ABSTRACT
The morphology and polarized growth of cells depend on pathways that control the asymmetric distribution of regulatory factors. The evolutionarily conserved Ndr kinases play important roles in cell polarity and morphogenesis in yeast and invertebrates but it is unclear whether they perform a similar function in mammalian cells. Here, we analyze the function of mammalian Ndr1 and Ndr2 (also known as STK38 or STK38L, respectively) in the establishment of polarity in neurons. We show that they act downstream of the tumor suppressor Rassf5 and upstream of the polarity protein Par3 (also known as PARD3). Rassf5 and Ndr1 or Ndr2 are required during the polarization of hippocampal neurons to prevent the formation of supernumerary axons. Mechanistically, the Ndr kinases act by phosphorylating Par3 at Ser383 to inhibit its interaction with dynein, thereby polarizing the distribution of Par3 and reinforcing axon specification. Our results identify a novel Rassf5–Ndr–Par3 signaling cascade that regulates the transport of Par3 during the establishment of neuronal polarity. Their role in neuronal polarity suggests that Ndr kinases perform a conserved function as regulators of cell polarity.
KEY WORDS: Cell polarity, Axon formation, Ndr kinases, Par
INTRODUCTION
Cell size and morphology are controlled by conserved pathways that regulate growth, cell cycle and the polarized distribution of molecules. The nuclear Dbf2-related (Ndr) kinases act as components of one of these essential pathways and are conserved from yeast to human (Hergovich et al., 2006b; Yu and Guan, 2013). Ndr kinases play important roles in cell polarity and morphogenesis in yeast (Hergovich et al., 2006b), but their function in mammalian cells is less well studied. Mammalian Ndr1 (also known as STK38) and Ndr2 (also known as STK38L) share a conserved kinase domain with Drosophila Warts and mammalian Large tumor suppressor (Lats) 1 and 2 (Hergovich et al., 2006a; Hergovich et al., 2006b; Yu and Guan, 2013). The Lats/Warts kinases are central components of the Hippo signaling pathway, which controls cell growth and organ size by promoting cell death and differentiation and inhibiting proliferation (Harvey and Tapon, 2007; Pan, 2007; Yu and Guan, 2013; Zhao et al., 2011). In the mammalian Hippo pathway, Mst1 and Mst2 (also known as STK4 and STK3, respectively), the homologs of Drosophila Hippo, together with Mob1, activate Lats1 and Lats2, which phosphorylate the transcriptional regulators YAP and TAZ. Like the Lats kinases, Ndr1 and Ndr2 are activated by interaction with Mob1 and by Mst-kinase-mediated phosphorylation at conserved residues in their hydrophobic motif (T444 and T442, respectively) (Bichsel et al., 2004; Hergovich, 2011; Hergovich et al., 2009; Stegert et al., 2005; Stegert et al., 2004; Vichalkovski et al., 2008). Less well understood is the regulation of Mst kinases themselves, which depends not only on kinases but also on scaffolding proteins like the tumor suppressor proteins Ras-associated domain family 1 (Rassf1) and Rassf5 (Calvisi et al., 2006; Harvey and Tapon, 2007; Hesson et al., 2003; Hwang et al., 2007; Khokhlatchev et al., 2002; Pan, 2007; Praskova et al., 2004; Scheel and Hofmann, 2003; Zhao et al., 2011).
Ndr1 and Ndr2 show similar substrate specificity and appear to act redundantly as suggested by the phenotype of knockout mice (Cornils et al., 2010; Hergovich et al., 2006a). The Drosophila and Caenorhabditis elegans Ndr homologs Tricornered and SAX-1 are involved in patterning dendrites (Emoto et al., 2004; Emoto et al., 2006; Gallegos and Bargmann, 2004; He et al., 2005). C. elegans SAX-1 also regulates neurite initiation but a similar function has not been reported for the Drosophila Tricornered or mammalian Ndr kinases thus far (Zallen et al., 2000). Mammalian Ndr kinases have been implicated in the regulation of centrosome duplication and in dendrite arborization and spine formation during neuronal development (Hergovich et al., 2007; Ultanir et al., 2012).
A crucial step in neuronal differentiation is the transition from an unpolarized neuron with multiple short neurites to a polarized neuron with a single axon (Barnes and Polleux, 2009). At the molecular level, this establishment of neuronal polarity involves an asymmetric distribution of polarity factors. Elucidating how this asymmetric distribution is achieved is, therefore, essential to the understanding of axon formation. Hippocampal neurons go through five stages of differentiation (Banker and Cowan, 1977; Dotti et al., 1988). Stage 2 neurons are unpolarized and have several minor neurites of similar length that potentially all can become an axon. Neuronal polarity is established during the transition from stage 2 to stage 3, when one of the neurites is selected as the axon and elongates rapidly. The remaining neurites begin to differentiate into dendrites at stage 4. Several signaling cascades that direct the establishment of neuronal polarity have been identified (Arimura and Kaibuchi, 2007). One key pathway depends on the sequential activity of phosphatidylinositol 3-kinase and the GTPases Rap1 and Cdc42 that act through the Par polarity complex (Garvalov et al., 2007; Schwamborn and Püschel, 2004). This complex is formed by Partition defective 3 (Par3, also known as PARD3), Par6 (also known as PARD6) and atypical protein kinase C (aPKC) and is required for polarity not only in neurons but also many other cell types (Arimura and Kaibuchi, 2007; Jan and Jan, 2001; Kemphues, 2000; Khazaei and Püschel, 2009; Ohno, 2001; Shi et al., 2003). The function of Par3 depends on its specific subcellular localization; it is present in all neurites at stage 2 but becomes restricted to the axon at stage 3 (Schwamborn and Püschel, 2004; Shi et al., 2003). Disruption of Par3 localization by interfering with the kinesin-2-dependent transport of Par3 causes polarity defects in hippocampal neurons, showing that the restricted localization of Par3 to the axon is essential for neuronal polarity (Funahashi et al., 2013; Nishimura et al., 2004).
Here, we investigate the function of Ndr1 and Ndr2 in axon formation and show that they act in a novel signaling pathway that is required to polarize the distribution of Par3. Rassf5 acts upstream of the Ndr kinases and stimulates their activity. The loss of Rassf5 or simultaneous suppression of Ndr1 and 2 disrupts neuronal polarity and induces the formation of supernumerary axons. Ndr1 and Ndr2 regulate the distribution of Par3 by phosphorylating it at Ser383. This phosphorylation blocks its interaction with dynein light intermediate chain 2 (Dlic2, also known as DYNC1LI2) and thereby facilitates the asymmetric localization of Par3. By polarizing the distribution of Par3, the Rassf5–Ndr pathway prevents the formation of supernumerary axons.
RESULTS
Ndr kinases are required for neuronal polarity
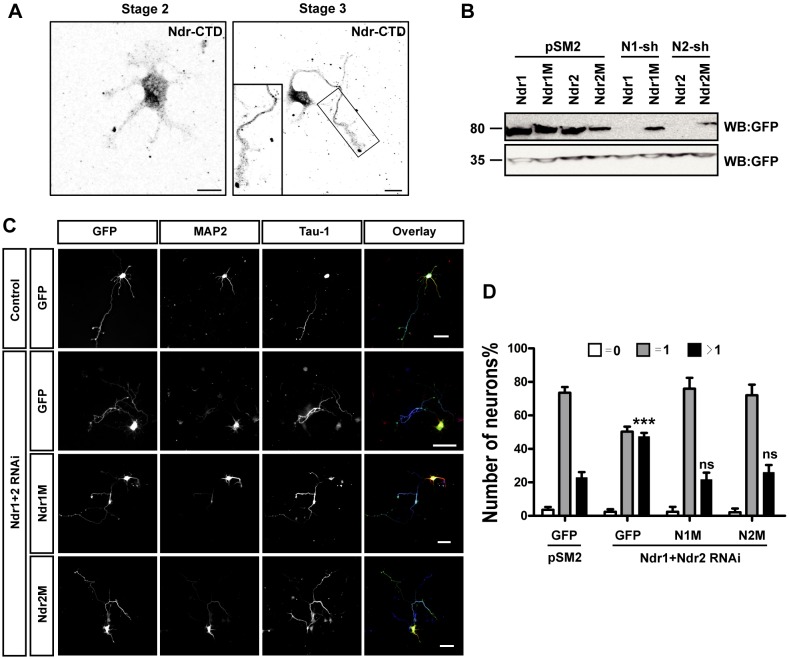
Staining with an antibody recognizing the conserved C-terminal region of both Ndr1 and Ndr2 (Hergovich et al., 2007) revealed Ndr1/2 immunoreactivity in all neurites of unpolarized neurons (Fig. 1A). After polarization, Ndr kinases were detectable mainly in the axon, and only weak signals were present in minor neurites (Fig. 1A). To analyze the function of Ndr, hippocampal neurons were transfected at 0 days in vitro (DIV) with vectors for short-hairpin RNAs (shRNAs) that efficiently suppress Ndr1 or Ndr2 (Fig. 1B). Neurons were stained with antibody against Tau-1 (also known as MAPT) as an axonal marker and with antibody against MAP2 as a dendritic marker at 3 DIV, and the effect on neuronal polarity was analyzed by quantifying the number of neurons without an axon, with a single axon or with multiple axons (Fig. 1C,D). When Ndr1 or Ndr2 were suppressed individually, no effect on the formation of axons was detected (data not shown). However, simultaneous knockdown of both Ndr1 and Ndr2 disrupted neuronal polarity and resulted in the formation of multiple Tau-1-positive axons. The percentage of neurons with a single axon was reduced from 74±2% (mean±s.e.m.) in controls to 50±2% after knockdown of both Ndr1 and Ndr2, whereas the proportion of neurons with supernumerary axons increased from 23±2% to 47±1%. This polarity defect was detectable only when neurons were transfected at 2 h after plating – transfection at a later time-point did not affect axon formation (data not shown). Co-transfection of neurons with vectors for RNAi-resistant Ndr1 or Ndr2 (Ndr1M and Ndr2M) and the shRNAs against Ndr1 and Ndr2 rescued the loss of Ndr kinases (Fig. 1C,D), confirming the specificity of the effect. Interestingly, the Ndr1/2 double knockdown phenotype was rescued by expression of only Ndr1M or Ndr2M alone, which indicates a redundant function of Ndr kinases in neuronal polarity (the percentage of the neurons forming multiple axons under each condition was as follows: control, 23±2%; Ndr1/2 shRNAs, 47±1%; Ndr1/2 shRNAs and Ndr1M, 22±3%; Ndr1/2 shRNAs and Ndr2M, 26±3%). Taken together, these results show that the Ndr kinases are required for neuronal polarity. Their loss leads to the formation of supernumerary axons, indicating that Ndr1 and Ndr2 restrict the number of axons that are extended during polarization.
Fig. 1.
Knockdown of Ndr1 and Ndr2 results in the formation of supernumerary axons. (A) Hippocampal neurons from E18 rat embryos were fixed at stage 2 and stage 3 and stained with an antibody against the Ndr-CTD. A higher magnification of the axon at stage 3 is shown in the lower-left corner. Scale bars: 10 µm. (B) HEK 293T cells were transfected with vectors for GFP, Ndr1, Ndr2 or shRNA-resistant Ndr1 (Ndr1M) or Ndr2 (Ndr2M) and shRNAs against Ndr1 (N1-sh) and Ndr2 (N2-sh) or pSM2 (control), as indicated. The expression of GFP–Ndr1 and GFP–Ndr2 was analyzed by western blotting (WB) using an anti-GFP antibody. Staining for GFP confirmed comparable transfection efficiencies and protein loading. (C) Hippocampal neurons were transfected at 0 DIV with vectors for GFP (green), shRNA-resistant Ndr1 (Ndr1M, N1M) or Ndr2 (Ndr2M, N2M) and shRNAs directed against Ndr1 and Ndr2 (Ndr1+2 RNAi). Neurons were fixed at 3 DIV and stained with the Tau-1 antibody (blue in overlay) and an anti-MAP2 antibody (red in overlay). Scale bars: 50 µm. (D) The percentage of unpolarized neurons without an axon (0, white), polarized neurons with a single axon (1, gray) and neurons with multiple axons (>1, black) is shown. Values are the mean±s.e.m. (three transfections, n>100); ns, non-significant; ***P≤0.001 compared with control (GFP+pSM2); two-way ANOVA.
Rassf5 is required for neuronal polarity and acts upstream of Ndr1/2
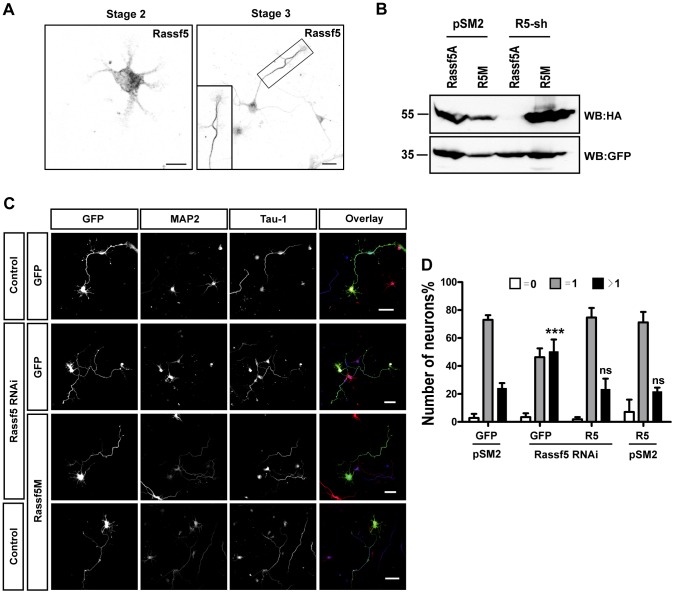
Ndr1 and 2 are regulated by different Mst kinases that act redundantly through the phosphorylation of Thr444 and 442, respectively, in the hydrophobic motif (Cornils et al., 2011b; Hergovich et al., 2009; Hesson et al., 2003; Oh et al., 2009; Song et al., 2010; Stegert et al., 2005; Vichalkovski et al., 2008; Zhou et al., 2009). To circumvent this redundancy directly upstream of Ndr1 and Ndr2, we targeted a known regulator of Mst kinases, the tumor suppressor Rassf5 (Hesson et al., 2003; Oh et al., 2009; Song et al., 2010; Zhou et al., 2009). Rassf5 is present throughout unpolarized neurons at stage 2 but was mainly detectable in the axon of stage 3 neurons (Fig. 2A). After transfection with a vector expressing Rassf5 shRNA (Fig. 2B), the number of neurons with multiple axons increased from 24±2% (mean±s.e.m.) in the control to 50±4% (Fig. 2C,D). The phenotype of the Rassf5 knockdown could be rescued by the coexpression of an RNAi-resistant version of Rassf5 (Rassf5M), which reduced the number of neurons with multiple axons to 23±4% (Fig. 2C,D). Thus, Rassf5 is required for neuronal polarity and its loss results in a phenotype that is similar to that of the Ndr1/2 double knockdown.
Fig. 2.
Rassf5 is required for neuronal polarity. (A) Neurons from the hippocampus of E18 rat embryos were fixed at stage 2 and stage 3, and stained with the anti-Rassf5 antibody. A higher magnification of the axon at stage 3 is shown in the lower-left corner. Scale bars: 10 µm. (B) HEK 293T cells were transfected with vectors for GFP, HA–Rassf5A, shRNA-resistant HA–Rassf5A (R5M) and an shRNA against Rassf5 (R5-sh) or pSM2 (control), as indicated. The expression of Rassf5A was analyzed by western blotting (WB) using an anti-HA antibody. Staining for GFP confirmed comparable transfection efficiencies and protein loading. (C) Hippocampal neurons were transfected at 0 DIV with vectors for GFP (green), shRNA-resistant Rassf5A (R5M) and an shRNA against Rassf5 or pSM2 (control). Neurons were fixed at 3 DIV and stained with the Tau-1 antibody (blue in overlay) and an anti-MAP2 antibody (red in overlay). Scale bars: 50 µm. (D) The percentage of unpolarized neurons without an axon (0, white), polarized neurons with a single axon (1, gray) and neurons with multiple axons (>1, black) is shown. Values are the mean±s.e.m. (three transfections, n>150); ns, non-significant; ***P≤0.001 compared with control (GFP+pSM2); two-way ANOVA.
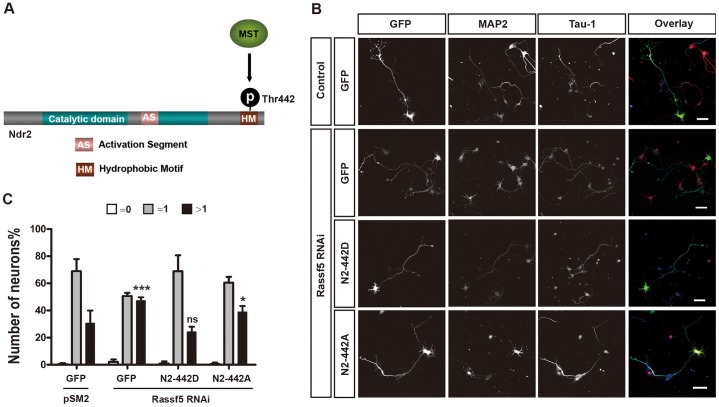
To confirm that Rassf5 and Ndr1/2 act in the same pathway to regulate neuronal polarity, we tested the ability of an Ndr2 mutant (Ndr2-T442D) that mimics phosphorylation by Mst kinases (Fig. 3A) to rescue the knockdown of Rassf5 (Fig. 3B,C). After suppression of Rassf5, 47±1% of the neurons formed multiple axons compared to 31±5% of control neurons. Expression of the phospho-mimic mutant Ndr2-T442D was sufficient to rescue the loss of Rassf5 and reduced the number of neurons with multiple axons (24±2%) to a level that was comparable to that of the control. The non-phosphorylatable mutant Ndr2-T442A did not rescue the loss of Rassf5, as the number of neurons with multiple axons (39±3%) was not significantly different from that of the Rassf5 knockdown. Thus, Rassf5 acts as an upstream regulator of Ndr2 in a novel pathway involved in neuronal polarity by stimulating the phosphorylation of Ndr2 at T442.
Fig. 3.
Ndr2-T442D is sufficient to rescue the suppression of Rassf5. (A) Schematic representation of Ndr2. Mst kinases phosphorylate Ndr2 at Thr442 (p) and activate its kinase activity. (B) Hippocampal neurons were transfected at 0 DIV with vectors for GFP (green), phospho-mimic Ndr2-Thr442 (N2-442D), non-phosphorylatable Ndr2-Thr442 (N2-442A) and an shRNA against Rassf5 or pSM2 (control), as indicated. Neurons were fixed at 3 DIV and stained with the Tau-1 antibody (blue) and an anti-MAP2 antibody (red). Scale bars: 50 µm. (C) The percentage of unpolarized neurons without an axon (0, white), polarized neurons with a single axon (1, gray) and neurons with multiple axons (>1, black) is shown. Values are the mean±s.e.m. (three transfections, n>150 neurons); ns, non-significant; *P<0.05; ***P≤0.001, compared with control (GFP+pSM2); two-way ANOVA.
Knockdown of Rassf5 blocks neuronal polarization in cortical slices
To investigate the function of Rassf5 in neuronal polarity in vivo, we analyzed Rassf5-knockout mice (Park et al., 2010). Rassf5−/− mice are viable (Park et al., 2010) and do not show any obvious defects in the development of cortical layers or hippocampal structures at embryonic day (E)17 (data not shown). Staining with an anti-neurofilament antibody did not show defects in axon formation. However, supernumerary axons might not be stable in the developing nervous system, and their formation could be difficult to detect by staining all axons. Therefore, we isolated hippocampal neurons from E17 Rassf5+/− and Rassf5−/− embryos and analyzed them at 3 DIV (supplementary material Fig. S1). We found that 41±2% (mean±s.e.m.) of the neurons from Rassf5−/− embryos formed multiple axons compared with 13±3% in cultures from Rassf5+/− brains, confirming the role of Rassf5 in axon formation.
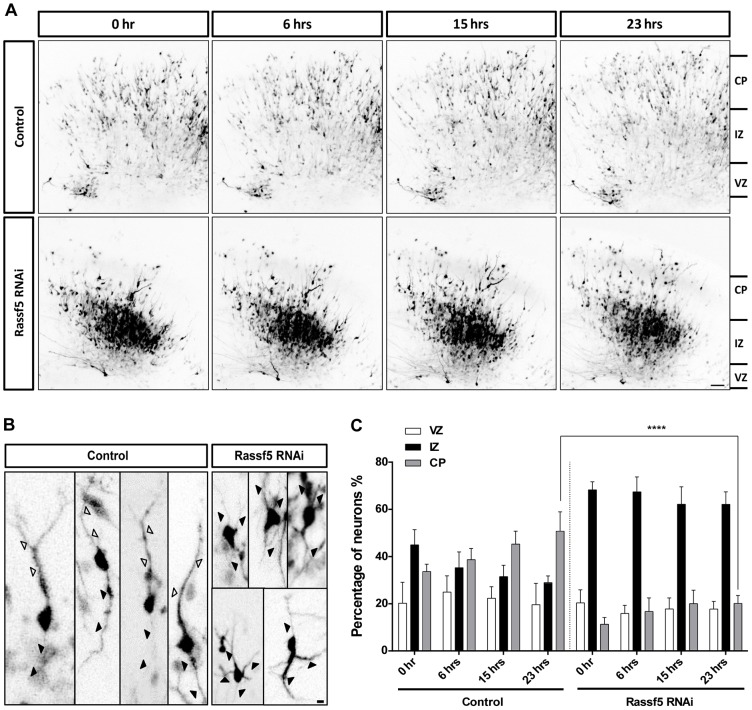
To test whether Rassf5 is required for neuronal polarization in the developing brain, we performed ex vivo electroporation of E14.5 embryonic brains and analyzed neuronal polarization by live-cell imaging of slice cultures at 36 h after transfection (Fig. 4). Cortical neurons arise in the ventricular zone from radial glial cells (RGCs) and leave the ventricular zone to generate the cortical plate (Barnes and Polleux, 2009). During their migration, newborn neurons first assume a multipolar morphology in the subventricular zone (SVZ) and intermediate zone. Subsequently, they become bipolar by extending a leading process that is required for radial migration and a trailing process that extends as the axon in the intermediate zone. To investigate the phenotype of Rassf5-knockdown neurons by live-cell imaging, the cortex of wild-type embryos was transfected with a vector that expresses both the shRNA against Rassf5 and GFP (Fig. 4). RGCs transfected by ex vivo electroporation gave rise to GFP-positive neurons that became bipolar and initiated their radial migration in control cultures (Fig. 4A,B). At 36 h after the transfection, 20±5% of the GFP-positive cells were present in the ventricular zone or SVZ, 45±4% in the intermediate zone and 34±2% had reached the cortical plate in the control slices (Fig. 4C). Over a period of 23 h, a large number of bipolar neurons migrated into the cortical plate (supplementary material Movies 1, 2; Fig. 4A,B). At the end of the imaging period, the percentage of neurons in the cortical plate had increased to 50±1% (Fig. 4C). By contrast, the migration from the intermediate zone into the cortical plate was blocked after transfection of the knockdown construct. The number of GFP-positive cells present in the ventricular zone or SVZ (20±3%) was comparable to that observed in controls, but 68±2% of the cells were found in the intermediate zone and only 11±2% had reached the cortical plate. This distribution did not change significantly during the imaging period. After 23 h, 62±3% of the transfected neurons remained in the intermediate zone and only 20±2% were present in the cortical plate. Many neurons in the intermediate zone extended multiple thin processes after knockdown of Rassf5, which might explain why they were unable to migrate (Fig. 4B). The morphology of these neurons indicates that they remain in the multipolar phase after knockdown of Rassf5. Taken together, these results suggest that Rassf5 is required in cortical neurons for the transition from a multipolar to a bipolar morphology. Suppression of Rassf5 blocks the formation of a single leading process and a single trailing axon.
Fig. 4.
The knockdown of Rassf5 interferes with the polarization of neurons. (A–C) E14.5 brains were transfected by ex vivo electroporation with empty pCAGGS-U6 (control) or pCAGGS-U6-Rassf5 with an shRNA against Rassf5 (Rassf5 RNAi), and live-cell imaging of slice cultures was performed at 36 h after electroporation. (A) Representative images (maximum-intensity projection) of cortical slices at the indicated time-points are shown. CP, cortical plate; IZ, intermediate zone; VZ, ventricular zone. Scale bars: 50 µm. (B) Representative images (maximum-intensity projection) of transfected neurons after 23 h of live-cell imaging from control and Rassf5-knockdown slices are shown. Open arrowheads mark leading processes, black arrowheads mark trailing axons or processes of unpolarized neurons after knockdown of Rassf5. Scale bars: 10 µm. (C) The percentage of GFP-positive cells in the ventricular and subventricular zone (VZ), intermediate zone and cortical plate at 0, 6, 12 and 23 h is shown. Values are the mean±s.e.m. (three experiments, n = 200–400 neurons per time-point); ****P<0.0001 compared with control; two-way ANOVA.
Ndr2 phosphorylates Par3 at Ser383 in hippocampal neurons
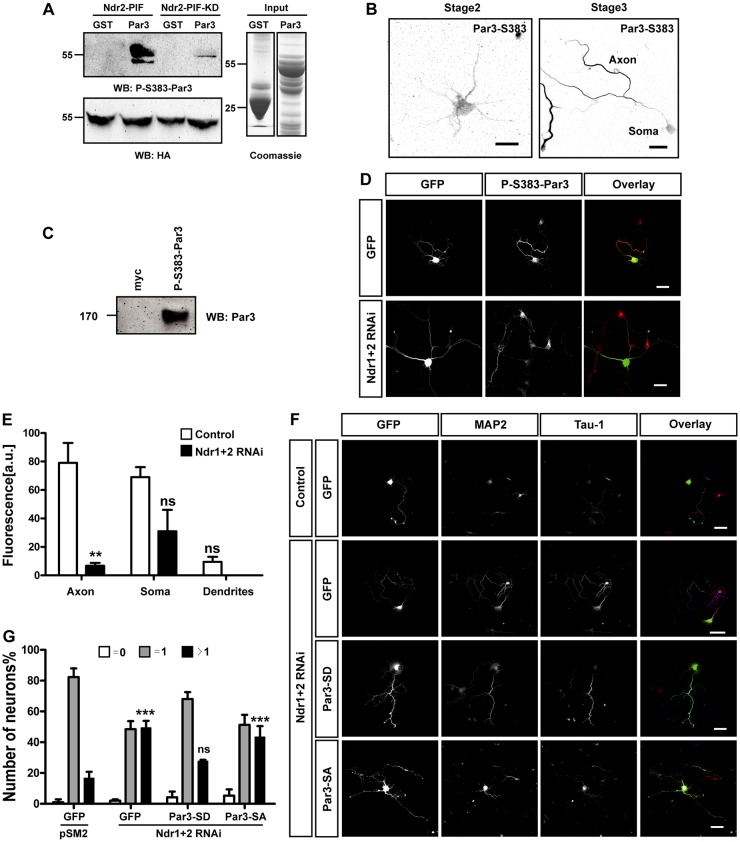
To elucidate how Ndr kinases regulate axon formation, we tested their interaction with proteins that are required for neuronal polarity, and we identified Par3 as an interaction partner of Ndr1 and Ndr2 (data not shown). GST pulldown assays with individual Par3 domains and GFP–Ndr1 or GFP–Ndr2 coexpressed with Mob1 and Mst1 in human embryonic kidney (HEK) 293T cells revealed that the CR domain, the PDZ1 domain and the C-terminus (amino acids 1116–1356) of Par3 act as binding sites for both kinases (supplementary material Fig. S2A,B). The same result was obtained when using bacterially expressed GST–Ndr1, GST–Ndr2 and GFP-fusion proteins for different Par3 domains expressed in HEK 293T cells, indicating that the interaction of Ndr kinases and Par3 is independent of Mob1 and Mst1 coexpression (supplementary material Fig. S2C). Mass spectrometry analysis of Par3 that was phosphorylated by Ndr2 using an in vitro kinase assay resulted in the identification of two phosphorylation sites in Par3 – Ser383 (SPGRFpSPD) in the PDZ1 domain and Ser1196 (SGRHpSVS) at the C-terminus.
To confirm the phosphorylation of Par3 by Ndr kinases, we generated an antibody that detects phosphorylation at Ser383. Attempts to obtain an antibody specific for phosphorylation of Ser1196 were not successful. Our further analysis showed that Ser383 is the main Ndr2 phosphorylation site that is essential for Par3 function in neuronal polarity (see below). The specificity of the antibody against phosphorylated (phospho)-Ser383-Par3 was confirmed in a heterologous system and in neurons. The phospho-specific antibody confirmed that Par3 is phosphorylated by Ndr2 in an in vitro phosphorylation assay using bacterially expressed GST–Par3-PDZ1/2 and constitutively active NDR2-PIF or a kinase-dead NDR2-PIF mutant (NDR2-PIF-KD) (Stegert et al., 2004) that were purified from HEK 293T cells (Fig. 5A). The anti-phospho-Ser383-Par3 antibody clearly detected the phosphorylation of GST–Par3-PDZ1/2 by Ndr2-PIF at Ser383, whereas there was only a weak signal detectable with Ndr2-PIF-KD. Par3 phosphorylation was also observed when GFP–Ndr2, Mob1, Mst1 and Myc–Par3 were expressed in HEK 293T cells and Par3 was precipitated using the anti-phospho-S383-Par3 antibody (data not shown). Strong signals for Par3 phosphorylation were detected for Myc–Par3 but not when non-phosphorylatable Myc–Par3-S383A was used as a control, confirming the specificity of the antibody for phosphorylated Par3.
Fig. 5.
Ndr kinases phosphorylate Par3 at Ser383. (A) Bacterially expressed GST or GST–Par3-PDZ1 (Par3) were purified (input, Coomassie Blue staining) and incubated with HA–Ndr2-PIF or HA–Ndr2-PIF-KD isolated from HEK 293T cells for an in vitro phosphorylation assay. Par3 phosphorylation was analyzed by western blotting (WB) using antibody against phosphorylated (P)-Ser383-Par3 antibody (upper panel). Blotting for HA confirmed comparable expression of Ndr2 constructs (lower panel). Numbers indicate the molecular mass in kDa. (B) Hippocampal neurons were stained with the anti-phospho-Ser383-Par3 antibody at stage 2 (unpolarized neuron, scale bar: 20 µm) and stage 3 (polarized neuron, scale bar: 50 µm). (C) Par3 was immunoprecipitated from lysates of E18 rat brain using the anti-phospho-S383-Par3 or an anti-Myc antibody as negative control, and the precipitation of phosphorylated protein was detected with an anti-Par3 antibody. (D) Hippocampal neurons were transfected at 0 DIV with vectors for GFP (green in overlay) and pSM2 (control), and shRNAs for Ndr1 and 2 (Ndr1+2 RNAi), fixed at 3 DIV and stained with the anti-phospho-Ser-383Par3 antibody (red in overlay). Scale bars: 50 µm. <@?show=[to]?>(E) The intensity of the immunofluorescence signal in the axon, soma and dendrites for staining with the anti-phospho-Ser383-Par3 antibody was quantified in arbitrary units (a.u.; control, white bars; Ndr1+2 RNAi, black bars). Values are the mean±s.e.m. (n>10); ns, non-significant; **P≤0.01 compared with control; two-way ANOVA. (F) Hippocampal neurons were transfected at 0 DIV with vectors for GFP (green), phospho-mimic Par3-S383,1196D (Par3-SD) or non-phosphorylatable Par3-S383,1196A (Par3-SA) and shRNAs directed against Ndr1 and Ndr2 (Ndr1+2 RNAi) or pSM2 (control), as indicated. Neurons were fixed at 3 DIV and stained with the Tau-1 antibody (blue in overlay) and an anti-MAP2 antibody (red in overlay). Scale bars: 50 µm. (G) The percentage of unpolarized neurons without an axon (0, white), polarized neurons with a single axon (1, gray) and neurons with multiple axons (>1, black) is shown. Values are the mean±s.e.m. (three transfections, n>180 neurons); ns, non-significant; ***P≤0.001 compared with the control (GFP+pSM2); two-way ANOVA.
The anti-phospho-Ser383-Par3 antibody revealed a uniform staining of the soma and dendrites in unpolarized neurons at stage 2 (Fig. 5B). Moreover, endogenous phospho-S383-Par3 could also be detected in lysates from E18 rat brain using the anti-phospho-S383-Par3 antibody, which indicates that Par3 is phosphorylated by Ndr kinases in the brain (Fig. 5C). At stage 3, a strong accumulation of phospho-Ser383-Par3 could be detected in the axon. After knockdown of Ndr1 and Ndr2, staining for Par3 phosphorylated at Ser383 was significantly reduced in the axon [control, 80±14 arbitrary units (a.u.); Ndr1/2 double knockdown, 7±2 a.u.] (Fig. 5D,E). When Par3 was suppressed by an shRNA, the signal for phospho-S383 Par3 was also almost completely lost (data not shown). Taken together, these results show that Par3 is phosphorylated at Ser383 by Ndr kinases specifically in the axon.
The phosphorylation of Par3 by Ndr1/2 is required for neuronal polarity
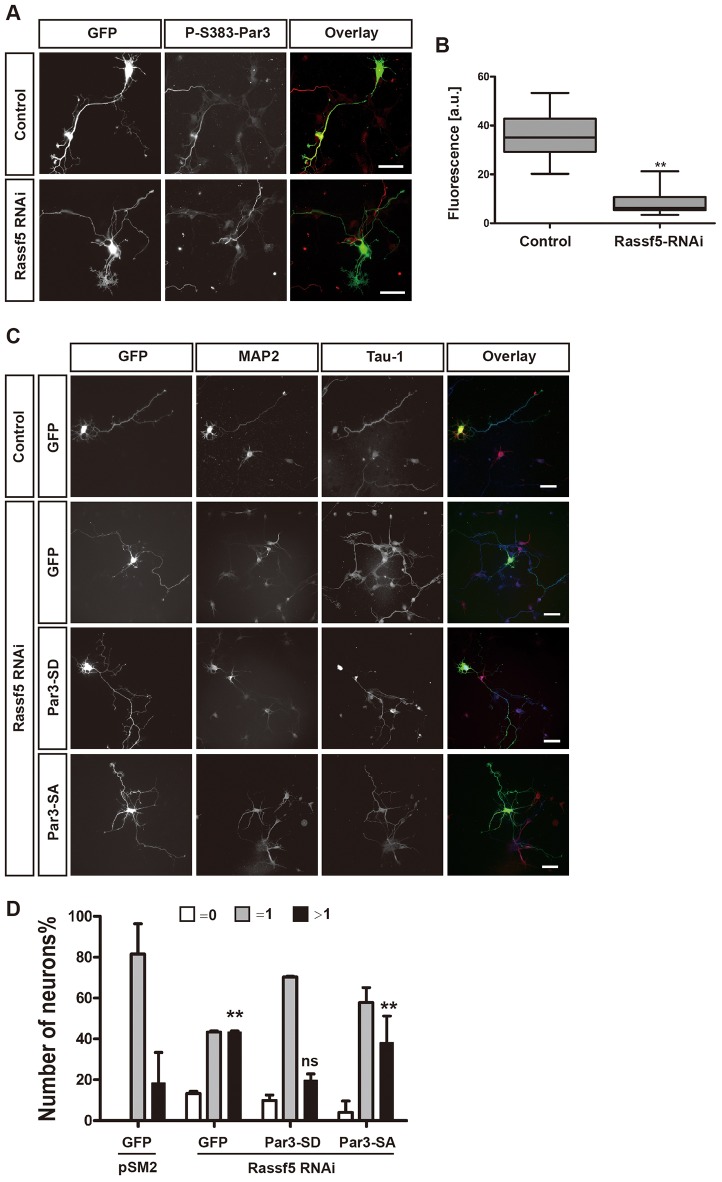
To address the function of Par3 phosphorylation, non-phosphorylatable (Par3-S383,1196A) and phospho-mimicking mutants (Par3-S383,1196D) were used to test their ability to rescue the loss of Ndr1 and Ndr2 (Fig. 5F). We found that 50±3% of the neurons formed multiple axons after double knockdown of Ndr1/2. This number was reduced to 28±1% when Par3-S383,1196D was coexpressed with the Ndr1/2 shRNAs. However, there was no significant change in the percentage of neurons with multiple axons when Par3-S383,1196A was coexpressed (44±4%) (Fig. 5G). These results show that the Par3 phospho-mimic is sufficient to rescue the loss of Ndr kinases and that the phosphorylation of Par3 by Ndr kinases is required for the establishment of neuronal polarity. Because Rassf5 regulates the activity of Ndr kinases in neurons, we investigated whether Ser383 phosphorylation also depends on Rassf5. We stained neurons after the knockdown of Rassf5 with the anti-phospho-Ser383-Par3 antibody to confirm that the loss of Rassf5 results in a reduction in the amount of Par3 phosphorylation. In control neurons, Par3 phosphorylation at Ser383 was observed in the distal axon (intensity, 36±4 a.u.). After suppression of Rassf5, phospho-Ser383-Par3 immunoreactivity was strongly reduced (9±2 a.u.) (Fig. 6A,B). This result confirmed that Par3 phosphorylation depends on Rassf5.
Fig. 6.
The phospho-mimic Par3-S383,1196D is sufficient to rescue the effects of Rassf5 knockdown. (A) Hippocampal neurons were transfected at 0 DIV with vectors for GFP (green) and an shRNA against Rassf5 (Rassf5 RNAi) or pSM2 (control). Neurons were fixed at 3 DIV and stained with antibody against phosphorylated (P)-Ser-383Par3 antibody (red in overlay). (B) The intensity of the immunofluorescence for staining with the anti-phospho-Ser383-Par3 antibody was quantified in arbitrary units (a.u.). Boxes show the median (midline), 25th percentile (control, 29.22; Rassf5-shRNA, 5) and 75th percentile (control, 43; Rassf5-shRNA, 11). Whiskers show the minimum and maximum (control, 20 to 53; Rassf5-shRNA, 3 to 21). n = 7; **P≤0.01 compared with control (Student's t-test). (C) Hippocampal neurons were transfected at 0 DIV with vectors for GFP (green), Par3-S383,1196A (Par3-SA) or Par3-S383,1196D (Par3-SD) and an shRNA against Rassf5 or pSM2 (control), as indicated. Neurons were stained at 3 DIV with the Tau-1 antibody (blue in overlay) and an anti-MAP2 antibody (red in overlay). Scale bars: 50 µm. (D) The percentage of unpolarized neurons without an axon (0, white), polarized neurons with a single axon (1, gray) and neurons with multiple axons (>1, black) is shown. Values are the mean±s.e.m. (three transfections, n>180 neurons); ns, non-significant; **P≤0.01 compared with the control (GFP+pSM2); two-way ANOVA.
Next, we tested whether expression of Par3-S383,1196D is able to rescue the loss of Rassf5. Hippocampal neurons were co-transfected with vectors for the Rassf5 shRNA and the pseudophosphorylated Par3-S383,1196D at 0 DIV and were analyzed at 3 DIV (Fig. 6C,D). After knockdown of Rassf5, 43±1% of the neurons formed multiple axons compared with 15±7% of control neurons. The expression of the phospho-mimic Par3-S383,1196D was sufficient to rescue the Rassf5-knockdown phenotype (19±2% of neurons formed multiple axons). By contrast, the expression of Par3-S383,1196A did not rescue the polarity defect caused by the Rassf5 knockdown (38±9% of neurons formed multiple axons). These results confirmed that Rassf5 acts upstream of the Ndr kinases and Par3 to regulate neuronal polarity. Taken together, these experiments strongly suggest that phosphorylation of Par3 downstream of Rassf5–Ndr signaling is essential for the establishment of neuronal polarity.
The phosphorylation of Par3 by Ndr1/2 changes its distribution
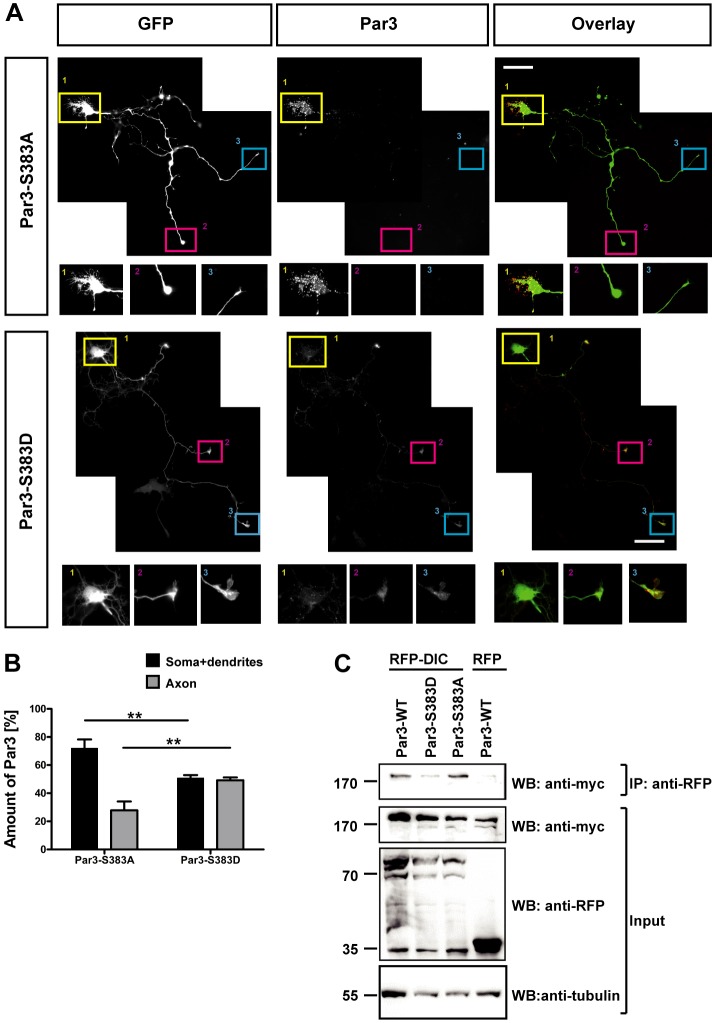
The localization of Par3 to the distal axon is important for its function in neuronal polarity (Shi et al., 2003). To investigate whether Par3 phosphorylation by Ndr kinases affects its subcellular localization, the phospho-mimic Myc–Par3-Ser383D or non-phosphorylatable Myc-Par3-Ser383A were expressed in hippocampal neurons. Neurons were fixed at different stages and stained with an anti-Myc antibody (Fig. 7A; supplementary material Fig. S3). In unpolarized stage 2 neurons, both the non-phosphorylatable and the phospho-mimic Par3 mutants were detected throughout all neurites (supplementary material Fig. S3). At the transition to stage 3, when one of the neurites is slightly longer than the others, Par3-Ser383A was present in all neurites, including the longest one, which presumably is the future axon. By contrast, Par3-Ser383D displayed a higher accumulation in the longest neurite. After the establishment of polarity, a clear difference in the distribution of Par3-S383A and Par3-S383D was observed (Fig. 7A). Par3-S383D was abundant in the axon, including the tip. Quantification of the staining intensity showed that 49±1% of the total signal for Par3-S383D was present in the axon. By contrast, the majority of Par3-S383A was present in the dendrites and soma (72±4% of the Par3-S383A signal) and much less was detected in the axon (29±4%) (Fig. 7B). The distribution of Par3-S1196A and Par3-S1196D did not show any difference between axons and minor neurites (data not shown). Thus, the phosphorylation of Par3 at Ser383 is required for its axonal localization. The Par3-S383D phospho-mimic preferentially localizes to the axon, whereas the non-phosphorylatable mutant is localized mainly to the soma and dendrites.
Fig. 7.
Par3-S383A and Par3-S383D show different patterns of subcellular localization. (A) Hippocampal neurons were transfected at 0 DIV with vectors encoding GFP and Myc–Par3-S383A or Myc–Par3-S383D. Neurons were fixed at 3 DIV and stained with an anti-Myc antibody (Par3). A higher magnification of the areas marked by squares is shown below each image. Scale bars: 50 µm. (B) The relative intensity of the immunofluorescence in the axon (gray bars) or soma and dendrites (black bars) for staining with the anti-phospho-Ser383-Par3 antibody was quantified as the percentage of the total signal for phospho-Ser383-Par3. Values are the mean±s.e.m. (n = 6); **P<0.01 between indicated pairs; two-way ANOVA. (C) RFP–Dlic2 (DIC) or RFP (negative control) were coexpressed with Myc-tagged wild-type (WT) Par3, phospho-mimic Par3-S383D or non-phosphorylatable Par3-S383A in HEK 293T cells. Dlic2 was immunoprecipitated (IP) from cell lysates with an anti-RFP antibody, and the bound proteins were detected by western blotting (WB) using an anti-Myc antibody. Blotting for RFP, Myc and tubulin (input) confirmed that comparable amounts of proteins were expressed and loaded. Numbers indicate the molecular mass in kDa.
To elucidate how Ser383 phosphorylation affects the distribution of Par3, we analyzed the interaction between Par3 and the kinesin-2 subunit KIF3A or the dynein subunit Dlic2, two motor proteins that interact with Par3 (Nishimura et al., 2004; Schmoranzer et al., 2009). While there was no significant difference in the binding of Par3-S383A and Par3-S383D to KIF3A (data not shown), the mutation of Ser383 clearly affected the interaction of Par3 with Dlic2 (Fig. 7C), which could play a role in the retrograde transport of Par3. Myc-tagged Par3, Par3-S383A and Par3-S383D were coexpressed with RFP-tagged Dlic2 in HEK 239T cells, and their interaction was analyzed by immunoprecipitation using an anti-RFP antibody. Wild-type Par3 and Par3-S383A showed a strong interaction as reported previously (Schmoranzer et al., 2009), but the binding of Par3-S383D to Dlic2 was almost completely abolished, which suggests that Par3 phosphorylation at Ser383 disrupts the interaction between Par3 and dynein. Therefore, a defect in dynein-mediated retrograde transport of Par3 might cause the aberrant distribution of pseudophosphorylated Par3. Taken together, these results show that the normal distribution of Par3 depends on its phosphorylation by Ndr kinases, which affects its interaction with dynein.
DISCUSSION
A novel pathway regulates Par3 localization and neuronal polarity
In this study, we identify a novel signaling cascade that regulates the establishment of neuronal polarity through Par3. In this Rassf5–Ndr–Par3 pathway, Rassf5 acts upstream of Ndr1 and Ndr2 to stimulate their kinase activity. Activated Ndr kinases then phosphorylate Par3 to polarize its distribution to the axon. Thus, they promote the establishment of neuronal polarity and prevent minor neurites from becoming axons. Inhibition of this pathway results in the formation of supernumerary axons. This function appears to be conserved, as neurons form ectopic neurites in mutants of the C. elegans Ndr homolog SAX-1 (Zallen et al., 2000).
Our results show that the loss of Rassf5 or the simultaneous knockdown of Ndr1 and Ndr2 in hippocampal neurons both result in the disruption of neuronal polarity and the formation of multiple axons. The analysis of a knockdown of Rassf5 in cortical slices confirms its function in neuronal polarity. An Ndr2 mutant mimicking phosphorylation by Mst kinases is sufficient to rescue the loss of Rassf5, indicating that Rassf5 acts indirectly to stimulate Ndr kinase activity. Finally, we identify Par3 as a novel target for the Ndr kinases, which phosphorylate Par3 at Ser383 in vitro and in neurons. This phosphorylation depends on Rassf5 and Ndr1/2, because the knockdown of Rassf5 or Ndr1/2 results in the loss of Par3 Ser383 phosphorylation. The functional relevance of this phosphorylation is confirmed by the ability of a Par3 construct mimicking phosphorylation by Ndr1/2 to rescue the knockdown of Ndr1/2 or Rassf5.
Par3 phosphorylated at Ser383 is strongly enriched in the axon, and this phosphorylation is required for its axonal localization. The phospho-mimic Par3-Ser383D reproduces the enrichment in the axon, whereas the non-phosphorylatable Par3-Ser383A mutant preferentially localizes to the soma and dendrites. The aberrant localization of non-phosphorylatable Par3-Ser383A in stage 3 neurons can be explained by its unregulated interaction with the dynein subunit Dlic2. Failure to block the binding of Par3-Ser383A to dynein results in the continuing retrograde transport of Par3 in the axon. Taken together, our data support the model that Rassf5 activates Ndr kinases, which, in turn, phosphorylate Par3 on Ser383 to regulate the Par3–Dlic2 interaction and polarize Par3 distribution.
Rassf5 activates Ndr kinases
Our analysis of the Rassf5–Ndr–Par3 pathway identifies a novel function of Ndr kinases that act redundantly at an early stage of neuronal differentiation to promote the establishment of neuronal polarity. The Ndr kinases appear to target intracellular transport processes; they regulate the interaction of Par3 with Dlic2, as reported here, and are also known to phosphorylate Aak1 and Rabin8 (also known as RAB3IP), two proteins involved in regulating trafficking in neurons (Chiba et al., 2013; Ultanir et al., 2012). This distinguishes Ndr kinases from the closely related Lats kinases that regulate cell growth and organ size at the transcriptional level through YAP and TAZ as central components of the Hippo pathway (Hergovich et al., 2006a; Hergovich et al., 2006b; Yu and Guan, 2013).
Here, we identify Rassf5 as a new upstream activator of Ndr kinases in neurons. The function of Rassf5 in neuronal polarity is confirmed by the knockdown of Rassf5 in cortical slices by ex vivo electroporation. Suppression of Rassf5 resulted in the arrest of neurons in the intermediate zone. Instead of forming a single leading process and a single trailing axon, neurons displayed multiple thin processes and were not able to migrate. The analysis of Rassf5-knockout mice did not reveal major defects in cortical organization, probably because a constitutive knockout throughout development allows sufficient time for compensatory changes to overcome the loss of Rassf5. This might involve one or several of the ten known Rassf proteins (Sherwood et al., 2010).
The Ndr2-T442D mutant that mimics phosphorylation by Mst kinases is sufficient to rescue the loss of Rassf5, whereas Ndr2-T442A is not able to restore normal polarity. Rassf5 has been shown to stimulate the activity of Mst kinases, suggesting that it most likely regulates Ndr1/2 indirectly by activating the Mst kinases during neuronal polarization (Khokhlatchev et al., 2002; Praskova et al., 2004). However, given that at least three different Mst kinases can function upstream of Ndr (Cornils et al., 2011b; Hergovich et al., 2009; Stegert et al., 2005; Stegert et al., 2004; Vichalkovski et al., 2008), future research is warranted to dissect the role of Rassf5 in more detail. One member of the Mst kinase family, Mst3 (also known as STK24), has been shown to regulate axon growth of cortical neurons and to mediate the growth-promoting effects of trophic factors during regeneration (Lorber et al., 2009). It will be interesting to test whether these functions also depend on the Ndr kinases, and whether Mst3 regulates Ndr in these settings.
Rassf5 has also been shown to bind to microtubules (Moshnikova et al., 2006; Moshnikova et al., 2008; van der Weyden et al., 2005). This suggests the interesting possibility that the activity of the Rassf5–Ndr pathway is regulated by changes in the organization of the neuronal cytoskeleton. Because the dynamics of microtubules differ in axons and dendrites, it is tempting to speculate that the microtubule-binding protein Rassf5 links the activity of Ndr kinases to specific aspects of microtubule dynamics and thereby preferentially activates these kinases in the axon (Witte et al., 2008).
Par3 is a novel substrate of Ndr kinases
So far, only few substrates for the Ndr kinases have been identified. They phosphorylate p21 to control the cell cycle, and Aak1 and Rabin mediate their function in dendrite arborization and spine development (Cornils et al., 2011a; Ultanir et al., 2012). We identified two phosphorylation sites in Par3 for Ndr kinases (Ser383 and Ser1196) using kinase assays and mass spectrometry. The Ndr phosphorylation sites in Par3 are novel and distinct from the sites for Aurora A, ROCK and Erk2 that regulate its function in neuronal polarity (Chen et al., 2006; Funahashi et al., 2013; Khazaei and Püschel, 2009; Nakayama et al., 2008). By using a phospho-specific antibody, we confirmed Ser383 as the major regulatory site involved in neuronal polarization but we did not observe a role for Ser1196 phosphorylation in Par3 localization. Par3 phosphorylated at Ser383 is highly concentrated in the distal axon and largely absent from dendrites, which is consistent with the function of Par3 in axon specification. Ser383 phosphorylation of Par3 depends on Ndr1/2 and Rassf5, and only the Ser383 Par3 phospho-mimic is sufficient to rescue the loss of Ndr kinases or the loss of Rassf5, whereas the non-phosphorylatable mutant did not show rescuing activity. This confirms the functional importance of Par3 phosphorylation by Ndr kinases for the normal establishment of neuronal polarity.
The Par3-S383A and Par3-S383D mutants exhibited different subcellular localizations when expressed in hippocampal neurons. Par3-S383D is located preferentially in the axon, whereas the majority of Par3-S383A remains in the soma. This difference in the distribution pattern suggests that Ser383 phosphorylation affects the localization and/or transport of Par3. The asymmetric distribution of Par3 is crucial for the polarization of neurons (Nishimura et al., 2004; Shi et al., 2003). In stage 2 neurons, Par3 is present in all neurites but becomes restricted to the axon upon its formation at stage 3. A uniform distribution after overexpression of Par3 or a block of its KIF3A-dependent anterograde transport into the axon disrupts the establishment of neuronal polarity (Nishimura et al., 2004; Shi et al., 2003). Par3 interacts not only with KIF3A but also with the dynein subunit Dlic2 (Schmoranzer et al., 2009). The dynein-dependent transport of the Drosophila Par3 homolog Bazooka is required for the segregation of apically localized proteins during the establishment of epithelial polarity (Harris and Peifer, 2005). Thus, dynein might perform a similar function in neurons to mediate the retrograde transport of Par3 and segregate dendritic and axonal properties. Par3-Ser383D shows a strongly reduced interaction with Dlic2 compared with that of Par3-Ser383A, suggesting that Par3 phosphorylated at Ser383 does not undergo dynein-mediated retrograde transport. Their differential interaction with dynein is a possible explanation for the difference in the distribution of Par-Ser383D and Par-Ser383A.
Ndr kinases and Par3 have been implicated in the regulation of centrosome duplication and orientation, respectively (Hergovich et al., 2007; Schmoranzer et al., 2009). It has been suggested that the centrosome induces the formation of axons (de Anda et al., 2010; de Anda et al., 2005) but subsequent experiments have indicated that its role in the initiation of polarity is more complicated than initially suggested (Gärtner et al., 2012; Sakakibara et al., 2014). The suppression of Ndr kinases in dividing cells negatively affects centrosome duplication (Hergovich et al., 2007) and, thus, would be expected to result in the loss of axons due to reduced centrosome numbers but not the formation of multiple axons as observed after the knockdown of Ndr1/2 in neurons. Therefore, it is unlikely that the Rassf5–Ndr–Par3 signaling cascade regulates neuronal polarity through an effect on centrosomes. Recently, it was shown that Par3 also acts as a microtubule-binding protein that bundles and stabilizes microtubules to promote axon extension (Chen et al., 2013). Its concentration-dependent oligomerization determines its ability to bind microtubules. A reduced retrograde transport of Par3 would increase its local concentration in the axon, favoring its oligomerization and microtubule-binding and thereby promoting axon extension.
The Rassf5–Ndr pathway suppresses supernumerary axon formation by regulating Par3 localization
Our results indicate that Rassf5 and Ndr regulate the localization of Par3 to the axon by changing the balance between anterograde and retrograde transport to favor Par3 retention in the axon. The interaction with kinesin and dynein subunits initially distributes Par3 uniformly to all neurites at stage 2. During the establishment of polarity, kinesin-2 transports Par3 into the axon where it is released after phosphorylation by Erk2 (Funahashi et al., 2013). Phosphorylation at Ser383 by Ndr kinases then prevents the interaction of Par3 with dynein and thereby blocks its retrograde transport to the soma, which allows the accumulation of Par3 in a single neurite to promote axon formation. By contrast, Par3 Ser383 is not phosphorylated in the remaining minor neurites, allowing its depletion from these processes by continued retrograde transport. Thus, the suppression of retrograde Par3 transport together with kinesin-2-mediated anterograde transport establishes the enrichment of Par3 in a single neurite. In the absence of Ndr kinases, Par3 Ser383 remains unphosphorylated and the retrograde transport of Par3 persists. As a consequence, Par3 continues to be equally distributed to all neurites, which results in the formation of supernumerary axons. Taken together, we identify a novel function of Rassf5 and Ndr kinases, which might also play important roles in other types of polarized cells, such as epithelial cells, where Par3 performs an important function.
MATERIALS AND METHODS
Cell culture and transfection
E18 hippocampal neurons were prepared and transfected by using calcium phosphate co-precipitation as described previously (Schwamborn and Püschel, 2004). Briefly, E18 hippocampal neurons were transfected by adding the DNA and calcium solution (6 µg of DNA, 6.25 µl of 1 M CaCl2 and ddH2O in a total volume of 25 µl) at 2–6 h after plating. The culture medium (Neurobasal Medium, Life Technologies) was replaced with 400 µl of Opti-MEM (Life Technologies) before the DNA mixture was added. After incubation for 45 min at 37°C under 5% CO2, the neurons were washed for 15 min with 1 ml of Opti-MEM, which had been pre-incubated at 37°C under 10% CO2, and the conditioned Neurobasal medium was added back to the cells.
Immunostaining and imaging of hippocampal cultures
Neurons were fixed at 3 DIV and permeabilized with a solution of 0.1% Triton X-100 and 0.1% sodium citrate in PBS for 3 min on ice. After three washes with PBS, fixed cells were blocked for 1 h at room temperature with 10% normal goat serum in PBS. Incubation with primary and secondary antibodies was performed in the blocking buffer. Antibodies against the following proteins were used: Tau-1 (Chemicon, MAB3420; 1∶500), MAP2 (Chemicon, AB5622; 1∶1000), Myc (Cell Signaling Technology, 2272; 1∶500), Par3 (Upstate, 07-330; 1∶750), phosphorylated Par3 (Ser383) (Eurogentec, 1∶500), NDR-CTD (Hergovich et al., 2007) and Rassf5 (Bioss, bs-11168R; 1∶500). Alexa-Fluor-conjugated secondary antibodies were also used (Molecular Probes; 1∶1000). The polyclonal rabbit antibody against phospho-Par3 (Ser383) was generated using the peptide SPGRF-S[PO3H2]-PDSHC, derived from the mouse Par3 sequence (Eurogenetec). The stage of neuronal differentiation and axon formation was determined according to published criteria (Schwamborn and Püschel, 2004). Statistical significance was determined using a two-way ANOVA.
Biochemistry
Pulldown assays, immunoprecipitation and western blotting were performed as described previously (Khazaei and Püschel, 2009). For pulldown assays, bacterially expressed GST-fusion proteins were immobilized on glutathione–Sepharose (GE Healthcare). After washing the beads with lysis buffer [50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM DTT, 10 mM MgCl2, 4 mM EDTA, 10% glycerol, 1% Triton X-100 and complete protease inhibitor cocktail (Roche)], they were incubated with lysates from transfected HEK 293T cells and washed, and the bound proteins were eluted with sample buffer.
For immunoprecipitation, transfected HEK 293T cells were lysed at 48 h after transfection. The cell lysate was incubated with 3–5 µg of antibody at 4°C for 3 h or overnight with shaking. A total of 50 µl of Protein-G−agarose beads (Roche) was added to the lysate and incubated at 4°C for 3–5 h with shaking. After three washes of the beads with immunoprecipitation wash buffer [20 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM MgCl2, 2 mM EDTA, 10% (v/v) glycerol, 0.1% (v/v) NP-40], bound proteins were eluted with 30–50 µl of 2× SDS sample buffer, boiled at 95°C for 5 min and analyzed by western blotting.
Western blotting was performed using the following antibodies: mouse anti-FLAG M2 (Sigma, F3165; 1∶1000), mouse anti-GFP (BabCo, MMS-118P; 1∶1000), mouse anti-HA (Sigma, H6908; 1∶200 and Roche, 1867423; 1∶500), rabbit anti-Par3 (Upstate, 07-330; 1∶750), mouse anti-Myc (Roche, 1667149; 1∶1000), rabbit anti-RFP (Evrogen, 23402290601; 1∶5000) and HRP-coupled secondary antibodies (Dianova; 1∶1000). For kinase assays, proteins were expressed in BL21-StarTM (Rosetta+) bacteria or HEK 293T cells and purified by pulldown or immunoprecipitation, respectively, as described previously (Khazaei and Püschel, 2009). The kinase was incubated with substrate proteins in 20 µl of kinase buffer containing 1 µM ATP at 30°C for 30 min (Khazaei and Püschel, 2009). The reaction was terminated by the addition of sample buffer followed by boiling for 5 min and was then analyzed by western blotting or staining with Coomassie Blue. The bands corresponding to the phosphorylated substrates were isolated, digested and analyzed by mass spectrometry at the Zentrale Bioanalytic, Zentrum für Molekulare Medizin Köln.
Plasmids
Expression vectors for FLAG–Mst1 (Lin et al., 2002), FLAG–Ndr1, FLAG–Ndr2 (Devroe et al., 2004) and HA–Rassf5 (Ortiz-Vega et al., 2002) were obtained from Addgene (plasmid numbers 1965, 8972, 8931 and 1979, respectively). pEGFP-Mob1b, FLAG–Ndr1-K118A and FLAG–Ndr2-119A (Devroe et al., 2005) were kindly provided by Alan Engelman. HA–NDR2-PIF and HA–NDR2-PIF-KD were described previously (Stegert et al., 2004). GFP-tagged Mus musculus dynein cytoplasmic 1 light intermediate chain 2 (DYNC1LI2) was purchased from Origene (catalog number MG207876) and cloned into a pTagRFP-N vector from Evrogen (catalog number FP142) using the XhoI and NotI sites. To generate the shRNA vectors (Paddison et al., 2002), the following target sequences were used: NDR1, 5′-GACATGATGACTTTATTGA-3′; Ndr2, 5′-GCAGCATGCTCGTAAAGAA-3′; Rassf5, 5′-GACAAGTGCT CTTCCAGAA-3′. The amplified PCR fragments were cloned into the XhoI and EcoRI sites of the pSHAG-MAGIC2 or pCAGGS-U6 vector. RNAi-resistant Ndr1, Ndr2 (Ndr1M and Ndr2M) and Rassf5 (Rassf5M) were generated by site-directed mutagenesis using the QuikChange Site-Directed Mutagenesis kit (Stratagene) according to the manufacturer's instructions. The shRNA target sequences containing the two mutations were used as primers in a PCR: Ndr1M, 5′-GCCTGGAGGGGACATGATGACCTTGTTGATGAAAAAAGATACT-3′; Ndr2M, 5′-GTTACGTCGATCGCAGCATGCCCGGAAAGAAACAGAG-3′; Rassf5M, 5-CAAAGATGGACAAGTCCTGTTCCAGAAACTCTCCATTGCT-3′. Positive colonies were confirmed by sequencing. The constructs for phospho-mimic or non-phosphorylatable Ndr2 and Par3 were generated by site-directed mutagenesis using the QuikChange Site-Directed Mutagenesis kit (Stratagene) according to the manufacturer's instructions using the following primers: Ndr2-T442A, 5′-CTGGGTTTTTCTCAATTACGCCTACAAAAGGTTTGAAG-3′; Ndr2-T442D, 5′-CTGGGTTTTTCTCAATTACGACTACAAAAGGTTTGAAG-3′; Par3-S383A, 5′-CAATTACTATTCAAGCCGTTTTGCCCCTGACAGCCAGTAT-3′; Par3-S383D, 5′-CAATTACTATTCAAGCCGTTTTGACCCTGACAGCCAGTAT-3′; Par3-S1196A, 5′-AGCGGGCGACACGCGGTGTCCGTGGAG-3′; Par3-S1196D, 5′-AGCGGGCGACACGACGTGTCCGTGGAG-3′: The Par3 constructs CR (amino acids 1–200), PDZ1 (amino acids 201–400), PDZ2 (amino acids 401–560), PDZ3 (amino acids 561–780), aPKC-binding domain (aPKC BD, amino acids 700–1356), coiled-coil region 1 (CC1) (amino acids 1116–1184), CC2 (amino acids 1185–1247), CC3 (amino acids 1186–1356) and ABD-Z (amino acids 1116–1356) have been described previously (Khazaei and Püschel, 2009).
Rassf5-knockout mice
Rassf5-knockout mice were generously provided by Sean Bong Lee (Park et al., 2010). Genotyping of Rassf5-knockout mice was performed using the following primers for the wild-type (177-bp product); Rassf5_WT_S1, 5′-CCCAGTGTTCTCACCTGGAGAATC-3′ and Rassf5_WT_AS1, 5′-GTCAGCTCATGTCACTGGCAATAAGC-3′, and the following primers for the mutant (228-bp product); Rassf5_MUT_S1, 5′-CCCAGTGTTCTCACCTGGAGAATC-3′ and Rassf5_MUT_AS1, 5′-CCAGACTGCCTTGGGAAAAGC G-3′. The conditions were 35 cycles of 95°C for 30 s, 58°C for 30 s and 72°C for 30 s.
Ex vivo electroporation and live-cell imaging
Brains from wild-type CD1 E14.5 embryos were used for ex vivo electroporation. Briefly, plasmids were mixed with Fast Green dye (0.5%) and injected into the lateral ventricle. Embryos were transfected by five pulses at 54 V for 50 ms at 1 s intervals, using the ECM-830 BTX square wave electroporator (BTX, Gentronic). The brains were embedded in 3% low-melting agarose (Biozym). Slices with a thickness of 290 µm were cut using a vibratome (Leica), placed onto the membrane of a Millicell tissue culture insert (0.4 µm, 30 mm; Millipore) and cultured at the air–liquid interface using neurobasal medium supplemented with B27 and N2 in 35-mm tissue culture dishes at 37°C under 5% CO2 and 40% O2. Imaging was performed in an incubation chamber at 37°C and under 5% CO2 at 36 h after electroporation, using a Zeiss LSM 700 laser-scanning microscope (Carl Zeiss MicroImaging, Jena, Germany) equipped with the Zeiss ZEN Software (Carl Zeiss MicroImaging). Images were taken every 30 min for a period of 24 h. The distribution of transfected neurons was analyzed by determining the percentage of GFP-positive cells in the ventricular zone or SVZ, intermediate zone and cortical plate.
Supplementary Material
Acknowledgments
We thank Alan Engelman (Harvard Medical School, Cambridge, MA) for plasmids, Sean Bong Lee (NIDDK, Bethesda, MD) for knockout mice and Christian Klämbt (Institute of Neurobiology, Münster, Germany) for comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
R.Y., E.K. and J.J. performed the experiments and analyzed data. R.Y., A.H. and A.W.P. wrote the manuscript.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft [grant number PU 102/12-1 to A.W.P.]; and a German Academic Exchange Service (DAAD) fellowship to E.K. A.H. is a Wellcome Trust Research Career Development fellow [grant number 090090/Z/09/Z] at the UCL Cancer Institute. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.146696/-/DC1
References
- Arimura N., Kaibuchi K. (2007). Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 8, 194–205 10.1038/nrn2056 [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. (1977). Rat hippocampal neurons in dispersed cell culture. Brain Res. 126, 397–425 10.1016/0006-8993(77)90594-7 [DOI] [PubMed] [Google Scholar]
- Barnes A. P., Polleux F. (2009). Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 32, 347–381 10.1146/annurev.neuro.31.060407.125536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichsel S. J., Tamaskovic R., Stegert M. R., Hemmings B. A. (2004). Mechanism of activation of NDR (nuclear Dbf2-related) protein kinase by the hMOB1 protein. J. Biol. Chem. 279, 35228–35235 10.1074/jbc.M404542200 [DOI] [PubMed] [Google Scholar]
- Calvisi D. F., Ladu S., Gorden A., Farina M., Conner E. A., Lee J. S., Factor V. M., Thorgeirsson S. S. (2006). Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology 130, 1117–1128 10.1053/j.gastro.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Chen Y. M., Wang Q. J., Hu H. S., Yu P. C., Zhu J., Drewes G., Piwnica-Worms H., Luo Z. G. (2006). Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proc. Natl. Acad. Sci. USA 103, 8534–8539 10.1073/pnas.0509955103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Chen J., Shi H., Wei M., Castaneda-Castellanos D. R., Bultje R. S., Pei X., Kriegstein A. R., Zhang M., Shi S. H. (2013). Regulation of microtubule stability and organization by mammalian Par3 in specifying neuronal polarity. Dev. Cell 24, 26–40 10.1016/j.devcel.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Amagai Y., Homma Y., Fukuda M., Mizuno K. (2013). NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. EMBO J. 32, 874–885 10.1038/emboj.2013.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornils H., Stegert M. R., Hergovich A., Hynx D., Schmitz D., Dirnhofer S., Hemmings B. A. (2010). Ablation of the kinase NDR1 predisposes mice to the development of T cell lymphoma. Sci. Signal. 3, ra47 10.1126/scisignal.2000681 [DOI] [PubMed] [Google Scholar]
- Cornils H., Kohler R. S., Hergovich A., Hemmings B. A. (2011a). Downstream of human NDR kinases: impacting on c-myc and p21 protein stability to control cell cycle progression. Cell Cycle 10, 1897–1904 10.4161/cc.10.12.15826 [DOI] [PubMed] [Google Scholar]
- Cornils H., Kohler R. S., Hergovich A., Hemmings B. A. (2011b). Human NDR kinases control G(1)/S cell cycle transition by directly regulating p21 stability. Mol. Cell. Biol. 31, 1382–1395 10.1128/MCB.01216-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Anda F. C., Pollarolo G., Da Silva J. S., Camoletto P. G., Feiguin F., Dotti C. G. (2005). Centrosome localization determines neuronal polarity. Nature 436, 704–708 10.1038/nature03811 [DOI] [PubMed] [Google Scholar]
- de Anda F. C., Meletis K., Ge X., Rei D., Tsai L. H. (2010). Centrosome motility is essential for initial axon formation in the neocortex. J. Neurosci. 30, 10391–10406 10.1523/JNEUROSCI.0381-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devroe E., Erdjument-Bromage H., Tempst P., Silver P. A. (2004). Human Mob proteins regulate the NDR1 and NDR2 serine-threonine kinases. J. Biol. Chem. 279, 24444–24451 10.1074/jbc.M401999200 [DOI] [PubMed] [Google Scholar]
- Devroe E., Silver P. A., Engelman A. (2005). HIV-1 incorporates and proteolytically processes human NDR1 and NDR2 serine-threonine kinases. Virology 331, 181–189 10.1016/j.virol.2004.10.023 [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Sullivan C. A., Banker G. A. (1988). The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto K., He Y., Ye B., Grueber W. B., Adler P. N., Jan L. Y., Jan Y-N. (2004). Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell 119, 245–256 10.1016/j.cell.2004.09.036 [DOI] [PubMed] [Google Scholar]
- Emoto K., Parrish J. Z., Jan L. Y., Jan Y. N. (2006). The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature 443, 210–213 10.1038/nature05090 [DOI] [PubMed] [Google Scholar]
- Funahashi Y., Namba T., Fujisue S., Itoh N., Nakamuta S., Kato K., Shimada A., Xu C., Shan W., Nishioka T. et al. (2013). ERK2-mediated phosphorylation of Par3 regulates neuronal polarization. J. Neurosci. 33, 13270–13285 10.1523/JNEUROSCI.4210-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos M. E., Bargmann C. I. (2004). Mechanosensory neurite termination and tiling depend on SAX-2 and the SAX-1 kinase. Neuron 44, 239–249 10.1016/j.neuron.2004.09.021 [DOI] [PubMed] [Google Scholar]
- Gärtner A., Fornasiero E. F., Munck S., Vennekens K., Seuntjens E., Huttner W. B., Valtorta F., Dotti C. G. (2012). N-cadherin specifies first asymmetry in developing neurons. EMBO J. 31, 1893–1903 10.1038/emboj.2012.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvalov B. K., Flynn K. C., Neukirchen D., Meyn L., Teusch N., Wu X., Brakebusch C., Bamburg J. R., Bradke F. (2007). Cdc42 regulates cofilin during the establishment of neuronal polarity. J. Neurosci. 27, 13117–13129 10.1523/JNEUROSCI.3322-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J., Peifer M. (2005). The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J. Cell Biol. 170, 813–823 10.1083/jcb.200505127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K., Tapon N. (2007). The Salvador-Warts-Hippo pathway – an emerging tumour-suppressor network. Nat. Rev. Cancer 7, 182–191 10.1038/nrc2070 [DOI] [PubMed] [Google Scholar]
- He Y., Fang X., Emoto K., Jan Y. N., Adler P. N. (2005). The tricornered Ser/Thr protein kinase is regulated by phosphorylation and interacts with furry during Drosophila wing hair development. Mol. Biol. Cell 16, 689–700 10.1091/mbc.E04-09-0828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A. (2011). MOB control: reviewing a conserved family of kinase regulators. Cell. Signal. 23, 1433–1440 10.1016/j.cellsig.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A., Schmitz D., Hemmings B. A. (2006a). The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem. Biophys. Res. Commun. 345, 50–58 10.1016/j.bbrc.2006.03.244 [DOI] [PubMed] [Google Scholar]
- Hergovich A., Stegert M. R., Schmitz D., Hemmings B. A. (2006b). NDR kinases regulate essential cell processes from yeast to humans. Nat. Rev. Mol. Cell Biol. 7, 253–264 10.1038/nrm1891 [DOI] [PubMed] [Google Scholar]
- Hergovich A., Lamla S., Nigg E. A., Hemmings B. A. (2007). Centrosome-associated NDR kinase regulates centrosome duplication. Mol. Cell 25, 625–634 10.1016/j.molcel.2007.01.020 [DOI] [PubMed] [Google Scholar]
- Hergovich A., Kohler R. S., Schmitz D., Vichalkovski A., Cornils H., Hemmings B. A. (2009). The MST1 and hMOB1 tumor suppressors control human centrosome duplication by regulating NDR kinase phosphorylation. Curr. Biol. 19, 1692–1702 10.1016/j.cub.2009.09.020 [DOI] [PubMed] [Google Scholar]
- Hesson L., Dallol A., Minna J. D., Maher E. R., Latif F. (2003). NORE1A, a homologue of RASSF1A tumour suppressor gene is inactivated in human cancers. Oncogene 22, 947–954 10.1038/sj.onc.1206191 [DOI] [PubMed] [Google Scholar]
- Hwang E., Ryu K. S., Pääkkönen K., Güntert P., Cheong H. K., Lim D. S., Lee J. O., Jeon Y. H., Cheong C. (2007). Structural insight into dimeric interaction of the SARAH domains from Mst1 and RASSF family proteins in the apoptosis pathway. Proc. Natl. Acad. Sci. USA 104, 9236–9241 10.1073/pnas.0610716104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. (2001). Asymmetric cell division in the Drosophila nervous system. Nat. Rev. Neurosci. 2, 772–779 10.1038/35097516 [DOI] [PubMed] [Google Scholar]
- Kemphues K. (2000). PARsing embryonic polarity. Cell 101, 345–348 10.1016/S0092-8674(00)80844-2 [DOI] [PubMed] [Google Scholar]
- Khazaei M. R., Püschel A. W. (2009). Phosphorylation of the par polarity complex protein Par3 at serine 962 is mediated by aurora a and regulates its function in neuronal polarity. J. Biol. Chem. 284, 33571–33579 10.1074/jbc.M109.055897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlatchev A., Rabizadeh S., Xavier R., Nedwidek M., Chen T., Zhang X. F., Seed B., Avruch J. (2002). Identification of a novel Ras-regulated proapoptotic pathway. Curr. Biol. 12, 253–265 10.1016/S0960-9822(02)00683-8 [DOI] [PubMed] [Google Scholar]
- Lin Y., Khokhlatchev A., Figeys D., Avruch J. (2002). Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis. J. Biol. Chem. 277, 47991–48001 10.1074/jbc.M202630200 [DOI] [PubMed] [Google Scholar]
- Lorber B., Howe M. L., Benowitz L. I., Irwin N. (2009). Mst3b, an Ste20-like kinase, regulates axon regeneration in mature CNS and PNS pathways. Nat. Neurosci. 12, 1407–1414 10.1038/nn.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshnikova A., Frye J., Shay J. W., Minna J. D., Khokhlatchev A. V. (2006). The growth and tumor suppressor NORE1A is a cytoskeletal protein that suppresses growth by inhibition of the ERK pathway. J. Biol. Chem. 281, 8143–8152 10.1074/jbc.M511837200 [DOI] [PubMed] [Google Scholar]
- Moshnikova A., Kuznetsov S., Khokhlatchev A. V. (2008). Interaction of the growth and tumour suppressor NORE1A with microtubules is not required for its growth-suppressive function. BMC Res. Notes 1, 13 10.1186/1756-0500-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M., Goto T. M., Sugimoto M., Nishimura T., Shinagawa T., Ohno S., Amano M., Kaibuchi K. (2008). Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev. Cell 14, 205–215 10.1016/j.devcel.2007.11.021 [DOI] [PubMed] [Google Scholar]
- Nishimura T., Kato K., Yamaguchi T., Fukata Y., Ohno S., Kaibuchi K. (2004). Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat. Cell Biol. 6, 328–334 10.1038/ncb1118 [DOI] [PubMed] [Google Scholar]
- Oh S., Lee D., Kim T., Kim T. S., Oh H. J., Hwang C. Y., Kong Y. Y., Kwon K. S., Lim D. S. (2009). Crucial role for Mst1 and Mst2 kinases in early embryonic development of the mouse. Mol. Cell. Biol. 29, 6309–6320 10.1128/MCB.00551-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. (2001). Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr. Opin. Cell Biol. 13, 641–648 10.1016/S0955-0674(00)00264-7 [DOI] [PubMed] [Google Scholar]
- Ortiz-Vega S., Khokhlatchev A., Nedwidek M., Zhang X. F., Dammann R., Pfeifer G. P., Avruch J. (2002). The putative tumor suppressor RASSF1A homodimerizes and heterodimerizes with the Ras-GTP binding protein Nore1. Oncogene 21, 1381–1390 10.1038/sj.onc.1205192 [DOI] [PubMed] [Google Scholar]
- Paddison P. J., Caudy A. A., Bernstein E., Hannon G. J., Conklin D. S. (2002). Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 16, 948–958 10.1101/gad.981002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. (2007). Hippo signaling in organ size control. Genes Dev. 21, 886–897 10.1101/gad.1536007 [DOI] [PubMed] [Google Scholar]
- Park J., Kang S. I., Lee S. Y., Zhang X. F., Kim M. S., Beers L. F., Lim D. S., Avruch J., Kim H. S., Lee S. B. (2010). Tumor suppressor ras association domain family 5 (RASSF5/NORE1) mediates death receptor ligand-induced apoptosis. J. Biol. Chem. 285, 35029–35038 10.1074/jbc.M110.165506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praskova M., Khoklatchev A., Ortiz-Vega S., Avruch J. (2004). Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem. J. 381, 453–462 10.1042/BJ20040025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A., Sato T., Ando R., Noguchi N., Masaoka M., Miyata T. (2014). Dynamics of centrosome translocation and microtubule organization in neocortical neurons during distinct modes of polarization. Cereb. Cortex 24, 1301–1310 10.1093/cercor/bhs411 [DOI] [PubMed] [Google Scholar]
- Scheel H., Hofmann K. (2003). A novel interaction motif, SARAH, connects three classes of tumor suppressor. Curr. Biol. 13, R899–R900 10.1016/j.cub.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Schmoranzer J., Fawcett J. P., Segura M., Tan S., Vallee R. B., Pawson T., Gundersen G. G. (2009). Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr. Biol. 19, 1065–1074 10.1016/j.cub.2009.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwamborn J. C., Püschel A. W. (2004). The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat. Neurosci. 7, 923–929 10.1038/nn1295 [DOI] [PubMed] [Google Scholar]
- Sherwood V., Recino A., Jeffries A., Ward A., Chalmers A. D. (2010). The N-terminal RASSF family: a new group of Ras-association-domain-containing proteins, with emerging links to cancer formation. Biochem. J. 425, 303–311 10.1042/BJ20091318 [DOI] [PubMed] [Google Scholar]
- Shi S. H., Jan L. Y., Jan Y. N. (2003). Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112, 63–75 10.1016/S0092-8674(02)01249-7 [DOI] [PubMed] [Google Scholar]
- Song H., Mak K. K., Topol L., Yun K., Hu J., Garrett L., Chen Y., Park O., Chang J., Simpson R. M. et al. (2010). Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl. Acad. Sci. USA 107, 1431–1436 10.1073/pnas.0911409107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegert M. R., Tamaskovic R., Bichsel S. J., Hergovich A., Hemmings B. A. (2004). Regulation of NDR2 protein kinase by multi-site phosphorylation and the S100B calcium-binding protein. J. Biol. Chem. 279, 23806–23812 10.1074/jbc.M402472200 [DOI] [PubMed] [Google Scholar]
- Stegert M. R., Hergovich A., Tamaskovic R., Bichsel S. J., Hemmings B. A. (2005). Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol. Cell. Biol. 25, 11019–11029 10.1128/MCB.25.24.11019-11029.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultanir S. K., Hertz N. T., Li G., Ge W. P., Burlingame A. L., Pleasure S. J., Shokat K. M., Jan L. Y., Jan Y. N. (2012). Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 Uncovers their roles in dendrite arborization and spine development. Neuron 73, 1127–1142 10.1016/j.neuron.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weyden L., Tachibana K. K., Gonzalez M. A., Adams D. J., Ng B. L., Petty R., Venkitaraman A. R., Arends M. J., Bradley A. (2005). The RASSF1A isoform of RASSF1 promotes microtubule stability and suppresses tumorigenesis. Mol. Cell. Biol. 25, 8356–8367 10.1128/MCB.25.18.8356-8367.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichalkovski A., Gresko E., Cornils H., Hergovich A., Schmitz D., Hemmings B. A. (2008). NDR kinase is activated by RASSF1A/MST1 in response to Fas receptor stimulation and promotes apoptosis. Curr. Biol. 18, 1889–1895 [DOI] [PubMed] [Google Scholar]
- Witte H., Neukirchen D., Bradke F. (2008). Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 180, 619–632 10.1083/jcb.200707042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. X., Guan K. L. (2013). The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 10.1101/gad.210773.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J. A., Peckol E. L., Tobin D. M. and Bargmann C. I. (2000). Neuronal cell shape and neurite initiation are regulated by the Ndr kinase SAX-1, a member of the Orb6/COT-1/warts serine/threonine kinase family. Mol. Biol. Cell 11, 3177–3190 10.1091/mbc.11.9.3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Tumaneng K., Guan K. L. (2011). The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 13, 877–883 10.1038/ncb2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Conrad C., Xia F., Park J. S., Payer B., Yin Y., Lauwers G. Y., Thasler W., Lee J. T., Avruch J. et al. (2009). Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 10.1016/j.ccr.2009.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.