Abstract
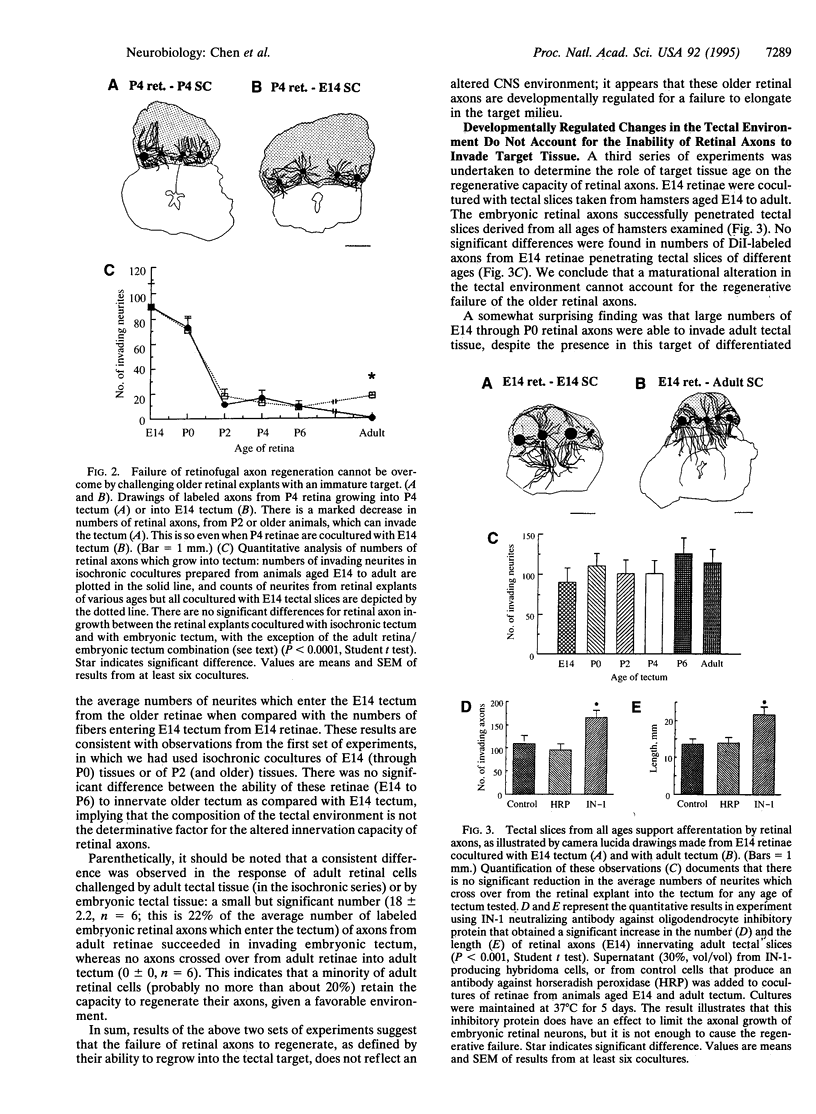
The failure of mature mammalian central nervous system axons to regenerate after transection is usually attributed to influences of the extraneuronal milieu. Using explant cocultures of retina and midbrain tectum from hamsters, we have found evidence that these influences account for failure of regrowth of only a small minority of retinal axons. For most of the axons, there is a programmed loss of ability to elongate in the central nervous system. We show that there is a precipitous decline in the ability of retinal axons to reinnervate tectal targets when the retina is derived from pups on or after postnatal day 2, even when the target is embryonic. By contrast, embryonic retinal axons can regrow into tectum of any age, overcoming growth-inhibiting influences of glial factors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo J., Valenzuela M. A., Wohllk N., Pineda G. Evidencia experimental que el TSH endogeno modula la expresion del antigeno microsomal: implicancia clinica. Rev Med Chil. 1993 Oct;121(10):1099–1104. [PubMed] [Google Scholar]
- Armson P. F., Bennett M. R., Raju T. R. Retinal ganglion cell survival and neurite regeneration requirements: the change from Müller cell dependence to superior colliculi dependence during development. Brain Res. 1987 Apr;429(2):207–216. doi: 10.1016/0165-3806(87)90101-5. [DOI] [PubMed] [Google Scholar]
- Bähr M., Eschweiler G. W. Regenerating adult rat retinal axons reconnect with target neurons in-vitro. Neuroreport. 1991 Oct;2(10):581–584. doi: 10.1097/00001756-199110000-00007. [DOI] [PubMed] [Google Scholar]
- Caeser M., Bonhoeffer T., Bolz J. Cellular organization and development of slice cultures from rat visual cortex. Exp Brain Res. 1989;77(2):234–244. doi: 10.1007/BF00274981. [DOI] [PubMed] [Google Scholar]
- Carter D. A., Bray G. M., Aguayo A. J. Long-term growth and remodeling of regenerated retino-collicular connections in adult hamsters. J Neurosci. 1994 Feb;14(2):590–598. doi: 10.1523/JNEUROSCI.14-02-00590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Burne J. F., McKinlay C., Winter J. The role of laminin and the laminin/fibronectin receptor complex in the outgrowth of retinal ganglion cell axons. Dev Biol. 1987 Aug;122(2):407–418. doi: 10.1016/0012-1606(87)90305-8. [DOI] [PubMed] [Google Scholar]
- Cohen J., Burne J. F., Winter J., Bartlett P. Retinal ganglion cells lose response to laminin with maturation. 1986 Jul 31-Aug 6Nature. 322(6078):465–467. doi: 10.1038/322465a0. [DOI] [PubMed] [Google Scholar]
- Collazo D., Takahashi H., McKay R. D. Cellular targets and trophic functions of neurotrophin-3 in the developing rat hippocampus. Neuron. 1992 Oct;9(4):643–656. doi: 10.1016/0896-6273(92)90028-c. [DOI] [PubMed] [Google Scholar]
- David S., Aguayo A. J. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981 Nov 20;214(4523):931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Davies A. M. Intrinsic differences in the growth rate of early nerve fibres related to target distance. Nature. 1989 Feb 9;337(6207):553–555. doi: 10.1038/337553a0. [DOI] [PubMed] [Google Scholar]
- Fawcett J. W. Intrinsic neuronal determinants of regeneration. Trends Neurosci. 1992 Jan;15(1):5–8. doi: 10.1016/0166-2236(92)90338-9. [DOI] [PubMed] [Google Scholar]
- Friedman B., Hockfield S., Black J. A., Woodruff K. A., Waxman S. G. In situ demonstration of mature oligodendrocytes and their processes: an immunocytochemical study with a new monoclonal antibody, rip. Glia. 1989;2(5):380–390. doi: 10.1002/glia.440020510. [DOI] [PubMed] [Google Scholar]
- Hankin M. H., Lund R. D. Directed early axonal outgrowth from retinal transplants into host rat brains. J Neurobiol. 1990 Dec;21(8):1202–1218. doi: 10.1002/neu.480210806. [DOI] [PubMed] [Google Scholar]
- Honig M. G., Hume R. I. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986 Jul;103(1):171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri S., Edwards M. A., Schneider G. E. Initial stages of retinofugal axon development in the hamster: evidence for two distinct modes of growth. Exp Brain Res. 1991;87(2):371–382. doi: 10.1007/BF00231854. [DOI] [PubMed] [Google Scholar]
- Jhaveri S., Erzurumlu R. S., Friedman B., Schneider G. E. Oligodendrocytes and myelin formation along the optic tract of the developing hamster: an immunohistochemical study using the Rip antibody. Glia. 1992;6(2):138–148. doi: 10.1002/glia.440060208. [DOI] [PubMed] [Google Scholar]
- McKeon R. J., Schreiber R. C., Rudge J. S., Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991 Nov;11(11):3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya K. L., Benowitz L. I., Jhaveri S., Schneider G. E. Changes in rapidly transported proteins in developing hamster retinofugal axons. J Neurosci. 1988 Dec;8(12):4445–4454. doi: 10.1523/JNEUROSCI.08-12-04445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya K. L., Jhaveri S., Schneider G. E., Benowitz L. I. Immunohistochemical localization of GAP-43 in the developing hamster retinofugal pathway. J Comp Neurol. 1989 Oct 1;288(1):51–58. doi: 10.1002/cne.902880105. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G., Doherty P., Walsh F. S., Crocker P. R., Filbin M. T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994 Sep;13(3):757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Schnell L., Schwab M. E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990 Jan 18;343(6255):269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Bandtlow C. E. Neurobiology. Inhibitory influences. Nature. 1994 Oct 20;371(6499):658–659. doi: 10.1038/371658a0. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Solomon A., Lavie V., Ben-Bassat S., Belkin M., Cohen A. Tumor necrosis factor facilitates regeneration of injured central nervous system axons. Brain Res. 1991 Apr 5;545(1-2):334–338. doi: 10.1016/0006-8993(91)91309-o. [DOI] [PubMed] [Google Scholar]
- So K. F., Schneider G. E., Ayres S. Lesions of the brachium of the superior colliculus in neonate hamsters: correlation of anatomy with behavior. Exp Neurol. 1981 May;72(2):379–400. doi: 10.1016/0014-4886(81)90231-4. [DOI] [PubMed] [Google Scholar]
- Tay D., So K. F., Jen L. S., Lau K. C. The postnatal development of the optic nerve in hamsters: an electron microscopic study. Brain Res. 1986 Dec;395(2):268–273. doi: 10.1016/s0006-8993(86)80206-2. [DOI] [PubMed] [Google Scholar]
- Thanos S., Thiel H. J. Mechanisms governing neuronal degeneration and axonal regeneration in the mature retinofugal system. J Cell Sci Suppl. 1991;15:125–134. doi: 10.1242/jcs.1991.supplement_15.17. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M., Bray G. M., Villegas-Pérez M. P., Thanos S., Aguayo A. J. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci. 1987 Sep;7(9):2894–2909. doi: 10.1523/JNEUROSCI.07-09-02894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Pérez M. P., Vidal-Sanz M., Bray G. M., Aguayo A. J. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988 Jan;8(1):265–280. doi: 10.1523/JNEUROSCI.08-01-00265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Sawai H., Fukuda Y. Axonal regeneration of retinal ganglion cells in the cat geniculocortical pathway. Brain Res. 1991 Sep 27;560(1-2):330–333. doi: 10.1016/0006-8993(91)91253-w. [DOI] [PubMed] [Google Scholar]
- Wikler K. C., Kirn J., Windrem M. S., Finlay B. L. Control of cell number in the developing visual system. II. Effects of partial tectal ablation. Brain Res. 1986 Jul;393(1):11–21. doi: 10.1016/0165-3806(86)90060-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Yamada K., Kurotani T., Toyama K. Laminar specificity of extrinsic cortical connections studied in coculture preparations. Neuron. 1992 Aug;9(2):217–228. doi: 10.1016/0896-6273(92)90161-6. [DOI] [PubMed] [Google Scholar]
- de Curtis I., Quaranta V., Tamura R. N., Reichardt L. F. Laminin receptors in the retina: sequence analysis of the chick integrin alpha 6 subunit. Evidence for transcriptional and posttranslational regulation. J Cell Biol. 1991 Apr;113(2):405–416. doi: 10.1083/jcb.113.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]