque-2 encodes a DNA-binding protein which activates the transcription of the b-32 gene. EMBO J. 1991 Mar;10(3):617–624. doi: 10.1002/j.1460-2075.1991.tb07989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendree W. L., Paul A. L., DeLisle A. J., Ferl R. J. In vivo and in vitro characterization of protein interactions with the dyad G-box of the Arabidopsis Adh gene. Plant Cell. 1990 Mar;2(3):207–214. doi: 10.1105/tpc.2.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Bloom K. S., Anderson J. N., Glover D. V., Larkins B. A. Analysis of the complexity and frequency of zein genes in the maize genome. Biochemistry. 1980 Apr 15;19(8):1644–1650. doi: 10.1021/bi00549a019. [DOI] [PubMed] [Google Scholar]
- Piette J., Hirai S., Yaniv M. Constitutive synthesis of activator protein 1 transcription factor after viral transformation of mouse fibroblasts. Proc Natl Acad Sci U S A. 1988 May;85(10):3401–3405. doi: 10.1073/pnas.85.10.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransone L. J., Verma I. M. Nuclear proto-oncogenes fos and jun. Annu Rev Cell Biol. 1990;6:539–557. doi: 10.1146/annurev.cb.06.110190.002543. [DOI] [PubMed] [Google Scholar]
- Rigaud G., Roux J., Pictet R., Grange T. In vivo footprinting of rat TAT gene: dynamic interplay between the glucocorticoid receptor and a liver-specific factor. Cell. 1991 Nov 29;67(5):977–986. doi: 10.1016/0092-8674(91)90370-e. [DOI] [PubMed] [Google Scholar]
- Schmidt R. J., Ketudat M., Aukerman M. J., Hoschek G. Opaque-2 is a transcriptional activator that recognizes a specific target site in 22-kD zein genes. Plant Cell. 1992 Jun;4(6):689–700. doi: 10.1105/tpc.4.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lefert P., Becker-André M., Schulz W., Hahlbrock K., Dangl J. L. Functional architecture of the light-responsive chalcone synthase promoter from parsley. Plant Cell. 1989 Jul;1(7):707–714. doi: 10.1105/tpc.1.7.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. S., Flavell R. B. Identification of an enhancer element for the endosperm-specific expression of high molecular weight glutenin. Plant Cell. 1990 Dec;2(12):1171–1180. doi: 10.1105/tpc.2.12.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. A., Larkins B. A. Structural elements regulating zein gene expression. Bioessays. 1989 Apr;10(4):108–113. doi: 10.1002/bies.950100404. [DOI] [PubMed] [Google Scholar]