Abstract
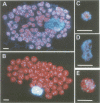
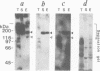
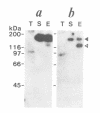
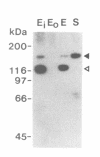
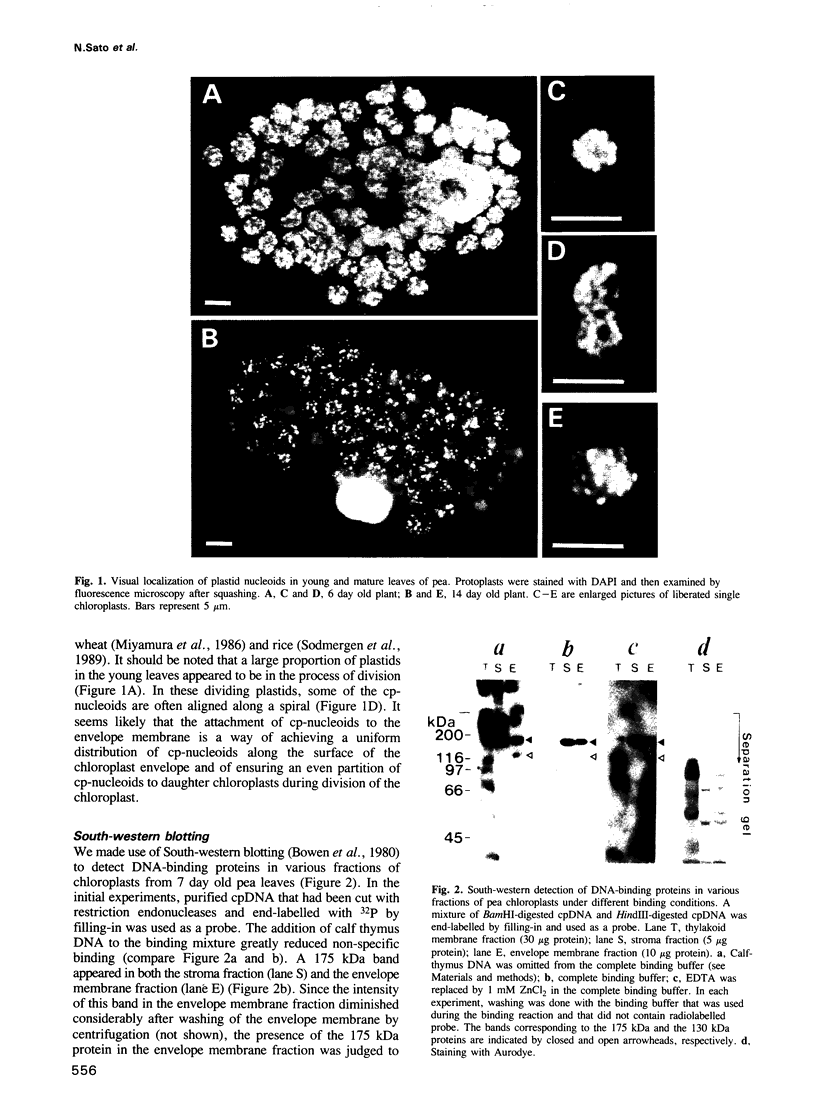
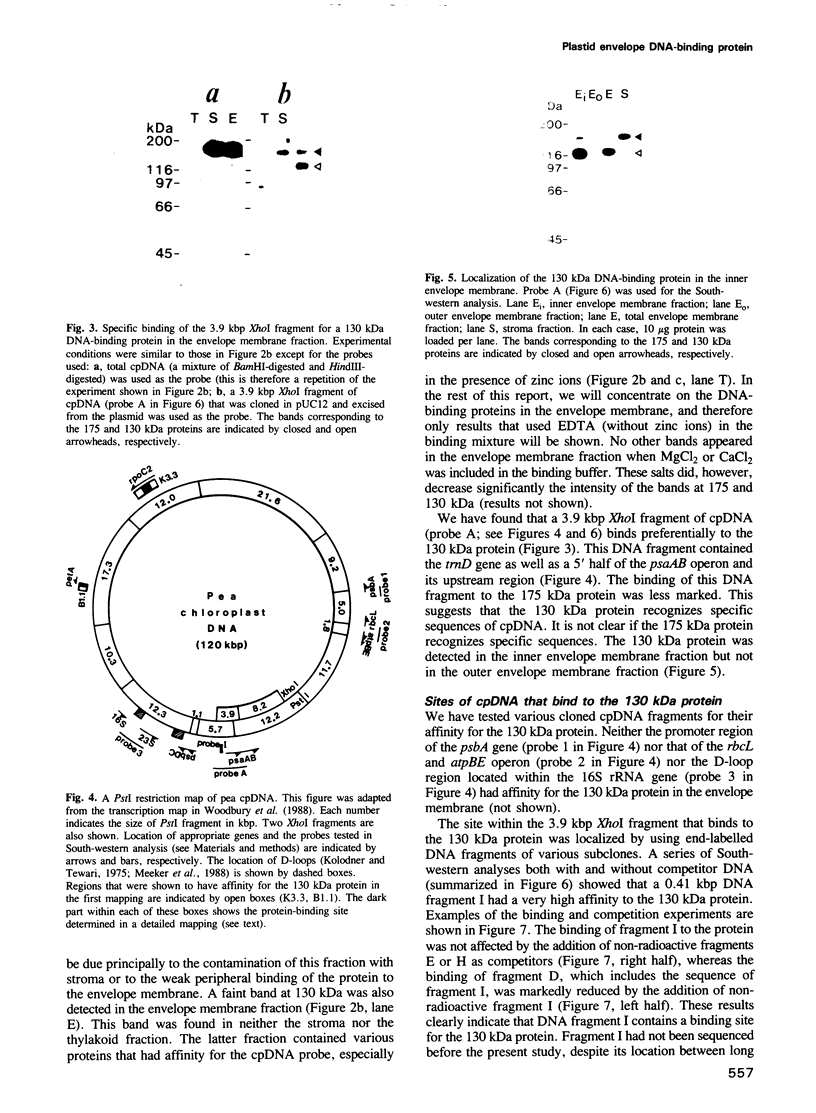
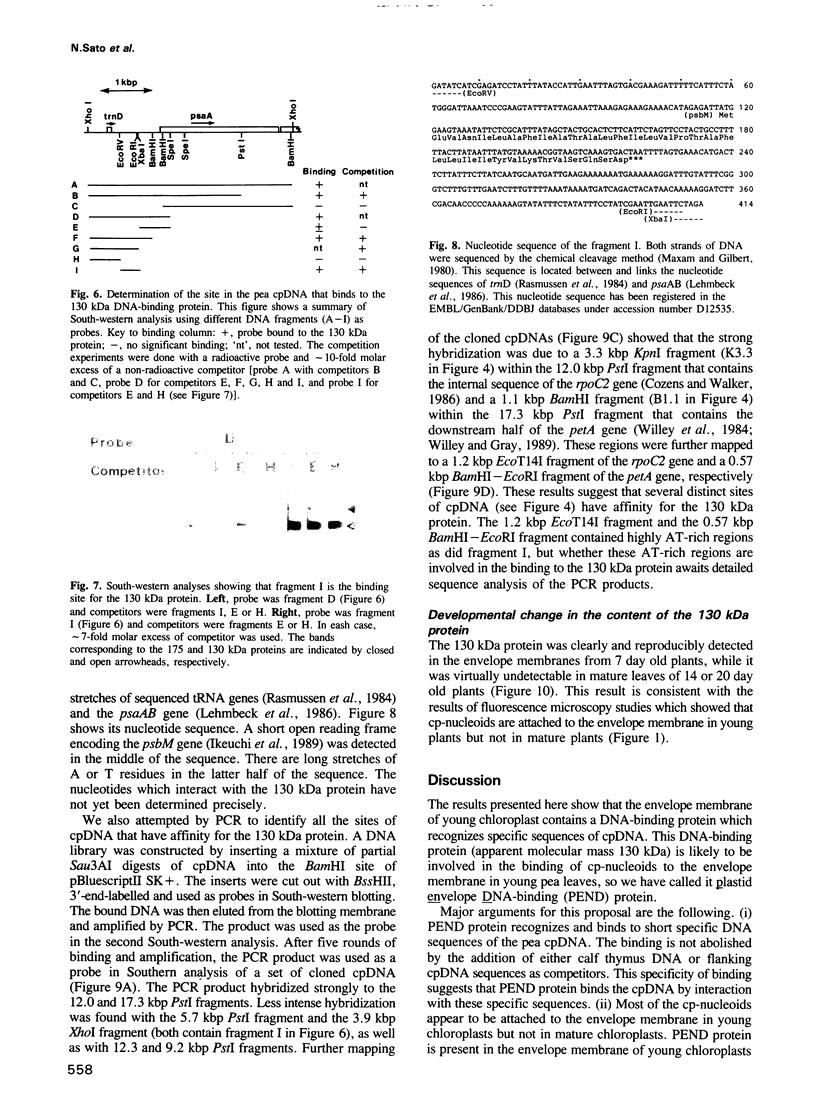
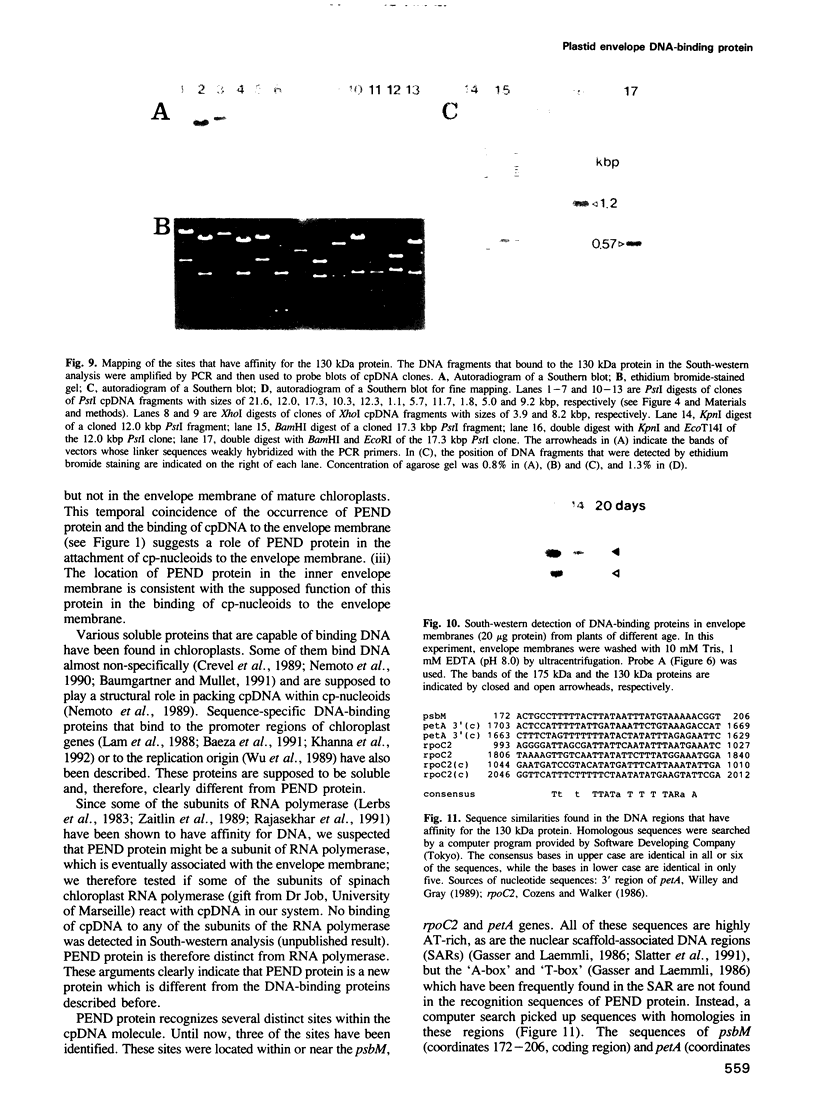
Chloroplast DNA (cpDNA) binds to the envelope membrane of actively dividing chloroplasts (plastids) in young pea leaves. South-western blotting was used to identify and characterize the protein involved in the binding of cpDNA to the envelope membrane. A 130 kDa protein in the inner chloroplast (plastid) envelope membrane binds specific sequences within the cpDNA. These included a 0.41 kbp sequence located upstream of the psaAB gene, a 0.57 kbp sequence located downstream of the petA gene and a 1.2 kbp sequence located within the rpoC2 gene. The protein was detected in the envelope membrane of young pea leaves in which the cpDNA had been located by fluorescence microscopy at the chloroplast periphery, whereas it was undetectable in mature leaves. We therefore propose that the 130 kDa protein is involved in the binding of cpDNA to the envelope membrane, and named it plastid envelope DNA-binding protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner B. J., Mullet J. E. Plastid DNA synthesis and nucleic acid-binding proteins in developing barley chloroplasts. J Photochem Photobiol B. 1991 Nov;11(2):203–218. doi: 10.1016/1011-1344(91)80261-f. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman A. W. Use of the fluorochrome 4'6-diamidino-2-phenylindole in genetic and developmental studies of chloroplast DNA. J Cell Biol. 1979 Jul;82(1):299–305. doi: 10.1083/jcb.82.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E. Pea chloroplast DNA encodes homologues of Escherichia coli ribosomal subunit S2 and the beta'-subunit of RNA polymerase. Biochem J. 1986 Jun 1;236(2):453–460. doi: 10.1042/bj2360453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevel G., Laine B., Sautière P., Galleron C. Isolation and characterization of DNA-binding proteins from the cyanobacterium Synechococcus sp. PCC 7002 (Agmenellum quadruplicatum) and from spinach chloroplasts. Biochim Biophys Acta. 1989 Jan 23;1007(1):36–43. doi: 10.1016/0167-4781(89)90127-9. [DOI] [PubMed] [Google Scholar]
- Echeverria M., Robert D., Carde J. P., Litvak S. Isolation from wheat mitochondria of a membrane-associated high molecular weight complex involved in DNA synthesis. Plant Mol Biol. 1991 Feb;16(2):301–315. doi: 10.1007/BF00020561. [DOI] [PubMed] [Google Scholar]
- Firshein W. Role of the DNA/membrane complex in prokaryotic DNA replication. Annu Rev Microbiol. 1989;43:89–120. doi: 10.1146/annurev.mi.43.100189.000513. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell. 1986 Aug 15;46(4):521–530. doi: 10.1016/0092-8674(86)90877-9. [DOI] [PubMed] [Google Scholar]
- Gerace L., Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Herrmann R. G., Kowallik K. V. Multiple amounts of DNA related to the size of chloroplasts. II. Comparison of electron-microscopic and autoradiographic data. Protoplasma. 1970;69(3):365–372. doi: 10.1007/BF01320301. [DOI] [PubMed] [Google Scholar]
- Hwang D. S., Kornberg A. A novel protein binds a key origin sequence to block replication of an E. coli minichromosome. Cell. 1990 Oct 19;63(2):325–331. doi: 10.1016/0092-8674(90)90165-b. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M., Koike H., Inoue Y. N-terminal sequencing of low-molecular-mass components in cyanobacterial photosystem II core complex. Two components correspond to unidentified open reading frames of plant chloroplast DNA. FEBS Lett. 1989 Aug 14;253(1-2):178–182. doi: 10.1016/0014-5793(89)80954-8. [DOI] [PubMed] [Google Scholar]
- Joyard J., Block M. A., Douce R. Molecular aspects of plastid envelope biochemistry. Eur J Biochem. 1991 Aug 1;199(3):489–509. doi: 10.1111/j.1432-1033.1991.tb16148.x. [DOI] [PubMed] [Google Scholar]
- Kawano S., Kuroiwa T. Isolation and characterization of a membrane-DNA complex in the mitochondria of Physarum polycephalum. Exp Cell Res. 1985 Dec;161(2):460–472. doi: 10.1016/0014-4827(85)90101-6. [DOI] [PubMed] [Google Scholar]
- Khanna N. C., Lakhani S., Tewari K. K. Identification of the template binding polypeptide in the pea chloroplast transcriptional complex. Nucleic Acids Res. 1992 Jan 11;20(1):69–74. doi: 10.1093/nar/20.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R. D., Tewari K. K. Chloroplast DNA from higher plants replicates by both the Cairns and the rolling circle mechanism. Nature. 1975 Aug 28;256(5520):708–711. doi: 10.1038/256708a0. [DOI] [PubMed] [Google Scholar]
- Lam E., Hanley-Bowdoin L., Chua N. H. Characterization of a chloroplast sequence-specific DNA binding factor. J Biol Chem. 1988 Jun 15;263(17):8288–8293. [PubMed] [Google Scholar]
- Landoulsi A., Malki A., Kern R., Kohiyama M., Hughes P. The E. coli cell surface specifically prevents the initiation of DNA replication at oriC on hemimethylated DNA templates. Cell. 1990 Nov 30;63(5):1053–1060. doi: 10.1016/0092-8674(90)90508-c. [DOI] [PubMed] [Google Scholar]
- Liu J. W., Rose R. J. The spinach chloroplast chromosome is bound to the thylakoid membrane in the region of the inverted repeat. Biochem Biophys Res Commun. 1992 Apr 30;184(2):993–1000. doi: 10.1016/0006-291x(92)90689-i. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meeker R., Nielsen B., Tewari K. K. Localization of replication origins in pea chloroplast DNA. Mol Cell Biol. 1988 Mar;8(3):1216–1223. doi: 10.1128/mcb.8.3.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA. I. Intramitochondrial distribution and structural relations of single- and double-length circular DNA. J Mol Biol. 1969 Jun 28;42(3):521–528. doi: 10.1016/0022-2836(69)90240-x. [DOI] [PubMed] [Google Scholar]
- Ogden G. B., Pratt M. J., Schaechter M. The replicative origin of the E. coli chromosome binds to cell membranes only when hemimethylated. Cell. 1988 Jul 1;54(1):127–135. doi: 10.1016/0092-8674(88)90186-9. [DOI] [PubMed] [Google Scholar]
- Pfaller R., Smythe C., Newport J. W. Assembly/disassembly of the nuclear envelope membrane: cell cycle-dependent binding of nuclear membrane vesicles to chromatin in vitro. Cell. 1991 Apr 19;65(2):209–217. doi: 10.1016/0092-8674(91)90155-r. [DOI] [PubMed] [Google Scholar]
- Rajasekhar V. K., Sun E., Meeker R., Wu B. W., Tewari K. K. Highly purified pea chloroplast RNA polymerase transcribes both rRNA and mRNA genes. Eur J Biochem. 1991 Jan 1;195(1):215–228. doi: 10.1111/j.1432-1033.1991.tb15697.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen O. F., Stummann B. M., Henningsen K. W. Nucleotide sequence of a 1.1 kb fragment of the pea chloroplast genome containing three tRNA genes, one of which is located within an open reading frame of 91 codons. Nucleic Acids Res. 1984 Dec 11;12(23):9143–9153. doi: 10.1093/nar/12.23.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. J., Possingham J. V. The localization of (3H) thymidine incorporation in the DNA of replicating spinach chloroplasts by electron-microscope autoradiography. J Cell Sci. 1976 Mar;20(2):341–355. doi: 10.1242/jcs.20.2.341. [DOI] [PubMed] [Google Scholar]
- Shearman C. W., Kalf G. F. DNA replication by a membrane-DNA complex from rat liver mitochondria. Arch Biochem Biophys. 1977 Aug;182(2):573–586. doi: 10.1016/0003-9861(77)90539-2. [DOI] [PubMed] [Google Scholar]
- Slatter R. E., Dupree P., Gray J. C. A scaffold-associated DNA region is located downstream of the pea plastocyanin gene. Plant Cell. 1991 Nov;3(11):1239–1250. doi: 10.1105/tpc.3.11.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey D. L., Auffret A. D., Gray J. C. Structure and topology of cytochrome f in pea chloroplast membranes. Cell. 1984 Feb;36(2):555–562. doi: 10.1016/0092-8674(84)90248-4. [DOI] [PubMed] [Google Scholar]
- Willey D. L., Gray J. C. Two small open reading frames are co-transcribed with the pea chloroplast genes for the polypeptides of cytochrome b-559. Curr Genet. 1989 Mar;15(3):213–220. doi: 10.1007/BF00435508. [DOI] [PubMed] [Google Scholar]
- Woodcock C. L., Fernández-Morán H. Electron microscopy of DNA conformations in spinach chloroplasts. J Mol Biol. 1968 Feb 14;31(3):627–631. doi: 10.1016/0022-2836(68)90435-x. [DOI] [PubMed] [Google Scholar]
- Wu M., Nie Z. Q., Yang J. The 18-kD protein that binds to the chloroplast DNA replicative origin is an iron-sulfur protein related to a subunit of NADH dehydrogenase. Plant Cell. 1989 May;1(5):551–557. doi: 10.1105/tpc.1.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitlin D., Hu J., Bogorad L. Binding and transcription of relaxed DNA templates by fractions of maize chloroplast extracts. Proc Natl Acad Sci U S A. 1989 Feb;86(3):876–880. doi: 10.1073/pnas.86.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]