Abstract
The coat protein of the RNA bacteriophage MS2 binds a specific stem-loop structure in viral RNA to accomplish encapsidation of the genome and translational repression of replicase synthesis. In order to identify the structural components of coat protein required for its RNA binding function, a series of repressor-defective mutants has been isolated. To ensure that the repressor defects were due to substitution of binding site residues, the mutant coat proteins were screened for retention of the ability to form virus-like particles. Since virus assembly presumably requires native structure, this approach eliminated mutants whose repressor defects were secondary consequences of protein folding or stability defects. Each of the variant coat proteins was purified and its ability to bind operator RNA in vitro was measured. DNA sequence analysis identified the nucleotide and amino acid substitutions responsible for reduced RNA binding affinity. Localization of the substituted sites in the three-dimensional structure of coat protein reveals that amino acid residues on three adjacent strands of the coat protein beta-sheet are required for translational repression and RNA binding. The sidechains of the affected residues form a contiguous patch on the interior surface of the viral coat.
Full text
PDF
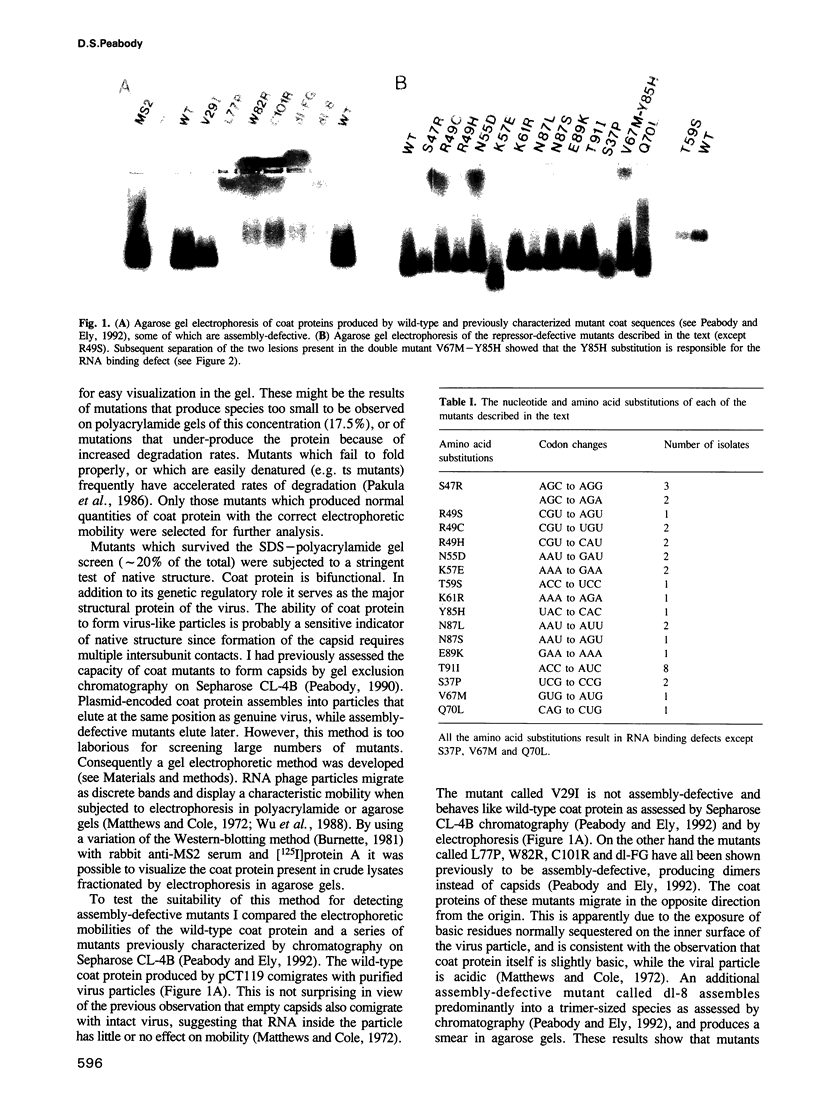

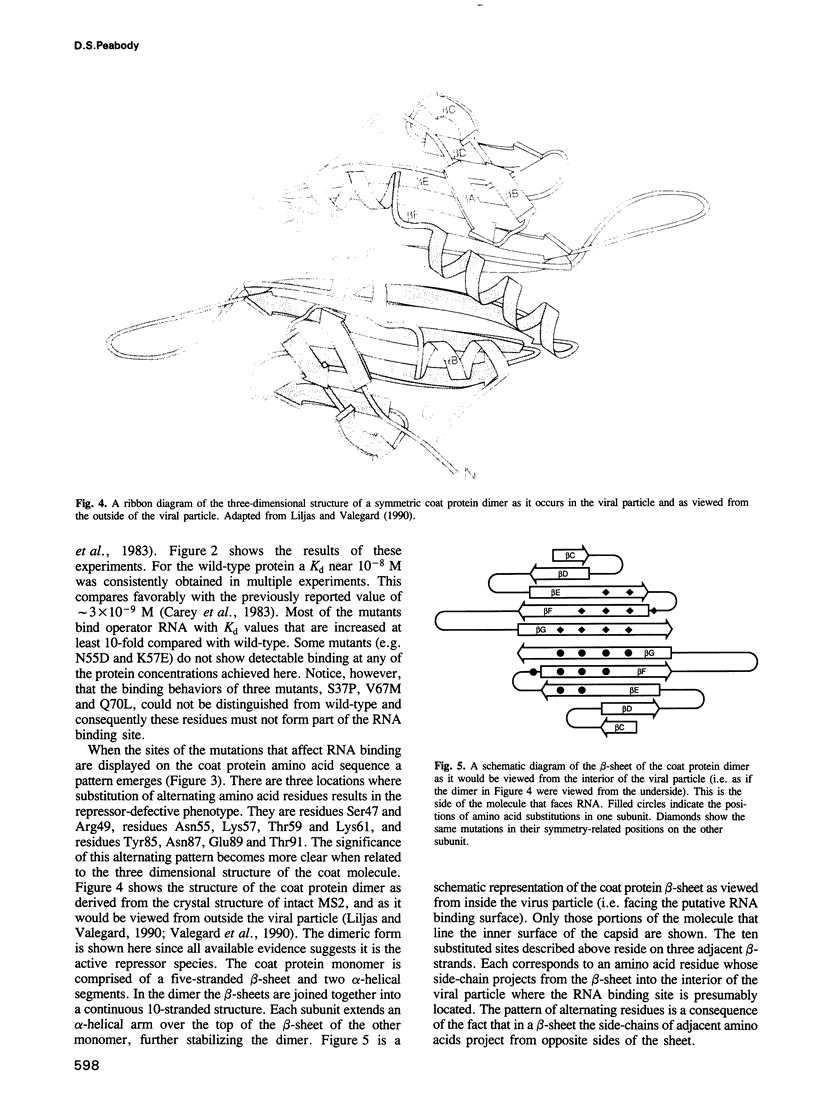


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckett D., Uhlenbeck O. C. Ribonucleoprotein complexes of R17 coat protein and a translational operator analog. J Mol Biol. 1988 Dec 20;204(4):927–938. doi: 10.1016/0022-2836(88)90052-6. [DOI] [PubMed] [Google Scholar]
- Bernardi A., Spahr P. F. Nucleotide sequence at the binding site for coat protein on RNA of bacteriophage R17. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3033–3037. doi: 10.1073/pnas.69.10.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshard H. R., Koch L. E., Hartley B. S. Aminoacyl-tRNA synthetases from Bacillus stearothermophilus. Asymmetry of substrate binding to tyrosyl-tRNA synthetase. Eur J Biochem. 1975 May 6;53(2):493–498. doi: 10.1111/j.1432-1033.1975.tb04091.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carey J., Cameron V., de Haseth P. L., Uhlenbeck O. C. Sequence-specific interaction of R17 coat protein with its ribonucleic acid binding site. Biochemistry. 1983 May 24;22(11):2601–2610. doi: 10.1021/bi00280a002. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J. A proposed model for interaction of polypeptides with RNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):283–287. doi: 10.1073/pnas.71.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Sussman J. L., Kim S. H. Secondary structural complementarity between DNA and proteins. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1458–1462. doi: 10.1073/pnas.74.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper D. E., White S. A., Kean J. M. Preparation of specific ribosomal RNA fragments. Methods Enzymol. 1988;164:221–237. doi: 10.1016/s0076-6879(88)64045-6. [DOI] [PubMed] [Google Scholar]
- Hoffman D. W., Query C. C., Golden B. L., White S. W., Keene J. D. RNA-binding domain of the A protein component of the U1 small nuclear ribonucleoprotein analyzed by NMR spectroscopy is structurally similar to ribosomal proteins. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2495–2499. doi: 10.1073/pnas.88.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matthews K. S., Cole R. D. Shell formation by capsid protein of f2 bacteriophage. J Mol Biol. 1972 Mar 14;65(1):1–15. doi: 10.1016/0022-2836(72)90487-1. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Lerman L. S., Maniatis T. A general method for saturation mutagenesis of cloned DNA fragments. Science. 1985 Jul 19;229(4710):242–247. doi: 10.1126/science.2990046. [DOI] [PubMed] [Google Scholar]
- Nagai K., Oubridge C., Jessen T. H., Li J., Evans P. R. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature. 1990 Dec 6;348(6301):515–520. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- Pakula A. A., Young V. B., Sauer R. T. Bacteriophage lambda cro mutations: effects on activity and intracellular degradation. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8829–8833. doi: 10.1073/pnas.83.23.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S., Ely K. R. Control of translational repression by protein-protein interactions. Nucleic Acids Res. 1992 Apr 11;20(7):1649–1655. doi: 10.1093/nar/20.7.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S. Translational repression by bacteriophage MS2 coat protein expressed from a plasmid. A system for genetic analysis of a protein-RNA interaction. J Biol Chem. 1990 Apr 5;265(10):5684–5689. [PubMed] [Google Scholar]
- Romaniuk P. J., Lowary P., Wu H. N., Stormo G., Uhlenbeck O. C. RNA binding site of R17 coat protein. Biochemistry. 1987 Mar 24;26(6):1563–1568. doi: 10.1021/bi00380a011. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valegård K., Liljas L., Fridborg K., Unge T. The three-dimensional structure of the bacterial virus MS2. Nature. 1990 May 3;345(6270):36–41. doi: 10.1038/345036a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wong I., Lohman T. M. Allosteric effects of nucleotide cofactors on Escherichia coli Rep helicase-DNA binding. Science. 1992 Apr 17;256(5055):350–355. doi: 10.1126/science.256.5055.350. [DOI] [PubMed] [Google Scholar]
- Wu H. N., Kastelic K. A., Uhlenbeck O. C. A comparison of two phage coat protein-RNA interactions. Nucleic Acids Res. 1988 Jun 10;16(11):5055–5066. doi: 10.1093/nar/16.11.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]





