Abstract
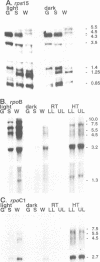
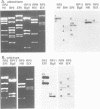
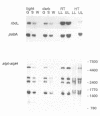
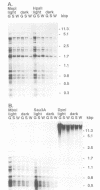
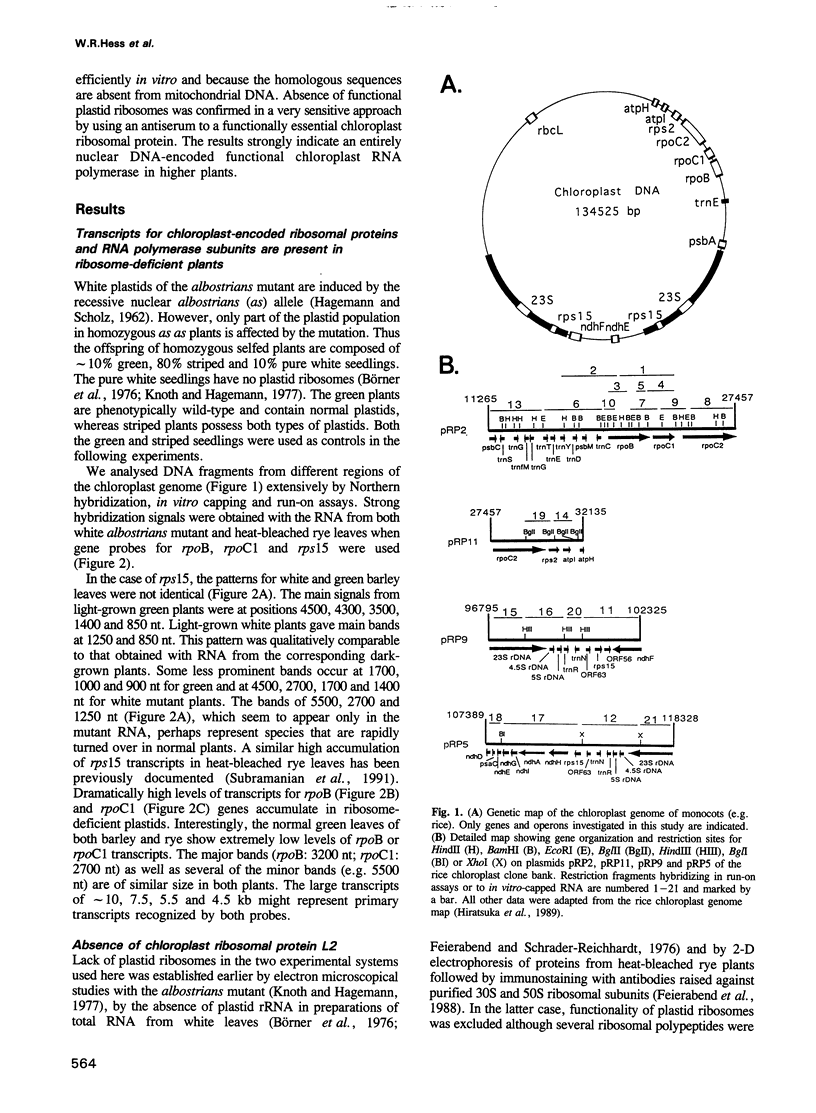
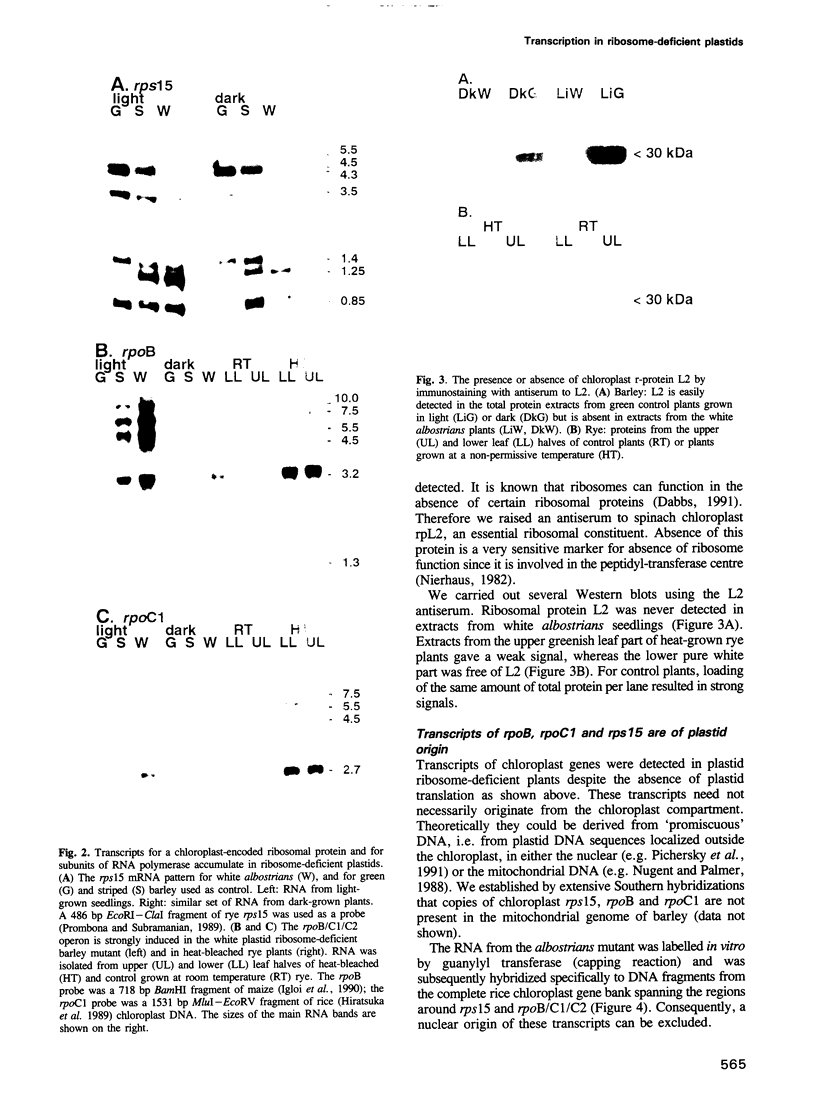
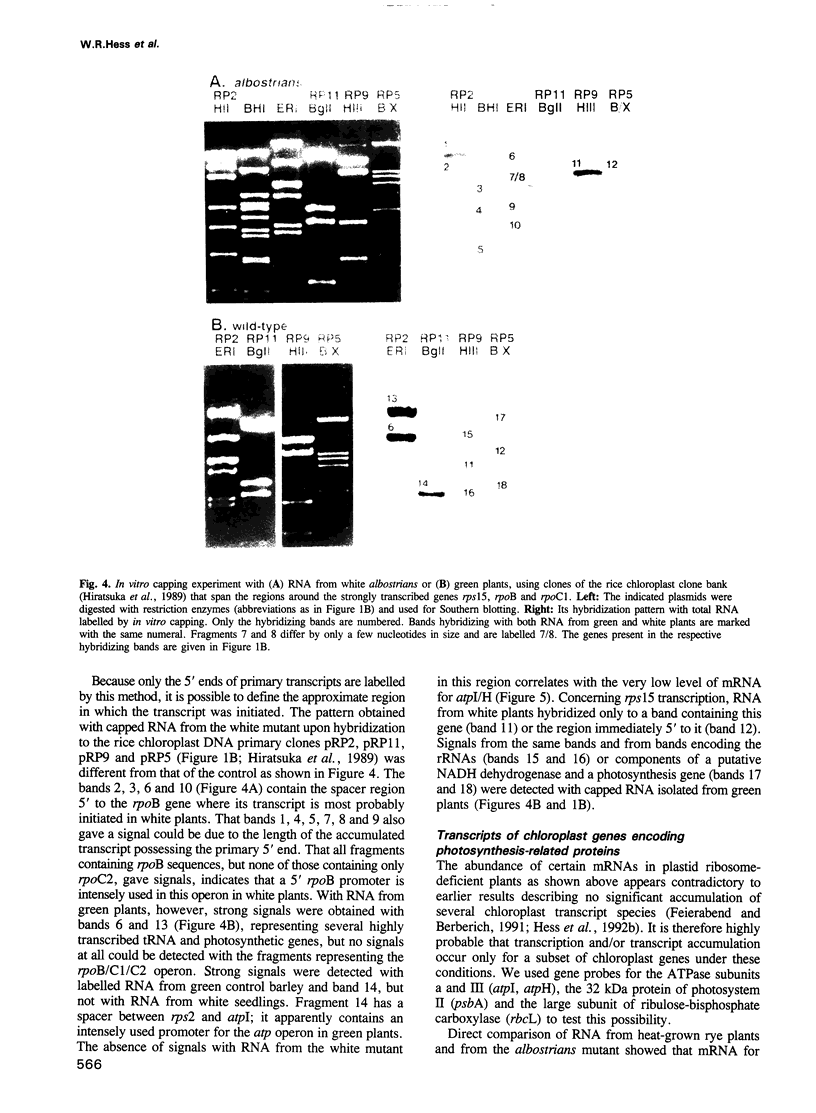
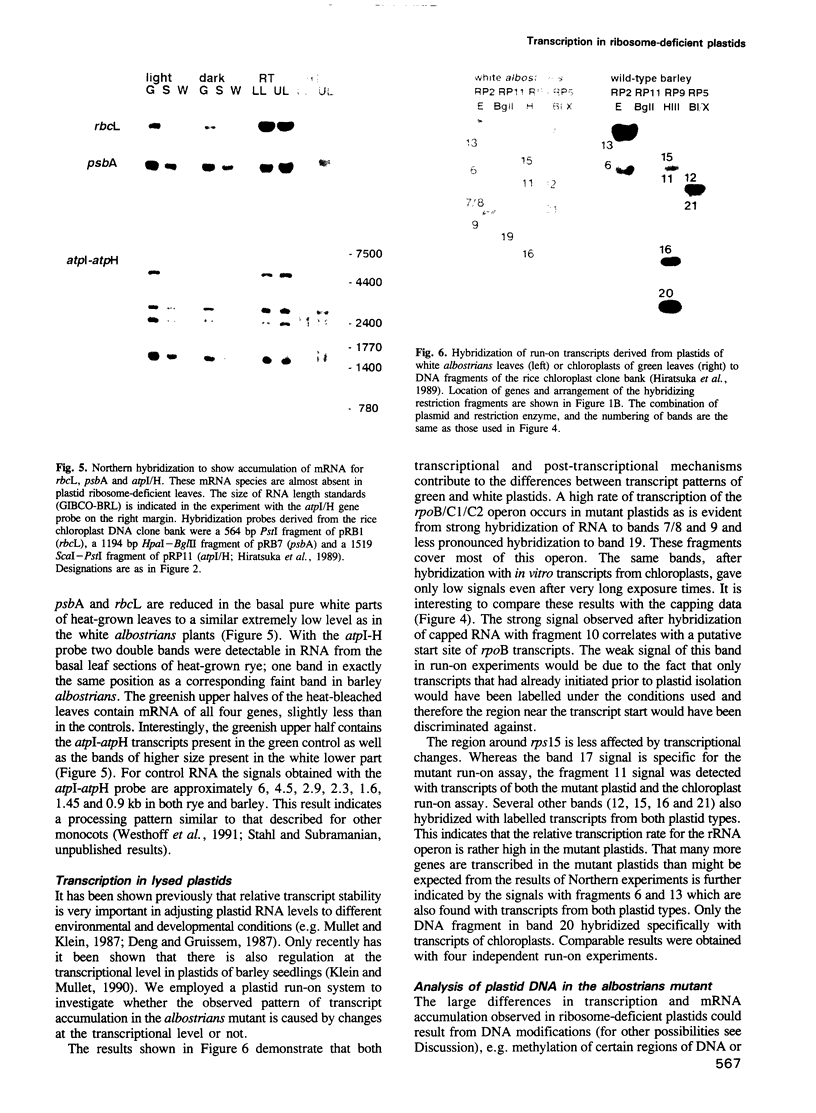
Transcription of plastid genes and transcript accumulation were investigated in white leaves of the albostrians mutant of barley (Hordeum vulgare) and in heat-bleached leaves of rye (Secale cereale) as well as in normal green leaves of both species. Cells of white leaves of the mutant and cells of heat-bleached leaves bear undifferentiated plastids lacking ribosomes and, consequently, plastid translation products, among them the subunits of a putative chloroplast RNA polymerase encoded by the plastid genes rpoA, B, C1 and C2. The following results were obtained. (i) Plastid genes are transcribed despite the lack of chloroplast gene-encoded RNA polymerase subunits. The plastid origin of these transcripts was proven. This finding provides evidence for the existence of a plastid RNA polymerase encoded entirely by nuclear genes. (ii) Transcripts of the rpo genes and of rps15, but not of genes involved in photosynthesis and related processes (psbA, rbcL, atpI-H), were abundantly accumulated in ribosome-deficient plastids. In contrast, chloroplasts accumulated transcripts of photosynthetic, but not of the rpo genes. (iii) Differences in transcript accumulation between chloroplasts and ribosome-deficient plastids are due to different relative transcription rates and different transcript stability. (iv) The observed differences in transcription are not caused by an altered pattern of methylation of plastid DNA. Thus, the prokaryotic plastid genome of higher plants is transcribed by two RNA polymerases. The observed differences in transcription between chloroplasts and undifferentiated plastids might reflect different functions of the two enzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audren H., Bisanz-Seyer C., Briat J. F., Mache R. Structure and transcription of the 5S rRNA gene from spinach chloroplasts. Curr Genet. 1987;12(4):263–269. doi: 10.1007/BF00435288. [DOI] [PubMed] [Google Scholar]
- Brown G. G., Auchincloss A. H., Covello P. S., Gray M. W., Menassa R., Singh M. Characterization of transcription initiation sites on the soybean mitochondrial genome allows identification of a transcription-associated sequence motif. Mol Gen Genet. 1991 Sep;228(3):345–355. doi: 10.1007/BF00260626. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R. Mutants lacking individual ribosomal proteins as a tool to investigate ribosomal properties. Biochimie. 1991 Jun;73(6):639–645. doi: 10.1016/0300-9084(91)90043-z. [DOI] [PubMed] [Google Scholar]
- Deng X. W., Gruissem W. Control of plastid gene expression during development: the limited role of transcriptional regulation. Cell. 1987 May 8;49(3):379–387. doi: 10.1016/0092-8674(87)90290-x. [DOI] [PubMed] [Google Scholar]
- Eisermann A., Tiller K., Link G. In vitro transcription and DNA binding characteristics of chloroplast and etioplast extracts from mustard (Sinapis alba) indicate differential usage of the psbA promoter. EMBO J. 1990 Dec;9(12):3981–3987. doi: 10.1002/j.1460-2075.1990.tb07619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B. M., Narita J. O., DeLuca-Flaherty C., Gruissem W., Rushlow K. A., Hallick R. B. Evidence for two RNA polymerase activities in Euglena gracilis chloroplasts. J Biol Chem. 1984 Dec 10;259(23):14880–14887. [PubMed] [Google Scholar]
- Herrmann R. G., Feierabend J. The presence of DNA in ribosome-deficient plastids of heat-bleached rye leaves. Eur J Biochem. 1980 Mar;104(2):603–609. doi: 10.1111/j.1432-1033.1980.tb04464.x. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Hu J., Bogorad L. Maize chloroplast RNA polymerase: the 180-, 120-, and 38-kilodalton polypeptides are encoded in chloroplast genes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1531–1535. doi: 10.1073/pnas.87.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi G. L., Meinke A., Döry I., Kössel H. Nucleotide sequence of the maize chloroplast rpo B/C1/C2 operon: comparison between the derived protein primary structures from various organisms with respect to functional domains. Mol Gen Genet. 1990 May;221(3):379–394. doi: 10.1007/BF00259403. [DOI] [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Light-induced transcription of chloroplast genes. psbA transcription is differentially enhanced in illuminated barley. J Biol Chem. 1990 Feb 5;265(4):1895–1902. [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Regulation of chloroplast-encoded chlorophyll-binding protein translation during higher plant chloroplast biogenesis. J Biol Chem. 1986 Aug 25;261(24):11138–11145. [PubMed] [Google Scholar]
- Kobayashi H., Ngernprasirtsiri J., Akazawa T. Transcriptional regulation and DNA methylation in plastids during transitional conversion of chloroplasts to chromoplasts. EMBO J. 1990 Feb;9(2):307–313. doi: 10.1002/j.1460-2075.1990.tb08112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerbs S., Bräutigam E., Parthier B. Polypeptides of DNA-dependent RNA polymerase of spinach chloroplasts: characterization by antibody-linked polymerase assay and determination of sites of synthesis. EMBO J. 1985 Jul;4(7):1661–1666. doi: 10.1002/j.1460-2075.1985.tb03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M. C., Hallick R. B. Chloroplast rpoA, rpoB, and rpoC genes specify at least three components of a chloroplast DNA-dependent RNA polymerase active in tRNA and mRNA transcription. J Biol Chem. 1988 Oct 5;263(28):14302–14307. [PubMed] [Google Scholar]
- Morden C. W., Wolfe K. H., dePamphilis C. W., Palmer J. D. Plastid translation and transcription genes in a non-photosynthetic plant: intact, missing and pseudo genes. EMBO J. 1991 Nov;10(11):3281–3288. doi: 10.1002/j.1460-2075.1991.tb04892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Klein R. R. Transcription and RNA stability are important determinants of higher plant chloroplast RNA levels. EMBO J. 1987 Jun;6(6):1571–1579. doi: 10.1002/j.1460-2075.1987.tb02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano Y., Ishikawa H., Matsuno R., Sasaki Y. Nucleotide sequence and expression of the ribosomal protein L2 gene in pea chloroplasts. Plant Mol Biol. 1991 Sep;17(3):541–545. doi: 10.1007/BF00040653. [DOI] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Kobayashi H., Akazawa T. DNA methylation as a mechanism of transcriptional regulation in nonphotosynthetic plastids in plant cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4750–4754. doi: 10.1073/pnas.85.13.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B. L., Rajasekhar V. K., Tewari K. K. Pea chloroplast DNA primase: characterization and role in initiation of replication. Plant Mol Biol. 1991 Jun;16(6):1019–1034. doi: 10.1007/BF00016074. [DOI] [PubMed] [Google Scholar]
- Nierhaus K. H. Structure, assembly, and function of ribosomes. Curr Top Microbiol Immunol. 1982;97:81–155. doi: 10.1007/978-3-642-68318-3_3. [DOI] [PubMed] [Google Scholar]
- Nugent J. M., Palmer J. D. Location, identity, amount and serial entry of chloroplast DNA sequences in crucifer mitochondrial DNAs. Curr Genet. 1988 Nov;14(5):501–509. doi: 10.1007/BF00521276. [DOI] [PubMed] [Google Scholar]
- Paulsen H., Bogorad L. Diurnal and Circadian Rhythms in the Accumulation and Synthesis of mRNA for the Light-Harvesting Chlorophyll a/b-Binding Protein in Tobacco. Plant Physiol. 1988 Dec;88(4):1104–1109. doi: 10.1104/pp.88.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E., Logsdon J. M., Jr, McGrath J. M., Stasys R. A. Fragments of plastid DNA in the nuclear genome of tomato: prevalence, chromosomal location, and possible mechanism of integration. Mol Gen Genet. 1991 Mar;225(3):453–458. doi: 10.1007/BF00261687. [DOI] [PubMed] [Google Scholar]
- Prombona A., Subramanian A. R. A new rearrangement of angiosperm chloroplast DNA in rye (Secale cereale) involving translocation and duplication of the ribosomal rpS15 gene. J Biol Chem. 1989 Nov 15;264(32):19060–19065. [PubMed] [Google Scholar]
- Purton S., Gray J. C. The plastid rpoA gene encoding a protein homologous to the bacterial RNA polymerase alpha subunit is expressed in pea chloroplasts. Mol Gen Genet. 1989 May;217(1):77–84. doi: 10.1007/BF00330945. [DOI] [PubMed] [Google Scholar]
- Rajasekhar V. K., Sun E., Meeker R., Wu B. W., Tewari K. K. Highly purified pea chloroplast RNA polymerase transcribes both rRNA and mRNA genes. Eur J Biochem. 1991 Jan 1;195(1):215–228. doi: 10.1111/j.1432-1033.1991.tb15697.x. [DOI] [PubMed] [Google Scholar]
- Ruf M., Kössel H. Structure and expression of the gene coding for the alpha-subunit of DNA-dependent RNA polymerase from the chloroplast genome of Zea mays. Nucleic Acids Res. 1988 Jul 11;16(13):5741–5754. doi: 10.1093/nar/16.13.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Nagano Y., Morioka S., Ishikawa H., Matsuno R. A chloroplast gene encoding a protein with one zinc finger. Nucleic Acids Res. 1989 Aug 11;17(15):6217–6227. doi: 10.1093/nar/17.15.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smooker P. M., Kruft V., Subramanian A. R. A ribosomal protein is encoded in the chloroplast DNA in a lower plant but in the nucleus in angiosperms. Isolation of the spinach L21 protein and cDNA clone with transit and an unusual repeat sequence. J Biol Chem. 1990 Sep 25;265(27):16699–16703. [PubMed] [Google Scholar]
- Winter U., Feierabend J. Multiple coordinate controls contribute to a balanced expression of ribulose-1,5-bisphosphate carboxylase/oxygenase subunits in rye leaves. Eur J Biochem. 1990 Jan 26;187(2):445–453. doi: 10.1111/j.1432-1033.1990.tb15324.x. [DOI] [PubMed] [Google Scholar]
- Zaitlin D., Hu J., Bogorad L. Binding and transcription of relaxed DNA templates by fractions of maize chloroplast extracts. Proc Natl Acad Sci U S A. 1989 Feb;86(3):876–880. doi: 10.1073/pnas.86.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dePamphilis C. W., Palmer J. D. Loss of photosynthetic and chlororespiratory genes from the plastid genome of a parasitic flowering plant. Nature. 1990 Nov 22;348(6299):337–339. doi: 10.1038/348337a0. [DOI] [PubMed] [Google Scholar]