Abstract
Polycomb repressive complex 2 (PRC2) is the epigenetic regulator that induces histone H3 lysine 27 methylation (H3K27me3) and silences specific gene transcription. Enhancer of zeste homolog 2 (EZH2) is an enzymatic subunit of PRC2, and evidence shows that EZH2 plays an essential role in cancer initiation, development, progression, metastasis, and drug resistance. EZH2 expression is indeed regulated by various oncogenic transcription factors, tumor suppressor miRNAs, and cancer-associated non-coding RNA. EZH2 activity is also controlled by post-translational modifications, which are deregulated in cancer. The canonical role of EZH2 is gene silencing through H3K27me3, but accumulating evidence shows that EZH2 methlyates substrates other than histone and has methylase-independent functions. These non-canonical functions of EZH2 are shown to play a role in cancer progression. In this review, we summarize current information on the regulation and roles of EZH2 in cancer. We also discuss various therapeutic approaches to targeting EZH2.
Keywords: EZH2, PRC2, Neoplasms, Genetic transcription, Untranslated RNA, MicroRNAs, Post-translational protein processing
Introduction
Polycomb group proteins are initially identified as regulators controlling the establishment of body segmentation by silencing hox genes expression in Drosophila. Later, they were foun to be epigenetic regulators that are critical for multiple cellular functions, including stem cell maintenance and differentiation [1]. Polycomb group proteins are well conserved between Drosophila and humans and are involved in gene silencing. Two major polycomb repressive complexes, Polycomb repressive complex (PRC) 1 and PRC2, control gene silencing through post-translational modifications of histone proteins [2]. PRC1 consists of Bmi1, Ring1b, CBX4, and PHC subunits and induces histone 2A lysine 119 ubiquitination (H2AK119ub1). In contrast, PRC2 consists primarily of enhancer of zeste homolog 2 (EZH2), EED, SUZ12, and RbAp48 and catalyzes the methylation of histone H3 lysine 27 (H3K27) to generate trimethyl-H3K27 (H3K27me3) [3]. PRC1 enhances the effects of PRC2 by recognizing H3K27me3 and interacting with it, but these complexes can also repress gene expression independently [2]. EZH2 is the catalytic subunit of PRC2, and growing evidence demonstrates that EZH2 is essential for cancer initiation, development, progression, metastasis, and drug resistance. Therefore, EZH2 is currently considered a promising drug target, and multiple inhibitors of EZH2 have been developed, some of which are in clinical trials. In this review, we introduce current information regarding the molecular mechanisms by which EZH2 expression/activity is regulated as well as the role of EZH2 in oncogenic signaling pathways. Moreover, we focus on the therapeutic potential of EZH2 and discuss possible approaches to targeting EZH2.
Regulation of EZH2 Expression and Activity in Cancer
EZH2 is frequently overexpressed in many cancer types and is critical for cancer cell proliferation and survival. Indeed, the regulators of EZH2 expression are also critical for cell proliferation, tumorigenesis, and stem cell maintenance (Fig. 1). For example, Myc binds to EZH2 promoter and directly activates its transcription, and EZH2 expression is correlated with Myc expression in prostate cancer [4]. Myc also upregulates EZH2 expression by downregulating miRNA 101 (miR-101), miR-26a, and miR-26b [4-7]. In contrast, c-Myc expression is also positively regulated by EZH2 in glioblastoma, although the underlying mechanism is uncertain [8]. In addition to Myc, another cell cycle regulator, E2F, positively controls EZH2 transcription, and EZH2 is critical for the regulation of pRB-E2F pathway [9]. ANCCA, a co-activator of androgen receptor (AR) and binding protein of E2F, can enhance E2F-mediated EZH2 transcription in prostate cancer cells [10,11]. In Ewing tumors, EWS-FLI1 fusion oncoprotein directly regulates EZH2 gene expression [12]. SOX4, one of the key regulators of stem cells, directly regulates the expression of EZH2 mRNA, which is critical for SOX4-mediated epithelial-mesenchymal transition (EMT) [13]. Moreover, NF-Y, STAT3, and ETS transcription factors directly regulate EZH2 transcription in epithelial ovarian, colorectal, and prostate cancer cells, respectively [14-16]. Both Elk-1 and HIF1 directly regulates EZH2 transcription that is associated with aggressive breast cancer [17,18].
Fig. 1.
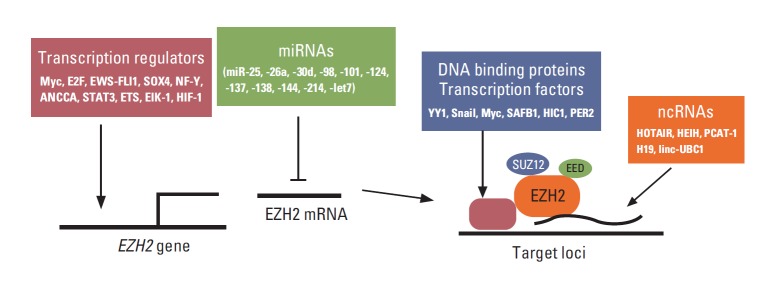
Regulators of EZH2 expression and DNA targeting in cancer. EZH2 expression is regulated by various oncogenic transcription factors and tumor suppressor miRNAs. Access to the specific DNA sites is regulated by various transcription factors and noncoding RNAs (ncRNAs).
In addition to transcriptional regulators, multiple miRNAs have been shown to directly regulate EZH2 expression, and many of them are deregulated in cancer. So far, miR-25, -26a, -30d, -98, -101, -124, -137, -138, -144, -214, -let-7, and -let-7a have been shown to be able to downregulate EZH2 expression directly in cancer cells. The downregulation of these miRNAs and the resulting upregulation of EZH2 seem to be critical for the aggressive behaviors of various cancers. These miRNAs include miR-25 and -30d in thyroid cancer [19]; miR-26a in lymphoma, nasopharyngeal carcinoma (NPC), and breast and prostate cancer [6,7,20,21]; mR-101 in NPC, glioblastoma multiforme (GBM), and prostate, bladder, gastric, head and neck (HN), and non-small cell lung cancer (NSCLC) [22-27]; miR-138 in HN cancer, GBM, and NSCLC [28-30]; let-7s in prostate cancer and NPC [31,32]; miR-124 in hepatocellular carcinoma (HCC) and gastric cancer [33,34]; miR-98 in NPC and gastric cancer [35,36]; miR-137 in melanoma [37]; miR-144 in bladder cancer [38]; and miR214 in gastric cancer and HCC [35,39]. These miRNAs are tumor suppressor like miRNA and, interestingly, miR-26a has been also shown to be regulated by epidermal growth factor receptor-mediated Ago2 phosphorylation under hypoxia condition [40].
Interaction Partners That Regulate the Recruitment of PRC2 to Specific Loci
EZH2, EED, SUZ12, and RbAp48 are core proteins in PRC2, but their DNA binding activity is weak. Thus, PRC2 requires other factors to recruit it to specific loci. Multiple transcription factors also interact with PRC2 to recruit it to specific loci, and some of them have been shown to play a critical role in cancer. Transcription factor Yin Yang 1 (YY1) interacts with EZH2 and recruits it to the specific sites to regulate gene silencing. YY1 and PRC2 are involved in muscle differentiation [41]. In endometrioid endometrial carcinoma, EZH2 and YY1 repress tumor suppressor APC and promote cell growth [42]. Snail forms a complex with EZH2 via histone deacetylase (HDAC)1/2 and recruits it to E-cadherin promoter to suppress E-cadherin expression in NPC [43]. c-Myc interacts with EZH2 and suppresses miR-101 expression in HCC, whereas MYCN interacts with EZH2 and inhibits tumor suppressor clusterin in neuroblastoma [5,44]. In addition to oncogenic transcription factor, PRC2 interacts with tumor suppressor proteins and contributes to tumor suppressor function. For example, tumor suppressor scaffold attachment factor B1 (SAFB1) interacts with PRC2 and AR and represses AR transcription machinery via H3K27me3 in prostate cancer cells [45]. Hypermethylated in cancer 1 (HIC1), which is a tumor suppressor gene that is frequently silenced or deleted in various cancers, recruits PRC2 to its target genes [46]. PER2 can interact with PRC2 and Oct1, and recruit them to Snail Slug and Twist promoters and inhibit their gene expression, thereby using PRC2 as a tumor suppressor [47]. Other transcription factors such as E2F6, Twist-1, RUNX3, and CCCTC binding factor interact with PRC2 and recruit it to repress specific target genes, but their roles in cancer are uncertain [48-51].
In addition to proteins, noncoding RNAs (ncRNAs) interact with EZH2 and play an important role in the recruitment of EZH2 to several specific loci. In cancer, HOTAIR is one of the most well described large intervening ncRNAs that interacts with EZH2 [52]. Overexpression of HOTAIR in breast cancer cells enhances cancer cell invasion and metastasis that require PRC2, while the loss of HOTAIR reduces them. HOTAIR plays an oncogenic role in colorectal cancer, pancreatic cancer, and NSCLC [53-55]. Remarkably, HOTAIR can interact with PRC2 and the LSD1/CoREST/REST repressor complex (which is responsible for the demethylation of H3K4me2), serving as a scaffold to recruit two distinct histone modifiers to the same loci [56].
In addition to HOTAIR, several ncRNAs have been shown to interact with EZH2 and are involved in EZH2-mediated cancer aggressiveness. These include HEIH in HCC [57], PCAT-1 in prostate cancer [58], and H19 and linc-UBC1 in bladder cancer [59,60]. Several other ncRNAs such as Xist, Six3OS, Meg3, AS1DHRS4, and ANCR have been shown to interact with PRC2 and regulate X-chromosome inactivation, cell differentiation, and stem cell maintenance [61-65], but their roles in cancer have not been identified. In addition to ncRNA, miR-320 directly interacts with EZH2 and argonaute-1 (AGO1) and recruits them to the promoter region of the cell cycle gene POLR3D and silences it [66]. Moreover, EZH2 also interacts with multiple intronic RNAs. Among them, the intronic RNA for SMYD3 (H3K4 methyltransferase) reduces SMYD3 expression, cell proliferation, and xenograft tumor growth in human colorectal cancer cells [67]. Interestingly, BRCA1 negatively regulates PRC2 activity by inhibiting the association between EZH2 and HOTAIR, and the loss of BRCA1 contributes to an aggressive breast cancer phenotype [68]. EZH2-HOTAIR or EZH2-Xist interaction is also regulated by CDK-mediated phosphorylation, as described in the next section [69]. PRC2 co-factor JARID2 also mediates the interaction of PRC2 and ncRNAs such as Xist and Meg3 [65,70].
Post-translational Modification of EZH2
Growing evidence shows that EZH2 activity and stability are regulated by post-translational modifications and that these modifications are critical for the biological function of PRC2 (Fig. 2). It has been reported that Akt phosphorylates EZH2 at serine 21 (S21) and inhibits its enzyme activity for H3K27me3 [71]. Later, this phosphorylation site was shown to be critical for the H3K27me3-independent function of EZH2 [72,73]. JAK2 phosphorylates EZH2 at tyrosine 641 (Y641), which promotes the interaction of EZH2 with β-TrCP and degradation of EZH2 [74]. Y641 is frequently mutated in B-cell lymphoma, and the stability and activity of the EZH2 Y641 mutant is higher than that of wild-type EZH2. Several studies have demonstrated that CDK1/2 phosphorylates EZH2 at multiple sites, including threonine 345, T416, and T487 [69,75-78]. The role of CDK-mediated phosphorylation in EZH2 function is diverse and may depend on cell types and conditions. T345 phosphorylation promotes the association between EZH2 and HOTAIR, whereas T416 phosphorylation induces the binding of NIPP1 to EZH2, and both T345 and T416 phosphorylation are critical for the recruitment of EZH2 to specific loci [69,78]. Moreover, NIPP1 maintains EZH2 phosphorylation by inhibiting its dephosphorylation by PP1 [78]. It has been showed that CDK1 phosphorylates EZH2 at T487 and that the phosphorylation induces the dissociation of EZH2 from PRC2, resulting in the inactivation of EZH2 and a reduction in cancer cell invasion [77]. In contrast, EZH2 phosphorylation at T345 promotes cell migration and proliferation [75]. T345 and T487 phosphorylation in EZH2 also promotes EZH2 ubiquitination and degradation [76].
Fig. 2.
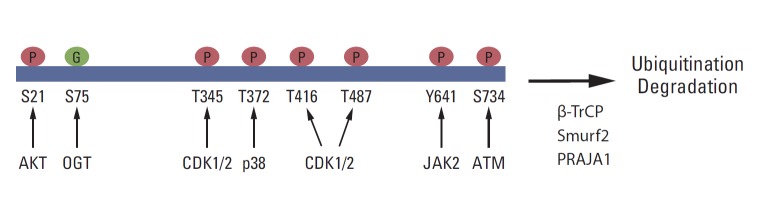
Post-translational modifications of EZH2. EZH2 is phosphorylated at S21, T345, T372, T416, T487, Y641, and S734 by the indicated kinases. S75 is glycosylated by O-linked N-acetylglucosamine transferase (OGT). In addition, EZH2 is ubiquitinated by Smurf2, β-TrCP, and PRAJA1 and undergoes degradation.
In neurons, ATM interacts with and phosphorylates EZH2 at S734, and S734 phosphorylation of EZH2 reduces PRC2 assembly, EZH2 stability, and cell death in neurons [79]. ATM-mediated phosphorylation of EZH2 is critical for neurodegeneration in ataxia-telangiectasia, which is caused by ATMmutation [79]. Moreover, p38 phosphorylates EZH2 at threonine 372 (T372) and promotes its interaction with YY1, which is critical for tumor necrosis factor-mediated Pax7 inhibition and muscle stem cell proliferation [80]. The role of ATM- or p38-mediated phosphorylation in cancer is not yet certain.
Recently, EZH2 was shown to interact with O-linked N-acetylglucosamine (GlcNAc) transferase (OGT) and to be O-GlcNAcylated at S75 in vivo. Interestingly, OGT upregulates cellular H3K27me3 levels, and S75 to alanine (S75A) mutant EZH2 is less stable than wild-type EZH2, suggesting that the O-GlcNAcylation of EZH2 may play a role in EZH2 stability and H3K27me3 [81]. EZH2 is also sumoylated in vivo and in vivo, but the functional significance of its sumoylation has not been determined [82]. EZH2 ubiquitination is critical for its protein stability. It has been shown that Smurf2 functions as an E3 ligase for EZH2 in human mesenchymal stem cells and promotes neuron differentiation [83]. β-TrCP and PRAJA1 also function as E3 ligases for EZH2 [74,84].
Function of EZH2 in Cancer
EZH2 is required for cancer cell proliferation, migration, invasion, and EMT, all of which are associated with cancer initiation, progression, and metastasis. More importantly, EZH2 is closely associated with stem cell properties, especially cancer stem cell properties, and tumor-initiating cell function [8,17,85].
In diffuse large B-cell lymphoma and follicular lymphoma, recurrent somatic mutations in the EZH2 gene have been identified, which changes amino acid tyrosine 641 (Y641) in EZH2, thereby altering its enzyme activity [86]. These mutations were originally considered a loss-of-function mutation because it reduces EZH2 methyltransferase activity toward an unmodified substrate. However, mono- to di- and di- to trimethylation activity is higher in Y641 mutant EZH2 than in wild-type EZH2. Y641 mutant EZH2 actually has higher activity of mono- to di- and di- to tri-methylation than wildtype EZH2 [87]. In addition, the Y641 mutation is always a heterogeneous mutation, and diffuse large B-cell lymphoma and follicular lymphoma with EZH2 mutation express both wild-type and Y641 mutant EZH2, resulting in higher H3K27me3 in mutant cancer cells than wild-type cells [87]. Thus, the EZH2 Y641 mutation is unique gain-of-function mutation. The oncogenic role of the Y641 mutation was further confirmed in an engineered mouse model in which conditional expression of mutant EZH2 in germinal center B-cells induced germinal center hyperplasia and promoted lymphoma formation in the presence of Bcl-2 overepxression [88]. In addition to Y641 mutation, A687V and A677G mutations have been identified as activating mutations of EZH2 in B-cell lymphoma [89,90]
Recently, a K27M mutation in one of the histone H3 variants, H3.3, was found in 50% of pediatric high-grade glioma [91,92]. The cells with H3.3K27M show reduced levels of global H3K27me3 because H3.3K27M binds to and inhibits EZH2. Interestingly, H3K27me3 and EZH2 were also shown to be locally increased in hundreds of genes in cells with the H3.3K27M mutation [93]. Therefore, alterations in H3K27me3 are closely associated with glioma.
Overexpression of EZH2 is frequently observed in multiple cancer types, including prostate, breast, bladder, ovarian, lung, liver, brain, kidney, gastric, esophageal, and pancreatic cancer and melanoma [94-104]. In many of these, EZH2 expression is also correlated with higher proliferation and aggressive behavior of cancer cells as well as poor prognosis. Indeed, multiple studies have shown that overexpression of EZH2 promotes cell proliferation, migration, and/or invasion in vivo [26,43,100,105]. Furthermore, overexpression of wild-type EZH2 in mammary epithelial cells in vivo results in epithelial hyperplasia and promotes mammary tumor initiation induced by human epidermal growth factor receptor 2/neu expression [106,107].
In some types of cancer, EZH2 functions as a tumor suppressor. Inactivating mutations of EZH2 are found in patients with myeloid malignancies including myelodysplastic syndrome and myeloproliferative neoplasms, and such EZH2 mutations are associated with poor patient survival [108,109]. Mice with conditional deletions of EZH2 and TET2 in hematopoietic stem cells, the mutations of which frequently co-exist in myeloid malignancies, develop myelodysplastic syndrome and myeloproliferative neoplasms [110]. In addition to myeloid malignacies, 25% of T-cell leukemia cases have been shown to have loss-of-function mutations and deletions of the EZH2 and SUZ12 genes [111]. Indeed, conditional deletion of EZH2 in bone marrow cells causes T-cell leukemia, indicating that EZH2 functions as a tumor suppressor in T-cell leukemia as well [112]. Moreover, mice with conditional deletion of EZH2 in the pancreatic epithelium also exhibit impaired pancreatic regeneration and acceleration of K-Ras-induced neoplasia [113]. Together, these results suggest that the role of EZH2 is cell context dependent, although EZH2 functions as an oncogenic factor in the majority of solid tumors.
EZH2 Targets in Cancer
So far, many EZH2 target genes have been identified, and HOX genes are well-known targets for EZH2 during embryonic development. Because EZH2 frequently functions as an oncogenic factor in many cancer types, most EZH2 targets identified in cancer are tumor suppressor genes. The INK4B-ARF-INK4A tumor suppressor locus is regulated by EZH2, PRC1, and PRC2, and the suppression of these genes is also critical for development of embryo as well as cancer [114-117]. Another critical target of EZH2 in multiple cancers is the E-cadherin gene (CHD1), the downregulation of which is critical for EMT and metastasis [118-121]. EZH2 also interacts with Snail to repress E-cadherin expression [43].
In addition to these proteins, multiple EZH2 target genes have been shown to be involved in EZH2-mediated cancer aggressiveness. These target genes include stathmin and Wnt antagonists in HCC [122,123]; bone morphogenetic protein receptor 1B in GBM [85]; p57 in breast and ovarian cancers [124,125]; DAB2IP, SLIT2, TIMP2/3, and CCN3/NOV in prostate cancer [126-129]; FOXC1, HOXC10, and RAD51 in breast cancer [130-132]; CXXC4 in gastric cancer [133]; MyoD in rhabdomyosarcoma [134]; rap1GAP in HN cancer [25]; CASZ1 in neuroblastoma [135]; and RUNX3 and KLF2 in multiple cancer types [136,137]. In addition, several molecules such as Bim, TRAIL, and FBXO32 play a role in apoptosis induced by the inhibition of EZH2 [138-140]. Vasohibin1 is regulated by EZH2 in tumor-associated endothelial cells, and this regulation plays a role in tumor angiogenesis [141]. EZH2 also regulates the expression other epigenetic regulators by silencing multiple miRNAs, which are critical for the oncogenic function of EZH2 [142,143].
H3K27me3-Independent Functions of EZH2
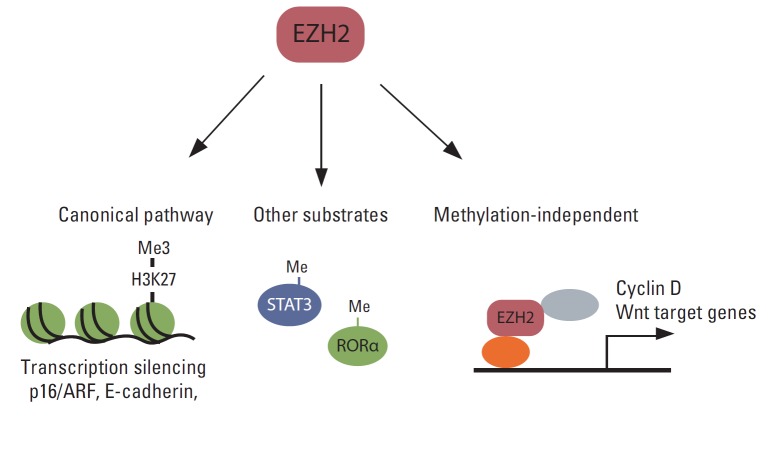
Although the primary function of EZH2 is gene silencing through the methylation of H3K27, accumulating evidence shows that EZH2 functions independently of H3K27me3 in various cancers (Fig. 3). Several reports have shown that EZH2 functions as a transcription activator. For example, EZH2 interacts with estrogen receptor (ER) α and β-catenin, and the complex regulates c-Myc and cyclin D1 expression in breast cancer cells [144]. Moreover, in a transgenic mouse model, EZH2 was shown to interact with β-catenin and promote its nuclear accumulation and activation in mammary epithelial cells [107]. In colon cancer cells, the DNA repair protein proliferating cell nuclear antigen (PCNA)-associated factor interacts with EZH2 and β-catenin and increases β-catenin target gene expression [145]. The effect of EZH2 on PCNA-associated factor-mediated activation of β-catenin does not require EZH2 methyltransferase activity. EZH2 also functions as a transcriptional co-activator with AR in castration-resistant prostate cancer cells [72]. Interestingly, this functional switch from a transcription silencer to an activator requires S21 phosphorylation of EZH2 by Akt, and activation of AR depends on EZH2 methyltransferase activity. In ER-negative basal-like breast cancer cells, EZH2 interacts with RelA/RelB and functions as a transcription co-activator of nuclear factor-kappa B. In contrast, EZH2 interacts with ER and represses nuclear factor-kappa B target gene expression by inducing H3K27me3 on their promoters in ER-positive luminal-like breast cancer cells [146]. In natural killer/T-cell lymphoma, EZH2, which is upregulated via Myc-mediated miRNA inhibition, directly activates cyclin D transcription and promotes cell proliferation independent of methyltransferase activity [147].
Fig. 3.
Various functions of EZH2 in human cancer. EZH2 silences multiple tumor suppressors such as INK4A/ARF and E-cadherin via canonical H3K27me3. EZH2 also methylates substrates other than H3K27, such as STAT3 and RORα. Furthermore, EZH2 has a methylase-independent function.
EZH2 also methylates proteins other than histone H3 and modulates their functions (Fig. 3). For example, EZH2 interacts with and methylates STAT3, resulting in increased tyrosine phosphorylation and activation of STAT3 [73]. Strikingly, AKT-mediated phosphorylation at S21 in EZH2 is critical for the interaction of EZH2 with STAT3, and this AKT-EZH2-STAT3 pathway is critical for the maintenance of glioblastoma stem cells and tumor progression. EZH2 also mono-methylates tumor suppressor, retinoic acid-related orphan nuclear receptor α (RORα) [148]. Mono-methylated RORα is recognized by the DCAF1/DDB1/CUL4 E3 ubiquitin ligase complex and undergoes ubiquitination and degradation. EZH2 also methylates GATA4 and inhibits its activity by inhibiting its interaction with p300 [149], although the role of this methylation in cancer has not been established.
EZH2 also regulates cellular functions other than transcription. Cytosolic EZH2, the level of which is higher in prostate cancer cells than in normal prostate cells, regulates actin polymerization [150,151]. However, the underlying molecular mechanism has not yet been identified. In addition, PRC2 is recruited to sites of DNA damage in a poly(ADP-ribose) polymerase-dependent manner and is involved in DNA damage repair [152]. EZH2 knockdown reduces DNA double-strand break repair and sensitizes cells to ionizing radiation. Interestingly, EZH2 and BRCA1 regulate each other and are involved in several cellular functions. Knockdown of EZH2 upregulates BRCA1 protein, which is important for the downregulation of proliferation induced by the inhibition of EZH2 in ER-negative breast cancer [153]. Consistently, EZH2 induces BRCA1 nuclear exclusion and inhibits its activity, which contributes to chromosome instability in breast cancer [154]. In contrast, BRCA1 also regulates EZH2 activity. BRCA1 inhibits EZH2-HOTAIR interaction as previously described herein [68]. Moreover BRCA1-deficient cells have higher EZH2 expression and are thereby more sensitive to EZH2 inhibition than BRCA1-proficient cells [155]. Thus, EZH2-BRCA1 interaction is complicated, and further studies may be necessary.
Therapeutic Implications of EZH2
Because EZH2 is a central regulator of proliferation, migration, invasion and stem cell properties of cancer cells, it is considered a potential drug target. 3-Deazaneplanocin A (DZNep), which is an inhibitor of S-adenosylhomocysteine hydrolase, downregulates PRC2 proteins including EZH2 and inhibits PRC2 activity [139]. DZNep treatment indeed induces the downregulation of H3K27me3, reactivates PRC2 target genes, and effectively induces apoptosis in cancer cells but not in normal cells [139]. This compound has been widely used in preclinical and in vitro studies to investigate the function of EZH2 in cancer and has been shown to effectively inhibit cell proliferation and tumor growth in various cancers [156-159]. Remarkably, the killing effect of DZNep is about 20-fold greater in BRCA1-deficient cells than in BRCA1-proficient mammary tumor cells although the underlying mechanism is not known [155]. DZNep was recently shown to induce erythroid differentiation independent of EZH2, suggesting that the effects of DZNep may be partially independent of EZH2 inhibition [160]. However, because DZNep downregulates EZH2 protein levels, it is expected to inhibit the methylation-independent functions of EZH2 [147].
Recently, several highly selective small molecule inhibitors against EZH2, such as GSK126, EPZ005687, EI1, and EPZ-6438, have been developed [161-164]. These inhibitors exhibit higher effects against the lymphoma with Y641 activation mutation of EZH2 than the one with wild-type EZH2. Currently, EPZ-6438 is being tested in clinical trials of patients with B-cell lymphoma and advanced solid tumors.
In addition to specific EZH2 inhibitors, several other drugs and compounds have been reported to be able to downregulate EZH2, and the downregulation of EZH2 is critical for their anti-cancer activity. These include curcumin [165,166], omega-3 polyunsaturated fatty acids [167], and sorafenib [168]. Moreover, inhibition of EZH2 also sensitizes cancer cells to various other anti-cancer drugs, such as HDAC inhibitors, imatinib, gemcitabine, paclitaxel, and cisplatin [27,98,140,169-174].
Conclusion
EZH2 is a critical regulator of cell proliferation, migration/invasion, and stemness in cancer and functions as an oncogenic factor in most solid tumors. Indeed, EZH2 inhibitors have shown promising anti-cancer activity against EZH2-active or -overexpressing cancer cells in multiple preclinical studies, and EPZ-6438 is currently under clinical trials. Inhibition of EZH2 also enhances several existing anticancer drugs, suggesting the potential for combination therapy using EZH2 inhibitors. Moreover, EZH2 is frequently overexpressed in multiple cancer types and is associated with poor prognosis. Therefore, EZH2 may serve as a valuable prognostic marker. In the future, additional studies will be required to establish effective combination treatment strategies and identify appropriate biomarkers in various cancer types to predict sensitivity to EZH2 inhibitors.
Herein, we introduced multiple mechanisms of EZH2 regulation, including transcriptional regulation, mRNA regulation by miRNAs, accessibility to DNA via DNA binding proteins and ncRNAs, and post-translational modifications. Because these upstream regulators of EZH2 most likely control multiple targets other than EZH2, the inhibition of these mechanisms may be an alternative approach to targeting EZH2 and even more effective than EZH2 inhibitors alone. For instance, the kinases that phosphorylate EZH2 also phosphorylate many substrates and activate other signaling pathways. Indeed, CDK inhibitors have shown anti-tumor activity in preclinical studies and are currently being tested in clinical trials. The effects of CDK inhibitors may be achieved partially through the attenuation of EZH2 activity, and EZH2 may serve as a biomarker for these drugs. Thus, the identification of upstream regulators of EZH2 may lead to effective therapeutic strategies for various cancers.
Acknowledgments
This work was supported by the following grants: National Institutes of Health (CA109311, CA099031); Ministry of Health and Welfare, China Medical University Hospital Cancer Research Center of Excellence (MOHW103-TD-B-111; Taiwan), the Program for Stem Cell and Regenerative Medicine Frontier Research (NSC102-2321-B-039; Taiwan).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Morey L, Helin K. Polycomb group protein-mediated repression of transcription. Trends Biochem Sci. 2010;35:323–32. doi: 10.1016/j.tibs.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 3.O'Meara MM, Simon JA. Inner workings and regulatory inputs that control Polycomb repressive complex 2. Chromosoma. 2012;121:221–34. doi: 10.1007/s00412-012-0361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh CM, Iwata T, Zheng Q, Bethel C, Yegnasubramanian S, De Marzo AM. Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms. Oncotarget. 2011;2:669–83. doi: 10.18632/oncotarget.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Zhang X, Jia LT, Hu SJ, Zhao J, Yang JD, et al. c-Mycmediated epigenetic silencing of MicroRNA-101 contributes to dysregulation of multiple pathways in hepatocellular carcinoma. Hepatology. 2014;59:1850–63. doi: 10.1002/hep.26720. [DOI] [PubMed] [Google Scholar]
- 6.Sander S, Bullinger L, Klapproth K, Fiedler K, Kestler HA, Barth TF, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112:4202–12. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- 7.Salvatori B, Iosue I, Djodji Damas N, Mangiavacchi A, Chiaretti S, Messina M, et al. Critical role of c-Myc in acute myeloid leukemia involving direct regulation of miR-26a and histone methyltransferase EZH2. Genes Cancer. 2011;2:585–92. doi: 10.1177/1947601911416357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suva ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle JC, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69:9211–8. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- 9.Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–35. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalashnikova EV, Revenko AS, Gemo AT, Andrews NP, Tepper CG, Zou JX, et al. ANCCA/ATAD2 overexpression identifies breast cancer patients with poor prognosis, acting to drive proliferation and survival of triple-negative cells through control of B-Myb and EZH2. Cancer Res. 2010;70:9402–12. doi: 10.1158/0008-5472.CAN-10-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan Z, Zou JX, Yang P, Wang Y, Borowsky AD, Gao AC, et al. Developmental and androgenic regulation of chromatin regulators EZH2 and ANCCA/ATAD2 in the prostate via MLL histone methylase complex. Prostate. 2013;73:455–66. doi: 10.1002/pros.22587. [DOI] [PubMed] [Google Scholar]
- 12.Richter GH, Plehm S, Fasan A, Rossler S, Unland R, Bennani-Baiti IM, et al. EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc Natl Acad Sci U S A. 2009;106:5324–9. doi: 10.1073/pnas.0810759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiwari N, Tiwari VK, Waldmeier L, Balwierz PJ, Arnold P, Pachkov M, et al. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–83. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Garipov A, Li H, Bitler BG, Thapa RJ, Balachandran S, Zhang R. NF-YA underlies EZH2 upregulation and is essential for proliferation of human epithelial ovarian cancer cells. Mol Cancer Res. 2013;11:360–9. doi: 10.1158/1541-7786.MCR-12-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YW, Ren LL, Xiong H, Du W, Yu YN, Sun TT, et al. Role of STAT3 and vitamin D receptor in EZH2-mediated invasion of human colorectal cancer. J Pathol. 2013;230:277–90. doi: 10.1002/path.4179. [DOI] [PubMed] [Google Scholar]
- 16.Kunderfranco P, Mello-Grand M, Cangemi R, Pellini S, Mensah A, Albertini V, et al. ETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3.1 in prostate cancer. PLoS One. 2010;5: doi: 10.1371/journal.pone.0010547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CJ, Yang JY, Xia W, Chen CT, Xie X, Chao CH, et al. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling. Cancer Cell. 2011;19:86–100. doi: 10.1016/j.ccr.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii S, Tokita K, Wada N, Ito K, Yamauchi C, Ito Y, et al. MEKERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes. Oncogene. 2011;30:4118–28. doi: 10.1038/onc.2011.118. [DOI] [PubMed] [Google Scholar]
- 19.Esposito F, Tornincasa M, Pallante P, Federico A, Borbone E, Pierantoni GM, et al. Down-regulation of the miR-25 and miR-30d contributes to the development of anaplastic thyroid carcinoma targeting the polycomb protein EZH2. J Clin Endocrinol Metab. 2012;97:E710–8. doi: 10.1210/jc.2011-3068. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, Liu XX, He JR, Zhou CX, Guo M, He M, et al. Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis. 2011;32:2–9. doi: 10.1093/carcin/bgq209. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q, et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225–33. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- 22.Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–9. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman JM, Liang G, Liu CC, Wolff EM, Tsai YC, Ye W, et al. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–9. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 24.Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT, Xia YJ, et al. MicroRNA-101 is down-regulated in gastric cancer and involved in cell migration and invasion. Eur J Cancer. 2010;46:2295–303. doi: 10.1016/j.ejca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee R, Mani RS, Russo N, Scanlon CS, Tsodikov A, Jing X, et al. The tumor suppressor gene rap1GAP is silenced by miR-101-mediated EZH2 overexpression in invasive squamous cell carcinoma. Oncogene. 2011;30:4339–49. doi: 10.1038/onc.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smits M, Nilsson J, Mir SE, van der Stoop PM, Hulleman E, Niers JM, et al. miR-101 is down-regulated in glioblastoma resulting in EZH2-induced proliferation, migration, and angiogenesis. Oncotarget. 2010;1:710–20. doi: 10.18632/oncotarget.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JG, Guo JF, Liu DL, Liu Q, Wang JJ. MicroRNA-101 exerts tumor-suppressive functions in non-small cell lung cancer through directly targeting enhancer of zeste homolog 2. J Thorac Oncol. 2011;6:671–8. doi: 10.1097/JTO.0b013e318208eb35. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Wang C, Chen Z, Jin Y, Wang Y, Kolokythas A, et al. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem J. 2011;440:23–31. doi: 10.1042/BJ20111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu S, Huang D, Yin D, Li F, Li X, Kung HF, et al. Suppression of tumorigenicity by microRNA-138 through inhibition of EZH2-CDK4/6-pRb-E2F1 signal loop in glioblastoma multiforme. Biochim Biophys Acta. 2013;1832:1697–707. doi: 10.1016/j.bbadis.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Zhang H, Zhao M, Lv Z, Zhang X, Qin X, et al. MiR-138 inhibits tumor growth through repression of EZH2 in nonsmall cell lung cancer. Cell Physiol Biochem. 2013;31:56–65. doi: 10.1159/000343349. [DOI] [PubMed] [Google Scholar]
- 31.Kong D, Heath E, Chen W, Cher ML, Powell I, Heilbrun L, et al. Loss of let-7 up-regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PLoS One. 2012;7: doi: 10.1371/journal.pone.0033729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai K, Wan Y, Sun G, Shi L, Bao X, Wang Z. Let-7a inhibits proliferation and induces apoptosis by targeting EZH2 in nasopharyngeal carcinoma cells. Oncol Rep. 2012;28:2101–6. doi: 10.3892/or.2012.2027. [DOI] [PubMed] [Google Scholar]
- 33.Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH, Chen SP, et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut. 2012;61:278–89. doi: 10.1136/gut.2011.239145. [DOI] [PubMed] [Google Scholar]
- 34.Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie Y, et al. microRNA- 124 inhibits proliferation and induces apoptosis by directly repressing EZH2 in gastric cancer. Mol Cell Biochem. 2014 Mar 22; doi: 10.1007/s11010-014-2028-0. [Epub]. http://dx.doi.org/10.1007/s11010-014-2028-0. [DOI] [PubMed] [Google Scholar]
- 35.Huang SD, Yuan Y, Zhuang CW, Li BL, Gong DJ, Wang SG, et al. MicroRNA-98 and microRNA-214 post-transcriptionally regulate enhancer of zeste homolog 2 and inhibit migration and invasion in human esophageal squamous cell carcinoma. Mol Cancer. 2012;11:51. doi: 10.1186/1476-4598-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alajez NM, Shi W, Hui AB, Bruce J, Lenarduzzi M, Ito E, et al. Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98. Cell Death Dis. 2010;1: doi: 10.1038/cddis.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo C, Tetteh PW, Merz PR, Dickes E, Abukiwan A, Hotz-Wagenblatt A, et al. miR-137 inhibits the invasion of melanoma cells through downregulation of multiple oncogenic target genes. J Invest Dermatol. 2013;133:768–75. doi: 10.1038/jid.2012.357. [DOI] [PubMed] [Google Scholar]
- 38.Guo Y, Ying L, Tian Y, Yang P, Zhu Y, Wang Z, et al. miR-144 downregulation increases bladder cancer cell proliferation by targeting EZH2 and regulating Wnt signaling. FEBS J. 2013;280:4531–8. doi: 10.1111/febs.12417. [DOI] [PubMed] [Google Scholar]
- 39.Xia H, Ooi LL, Hui KM. MiR-214 targets beta-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma. PLoS One. 2012;7: doi: 10.1371/journal.pone.0044206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim SO, et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature. 2013;497:383–7. doi: 10.1038/nature12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18:2627–38. doi: 10.1101/gad.1241904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Zhou L, Lu L, Wang L, Li X, Jiang P, et al. A novel miR-193a-5p-YY1-APC regulatory axis in human endometrioid endometrial adenocarcinoma. Oncogene. 2013;32:3432–42. doi: 10.1038/onc.2012.360. [DOI] [PubMed] [Google Scholar]
- 43.Tong ZT, Cai MY, Wang XG, Kong LL, Mai SJ, Liu YH, et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene. 2012;31:583–94. doi: 10.1038/onc.2011.254. [DOI] [PubMed] [Google Scholar]
- 44.Corvetta D, Chayka O, Gherardi S, D'Acunto CW, Cantilena S, Valli E, et al. Physical interaction between MYCN oncogene and polycomb repressive complex 2 (PRC2) in neuroblastoma: functional and therapeutic implications. J Biol Chem. 2013;288:8332–41. doi: 10.1074/jbc.M113.454280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukhopadhyay NK, Kim J, You S, Morello M, Hager MH, Huang WC, et al. Scaffold attachment factor B1 regulates the androgen receptor in concert with the growth inhibitory kinase MST1 and the methyltransferase EZH2. Oncogene. 2014;33:3235–45. doi: 10.1038/onc.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boulay G, Dubuissez M, Van Rechem C, Forget A, Helin K, Ayrault O, et al. Hypermethylated in cancer 1 (HIC1) recruits polycomb repressive complex 2 (PRC2) to a subset of its target genes through interaction with human polycomb-like (hPCL) proteins. J Biol Chem. 2012;287:10509–24. doi: 10.1074/jbc.M111.320234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang-Verslues WW, Chang PH, Jeng YM, Kuo WH, Chiang PH, Chang YC, et al. Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy. Proc Natl Acad Sci U S A. 2013;110:12331–6. doi: 10.1073/pnas.1222684110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Niu B, Hu JF, Ge S, Wang H, Li T, et al. Interruption of intrachromosomal looping by CCCTC binding factor decoy proteins abrogates genomic imprinting of human insulin-like growth factor II. J Cell Biol. 2011;193:475–87. doi: 10.1083/jcb.201101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leseva M, Santostefano KE, Rosenbluth AL, Hamazaki T, Terada N. E2f6-mediated repression of the meiotic Stag3 and Smc1beta genes during early embryonic development requires Ezh2 and not the de novo methyltransferase Dnmt3b. Epigenetics. 2013;8:873–84. doi: 10.4161/epi.25522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cakouros D, Isenmann S, Cooper L, Zannettino A, Anderson P, Glackin C, et al. Twist-1 induces Ezh2 recruitment regulating histone methylation along the Ink4A/Arf locus in mesenchymal stem cells. Mol Cell Biol. 2012;32:1433–41. doi: 10.1128/MCB.06315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciavatta DJ, Yang J, Preston GA, Badhwar AK, Xiao H, Hewins P, et al. Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis. J Clin Invest. 2010;120:3209–19. doi: 10.1172/JCI40034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, et al. Long noncoding RNA HOTAIR regulates polycombdependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 54.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–25. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W, et al. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21(WAF1/CIP1) expression. PLoS One. 2013;8: doi: 10.1371/journal.pone.0077293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang F, Zhang L, Huo XS, Yuan JH, Xu D, Yuan SX, et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–89. doi: 10.1002/hep.24563. [DOI] [PubMed] [Google Scholar]
- 58.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–9. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333:213–21. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 60.He W, Cai Q, Sun F, Zhong G, Wang P, Liu H, et al. linc-UBC1 physically associates with polycomb repressive complex 2 (PRC2) and acts as a negative prognostic factor for lymph node metastasis and survival in bladder cancer. Biochim Biophys Acta. 2013;1832:1528–37. doi: 10.1016/j.bbadis.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–6. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rapicavoli NA, Poth EM, Zhu H, Blackshaw S. The long noncoding RNA Six3OS acts in trans to regulate retinal development by modulating Six3 activity. Neural Dev. 2011;6:32. doi: 10.1186/1749-8104-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Q, Su Z, Xu X, Liu G, Song X, Wang R, et al. AS1DHRS4, a head-to-head natural antisense transcript, silences the DHRS4 gene cluster in cis and trans. Proc Natl Acad Sci U S A. 2012;109:14110–5. doi: 10.1073/pnas.1116597109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu L, Xu PC. Downregulated LncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochem Biophys Res Commun. 2013;432:612–7. doi: 10.1016/j.bbrc.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 65.Kaneko S, Bonasio R, Saldana-Meyer R, Yoshida T, Son J, Nishino K, et al. Interactions between JARID2 and noncoding RNAs regulate PRC2 recruitment to chromatin. Mol Cell. 2014;53:290–300. doi: 10.1016/j.molcel.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim DH, Saetrom P, Snove O, Jr, Rossi JJ. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci U S A. 2008;105:16230–5. doi: 10.1073/pnas.0808830105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guil S, Soler M, Portela A, Carrere J, Fonalleras E, Gomez A, et al. Intronic RNAs mediate EZH2 regulation of epigenetic targets. Nat Struct Mol Biol. 2012;19:664–70. doi: 10.1038/nsmb.2315. [DOI] [PubMed] [Google Scholar]
- 68.Wang L, Zeng X, Chen S, Ding L, Zhong J, Zhao JC, et al. BRCA1 is a negative modulator of the PRC2 complex. EMBO J. 2013;32:1584–97. doi: 10.1038/emboj.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaneko S, Li G, Son J, Xu CF, Margueron R, Neubert TA, et al. Phosphorylation of the PRC2 component Ezh2 is cell cycleregulated and up-regulates its binding to ncRNA. Genes Dev. 2010;24:2615–20. doi: 10.1101/gad.1983810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.da Rocha ST, Boeva V, Escamilla-Del-Arenal M, Ancelin K, Granier C, Matias NR, et al. Jarid2 is implicated in the initial Xist-induced targeting of PRC2 to the inactive X chromosome. Mol Cell. 2014;53:301–16. doi: 10.1016/j.molcel.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, et al. Aktmediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–10. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- 72.Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338:1465–9. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim E, Kim M, Woo DH, Shin Y, Shin J, Chang N, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23:839–52. doi: 10.1016/j.ccr.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahasrabuddhe AA, Chen X, Chung F, Velusamy T, Lim MS, Elenitoba-Johnson KS. Oncogenic Y641 mutations in EZH2 prevent Jak2/beta-TrCP-mediated degradation. Oncogene. 2014 Jan 27; doi: 10.1038/onc.2013.571. [Epub]. http://dx.doi.org/10.1038/onc.2013.571. [DOI] [PubMed] [Google Scholar]
- 75.Chen S, Bohrer LR, Rai AN, Pan Y, Gan L, Zhou X, et al. Cyclin-dependent kinases regulate epigenetic gene silencing through phosphorylation of EZH2. Nat Cell Biol. 2010;12:1108–14. doi: 10.1038/ncb2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu SC, Zhang Y. Cyclin-dependent kinase 1 (CDK1)-mediated phosphorylation of enhancer of zeste 2 (Ezh2) regulates its stability. J Biol Chem. 2011;286:28511–9. doi: 10.1074/jbc.M111.240515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei Y, Chen YH, Li LY, Lang J, Yeh SP, Shi B, et al. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol. 2011;13:87–94. doi: 10.1038/ncb2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minnebo N, Gornemann J, O'Connell N, Van Dessel N, Derua R, Vermunt MW, et al. NIPP1 maintains EZH2 phosphorylation and promoter occupancy at proliferation-related target genes. Nucleic Acids Res. 2013;41:842–54. doi: 10.1093/nar/gks1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, Hart RP, Mallimo EM, Swerdel MR, Kusnecov AW, Herrup K. EZH2-mediated H3K27 trimethylation mediates neurodegeneration in ataxia-telangiectasia. Nat Neurosci. 2013;16:1745–53. doi: 10.1038/nn.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palacios D, Mozzetta C, Consalvi S, Caretti G, Saccone V, Proserpio V, et al. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–69. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chu CS, Lo PW, Yeh YH, Hsu PH, Peng SH, Teng YC, et al. O-GlcNAcylation regulates EZH2 protein stability and function. Proc Natl Acad Sci U S A. 2014;111:1355–60. doi: 10.1073/pnas.1323226111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riising EM, Boggio R, Chiocca S, Helin K, Pasini D. The polycomb repressive complex 2 is a potential target of SUMO modifications. PLoS One. 2008;3: doi: 10.1371/journal.pone.0002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu YL, Chou RH, Shyu WC, Hsieh SC, Wu CS, Chiang SY, et al. Smurf2-mediated degradation of EZH2 enhances neuron differentiation and improves functional recovery after ischaemic stroke. EMBO Mol Med. 2013;5:531–47. doi: 10.1002/emmm.201201783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zoabi M, Sadeh R, de Bie P, Marquez VE, Ciechanover A. PRAJA1 is a ubiquitin ligase for the polycomb repressive complex 2 proteins. Biochem Biophys Res Commun. 2011;408:393–8. doi: 10.1016/j.bbrc.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 85.Lee J, Son MJ, Woolard K, Donin NM, Li A, Cheng CH, et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13:69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181–5. doi: 10.1038/ng.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sneeringer CJ, Scott MP, Kuntz KW, Knutson SK, Pollock RM, Richon VM, et al. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas. Proc Natl Acad Sci U S A. 2010;107:20980–5. doi: 10.1073/pnas.1012525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beguelin W, Popovic R, Teater M, Jiang Y, Bunting KL, Rosen M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23:677–92. doi: 10.1016/j.ccr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Majer CR, Jin L, Scott MP, Knutson SK, Kuntz KW, Keilhack H, et al. A687V EZH2 is a gain-of-function mutation found in lymphoma patients. FEBS Lett. 2012;586:3448–51. doi: 10.1016/j.febslet.2012.07.066. [DOI] [PubMed] [Google Scholar]
- 90.McCabe MT, Graves AP, Ganji G, Diaz E, Halsey WS, Jiang Y, et al. Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27) Proc Natl Acad Sci U S A. 2012;109:2989–94. doi: 10.1073/pnas.1116418109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lewis PW, Muller MM, Koletsky MS, Cordero F, Lin S, Banaszynski LA, et al. Inhibition of PRC2 activity by a gain-offunction H3 mutation found in pediatric glioblastoma. Science. 2013;340:857–61. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bender S, Tang Y, Lindroth AM, Hovestadt V, Jones DT, Kool M, et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell. 2013;24:660–72. doi: 10.1016/j.ccr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 93.Chan KM, Fang D, Gan H, Hashizume R, Yu C, Schroeder M, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27:985–90. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–73. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 95.Raman JD, Mongan NP, Tickoo SK, Boorjian SA, Scherr DS, Gudas LJ. Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res. 2005;11(24 Pt 1):8570–6. doi: 10.1158/1078-0432.CCR-05-1047. [DOI] [PubMed] [Google Scholar]
- 96.Matsukawa Y, Semba S, Kato H, Ito A, Yanagihara K, Yokozaki H. Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer. Cancer Sci. 2006;97:484–91. doi: 10.1111/j.1349-7006.2006.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kondo Y, Shen L, Suzuki S, Kurokawa T, Masuko K, Tanaka Y, et al. Alterations of DNA methylation and histone modifications contribute to gene silencing in hepatocellular carcinomas. Hepatol Res. 2007;37:974–83. doi: 10.1111/j.1872-034X.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 98.Ougolkov AV, Bilim VN, Billadeau DD. Regulation of pancreatic tumor cell proliferation and chemoresistance by the histone methyltransferase enhancer of zeste homologue 2. Clin Cancer Res. 2008;14:6790–6. doi: 10.1158/1078-0432.CCR-08-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee HW, Choe M. Expression of EZH2 in renal cell carcinoma as a novel prognostic marker. Pathol Int. 2012;62:735–41. doi: 10.1111/pin.12001. [DOI] [PubMed] [Google Scholar]
- 100.Rao ZY, Cai MY, Yang GF, He LR, Mai SJ, Hua WF, et al. EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-beta1 and is a predictor of outcome in ovarian carcinoma patients. Carcinogenesis. 2010;31:1576–83. doi: 10.1093/carcin/bgq150. [DOI] [PubMed] [Google Scholar]
- 101.Crea F, Hurt EM, Farrar WL. Clinical significance of Polycomb gene expression in brain tumors. Mol Cancer. 2010;9:265. doi: 10.1186/1476-4598-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamada A, Fujii S, Daiko H, Nishimura M, Chiba T, Ochiai A. Aberrant expression of EZH2 is associated with a poor outcome and P53 alteration in squamous cell carcinoma of the esophagus. Int J Oncol. 2011;38:345–53. doi: 10.3892/ijo.2010.868. [DOI] [PubMed] [Google Scholar]
- 103.Behrens C, Solis LM, Lin H, Yuan P, Tang X, Kadara H, et al. EZH2 protein expression associates with the early pathogenesis, tumor progression, and prognosis of non-small cell lung carcinoma. Clin Cancer Res. 2013;19:6556–65. doi: 10.1158/1078-0432.CCR-12-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alford SH, Toy K, Merajver SD, Kleer CG. Increased risk for distant metastasis in patients with familial early-stage breast cancer and high EZH2 expression. Breast Cancer Res Treat. 2012;132:429–37. doi: 10.1007/s10549-011-1591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crea F, Hurt EM, Mathews LA, Cabarcas SM, Sun L, Marquez VE, et al. Pharmacologic disruption of Polycomb repressive complex 2 inhibits tumorigenicity and tumor progression in prostate cancer. Mol Cancer. 2011;10:40. doi: 10.1186/1476-4598-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gonzalez ME, Moore HM, Li X, Toy KA, Huang W, Sabel MS, et al. EZH2 expands breast stem cells through activation of NOTCH1 signaling. Proc Natl Acad Sci U S A. 2014;111:3098–103. doi: 10.1073/pnas.1308953111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li X, Gonzalez ME, Toy K, Filzen T, Merajver SD, Kleer CG. Targeted overexpression of EZH2 in the mammary gland disrupts ductal morphogenesis and causes epithelial hyperplasia. Am J Pathol. 2009;175:1246–54. doi: 10.2353/ajpath.2009.090042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nikoloski G, Langemeijer SM, Kuiper RP, Knops R, Massop M, Tonnissen ER, et al. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. 2010;42:665–7. doi: 10.1038/ng.620. [DOI] [PubMed] [Google Scholar]
- 109.Ernst T, Chase AJ, Score J, Hidalgo-Curtis CE, Bryant C, Jones AV, et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010;42:722–6. doi: 10.1038/ng.621. [DOI] [PubMed] [Google Scholar]
- 110.Muto T, Sashida G, Oshima M, Wendt GR, Mochizuki-Kashio M, Nagata Y, et al. Concurrent loss of Ezh2 and Tet2 cooperates in the pathogenesis of myelodysplastic disorders. J Exp Med. 2013;210:2627–39. doi: 10.1084/jem.20131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ntziachristos P, Tsirigos A, Van Vlierberghe P, Nedjic J, Trimarchi T, Flaherty MS, et al. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat Med. 2012;18:298–301. doi: 10.1038/nm.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simon C, Chagraoui J, Krosl J, Gendron P, Wilhelm B, Lemieux S, et al. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes Dev. 2012;26:651–6. doi: 10.1101/gad.186411.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mallen-St Clair J, Soydaner-Azeloglu R, Lee KE, Taylor L, Livanos A, Pylayeva-Gupta Y, et al. EZH2 couples pancreatic regeneration to neoplastic progression. Genes Dev. 2012;26:439–44. doi: 10.1101/gad.181800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–30. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, et al. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–35. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reynolds PA, Sigaroudinia M, Zardo G, Wilson MB, Benton GM, Miller CJ, et al. Tumor suppressor p16INK4A regulates polycomb-mediated DNA hypermethylation in human mammary epithelial cells. J Biol Chem. 2006;281:24790–802. doi: 10.1074/jbc.M604175200. [DOI] [PubMed] [Google Scholar]
- 117.Sasaki M, Yamaguchi J, Itatsu K, Ikeda H, Nakanuma Y. Over-expression of polycomb group protein EZH2 relates to decreased expression of p16 INK4a in cholangiocarcinogenesis in hepatolithiasis. J Pathol. 2008;215:175–83. doi: 10.1002/path.2345. [DOI] [PubMed] [Google Scholar]
- 118.Wang C, Liu X, Chen Z, Huang H, Jin Y, Kolokythas A, et al. Polycomb group protein EZH2-mediated E-cadherin repression promotes metastasis of oral tongue squamous cell carcinoma. Mol Carcinog. 2013;52:229–36. doi: 10.1002/mc.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu H, Simons DL, Segall I, Carcamo-Cavazos V, Schwartz EJ, Yan N, et al. PRC2/EED-EZH2 complex is up-regulated in breast cancer lymph node metastasis compared to primary tumor and correlates with tumor proliferation in situ. PLoS One. 2012;7: doi: 10.1371/journal.pone.0051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fujii S, Ochiai A. Enhancer of zeste homolog 2 downregulates E-cadherin by mediating histone H3 methylation in gastric cancer cells. Cancer Sci. 2008;99:738–46. doi: 10.1111/j.1349-7006.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27:7274–84. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen Y, Lin MC, Yao H, Wang H, Zhang AQ, Yu J, et al. Lentivirus-mediated RNA interference targeting enhancer of zeste homolog 2 inhibits hepatocellular carcinoma growth through down-regulation of stathmin. Hepatology. 2007;46:200–8. doi: 10.1002/hep.21668. [DOI] [PubMed] [Google Scholar]
- 123.Cheng AS, Lau SS, Chen Y, Kondo Y, Li MS, Feng H, et al. EZH2-mediated concordant repression of Wnt antagonists promotes beta-catenin-dependent hepatocarcinogenesis. Cancer Res. 2011;71:4028–39. doi: 10.1158/0008-5472.CAN-10-3342. [DOI] [PubMed] [Google Scholar]
- 124.Yang X, Karuturi RK, Sun F, Aau M, Yu K, Shao R, et al. CDKN1C (p57) is a direct target of EZH2 and suppressed by multiple epigenetic mechanisms in breast cancer cells. PLoS One. 2009;4: doi: 10.1371/journal.pone.0005011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo J, Cai J, Yu L, Tang H, Chen C, Wang Z. EZH2 regulates expression of p57 and contributes to progression of ovarian cancer in vivo and in vivo. Cancer Sci. 2011;102:530–9. doi: 10.1111/j.1349-7006.2010.01836.x. [DOI] [PubMed] [Google Scholar]
- 126.Yu J, Cao Q, Yu J, Wu L, Dallol A, Li J, et al. The neuronal repellent SLIT2 is a target for repression by EZH2 in prostate cancer. Oncogene. 2010;29:5370–80. doi: 10.1038/onc.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu L, Runkle C, Jin HJ, Yu J, Li J, Yang X, et al. CCN3/NOV gene expression in human prostate cancer is directly suppressed by the androgen receptor. Oncogene. 2014;33:504–13. doi: 10.1038/onc.2012.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shin YJ, Kim JH. The role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cells. PLoS One. 2012;7: doi: 10.1371/journal.pone.0030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Min J, Zaslavsky A, Fedele G, McLaughlin SK, Reczek EE, De Raedt T, et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med. 2010;16:286–94. doi: 10.1038/nm.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Du J, Li L, Ou Z, Kong C, Zhang Y, Dong Z, et al. FOXC1, a target of polycomb, inhibits metastasis of breast cancer cells. Breast Cancer Res Treat. 2012;131:65–73. doi: 10.1007/s10549-011-1396-3. [DOI] [PubMed] [Google Scholar]
- 131.Pathiraja TN, Nayak SR, Xi Y, Jiang S, Garee JP, Edwards DP, et al. Epigenetic reprogramming of HOXC10 in endocrine-resistant breast cancer. Sci Transl Med. 2014;6:229ra41. doi: 10.1126/scitranslmed.3008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zeidler M, Varambally S, Cao Q, Chinnaiyan AM, Ferguson DO, Merajver SD, et al. The Polycomb group protein EZH2 impairs DNA repair in breast epithelial cells. Neoplasia. 2005;7:1011–9. doi: 10.1593/neo.05472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu H, Sun J, Wang F, Feng L, Ma Y, Shen Q, et al. Enhancer of zeste homolog 2 activates wnt signaling through downregulating CXXC finger protein 4. Cell Death Dis. 2013;4: doi: 10.1038/cddis.2013.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marchesi I, Fiorentino FP, Rizzolio F, Giordano A, Bagella L. The ablation of EZH2 uncovers its crucial role in rhabdomyosarcoma formation. Cell Cycle. 2012;11:3828–36. doi: 10.4161/cc.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang C, Liu Z, Woo CW, Li Z, Wang L, Wei JS, et al. EZH2 mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR. Cancer Res. 2012;72:315–24. doi: 10.1158/0008-5472.CAN-11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fujii S, Ito K, Ito Y, Ochiai A. Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by increasing histone H3 methylation. J Biol Chem. 2008;283:17324–32. doi: 10.1074/jbc.M800224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taniguchi H, Jacinto FV, Villanueva A, Fernandez AF, Yamamoto H, Carmona FJ, et al. Silencing of Kruppel-like factor 2 by the histone methyltransferase EZH2 in human cancer. Oncogene. 2012;31:1988–94. doi: 10.1038/onc.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu ZL, Zheng SS, Li ZM, Qiao YY, Aau MY, Yu Q. Polycomb protein EZH2 regulates E2F1-dependent apoptosis through epigenetically modulating Bim expression. Cell Death Differ. 2010;17:801–10. doi: 10.1038/cdd.2009.162. [DOI] [PubMed] [Google Scholar]
- 139.Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–63. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Carvalho DD, Binato R, Pereira WO, Leroy JM, Colassanti MD, Proto-Siqueira R, et al. BCR-ABL-mediated upregulation of PRAME is responsible for knocking down TRAIL in CML patients. Oncogene. 2011;30:223–33. doi: 10.1038/onc.2010.409. [DOI] [PubMed] [Google Scholar]
- 141.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18:185–97. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Asangani IA, Ateeq B, Cao Q, Dodson L, Pandhi M, Kunju LP, et al. Characterization of the EZH2-MMSET histone methyltransferase regulatory axis in cancer. Mol Cell. 2013;49:80–93. doi: 10.1016/j.molcel.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, Prensner JR, et al. Coordinated regulation of Polycomb group complexes through microRNAs in cancer. Cancer Cell. 2011;20:187–99. doi: 10.1016/j.ccr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shi B, Liang J, Yang X, Wang Y, Zhao Y, Wu H, et al. Integration of estrogen and Wnt signaling circuits by the Polycomb group protein EZH2 in breast cancer cells. Mol Cell Biol. 2007;27:5105–19. doi: 10.1128/MCB.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jung HY, Jun S, Lee M, Kim HC, Wang X, Ji H, et al. PAF and EZH2 induce Wnt/beta-catenin signaling hyperactivation. Mol Cell. 2013;52:193–205. doi: 10.1016/j.molcel.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee ST, Li Z, Wu Z, Aau M, Guan P, Karuturi RK, et al. Context-specific regulation of NF-kappaB target gene expression by EZH2 in breast cancers. Mol Cell. 2011;43:798–810. doi: 10.1016/j.molcel.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 147.Yan J, Ng SB, Tay JL, Lin B, Koh TL, Tan J, et al. EZH2 overexpression in natural killer/T-cell lymphoma confers growth advantage independently of histone methyltransferase activity. Blood. 2013;121:4512–20. doi: 10.1182/blood-2012-08-450494. [DOI] [PubMed] [Google Scholar]
- 148.Lee JM, Lee JS, Kim H, Kim K, Park H, Kim JY, et al. EZH2 generates a methyl degron that is recognized by the DCAF1/DDB1/CUL4 E3 ubiquitin ligase complex. Mol Cell. 2012;48:572–86. doi: 10.1016/j.molcel.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 149.He A, Shen X, Ma Q, Cao J, von Gise A, Zhou P, et al. PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes Dev. 2012;26:37–42. doi: 10.1101/gad.173930.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Su IH, Dobenecker MW, Dickinson E, Oser M, Basavaraj A, Marqueron R, et al. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell. 2005;121:425–36. doi: 10.1016/j.cell.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 151.Bryant RJ, Winder SJ, Cross SS, Hamdy FC, Cunliffe VT. The Polycomb group protein EZH2 regulates actin polymerization in human prostate cancer cells. Prostate. 2008;68:255–63. doi: 10.1002/pros.20705. [DOI] [PubMed] [Google Scholar]
- 152.Campbell S, Ismail IH, Young LC, Poirier GG, Hendzel MJ. Polycomb repressive complex 2 contributes to DNA doublestrand break repair. Cell Cycle. 2013;12:2675–83. doi: 10.4161/cc.25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gonzalez ME, Li X, Toy K, DuPrie M, Ventura AC, Banerjee M, et al. Downregulation of EZH2 decreases growth of estrogen receptor-negative invasive breast carcinoma and requires BRCA1. Oncogene. 2009;28:843–53. doi: 10.1038/onc.2008.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gonzalez ME, DuPrie ML, Krueger H, Merajver SD, Ventura AC, Toy KA, et al. Histone methyltransferase EZH2 induces Akt-dependent genomic instability and BRCA1 inhibition in breast cancer. Cancer Res. 2011;71:2360–70. doi: 10.1158/0008-5472.CAN-10-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Puppe J, Drost R, Liu X, Joosse SA, Evers B, Cornelissen-Steijger P, et al. BRCA1-deficient mammary tumor cells are dependent on EZH2 expression and sensitive to Polycomb repressive complex 2-inhibitor 3-deazaneplanocin A. Breast Cancer Res. 2009;11:R63. doi: 10.1186/bcr2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alimova I, Venkataraman S, Harris P, Marquez VE, Northcott PA, Dubuc A, et al. Targeting the enhancer of zeste homologue 2 in medulloblastoma. Int J Cancer. 2012;131:1800–9. doi: 10.1002/ijc.27455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kemp CD, Rao M, Xi S, Inchauste S, Mani H, Fetsch P, et al. Polycomb repressor complex-2 is a novel target for mesothelioma therapy. Clin Cancer Res. 2012;18:77–90. doi: 10.1158/1078-0432.CCR-11-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kalushkova A, Fryknas M, Lemaire M, Fristedt C, Agarwal P, Eriksson M, et al. Polycomb target genes are silenced in multiple myeloma. PLoS One. 2010;5: doi: 10.1371/journal.pone.0011483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gannon OM, Merida de Long L, Endo-Munoz L, Hazar-Rethinam M, Saunders NA. Dysregulation of the repressive H3K27 trimethylation mark in head and neck squamous cell carcinoma contributes to dysregulated squamous differentiation. Clin Cancer Res. 2013;19:428–41. doi: 10.1158/1078-0432.CCR-12-2505. [DOI] [PubMed] [Google Scholar]
- 160.Fujiwara T, Saitoh H, Inoue A, Kobayashi M, Okitsu Y, Katsuoka Y, et al. 3-Deazaneplanocin A (DZNep), an inhibitor of S-adenosylmethionine-dependent methyltransferase, promotes erythroid differentiation. J Biol Chem. 2014;289:8121–34. doi: 10.1074/jbc.M114.548651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–12. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 162.Knutson SK, Wigle TJ, Warholic NM, Sneeringer CJ, Allain CJ, Klaus CR, et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol. 2012;8:890–6. doi: 10.1038/nchembio.1084. [DOI] [PubMed] [Google Scholar]
- 163.Knutson SK, Kawano S, Minoshima Y, Warholic NM, Huang KC, Xiao Y, et al. Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol Cancer Ther. 2014;13:842–54. doi: 10.1158/1535-7163.MCT-13-0773. [DOI] [PubMed] [Google Scholar]
- 164.Qi W, Chan H, Teng L, Li L, Chuai S, Zhang R, et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci U S A. 2012;109:21360–5. doi: 10.1073/pnas.1210371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hua WF, Fu YS, Liao YJ, Xia WJ, Chen YC, Zeng YX, et al. Curcumin induces down-regulation of EZH2 expression through the MAPK pathway in MDA-MB-435 human breast cancer cells. Eur J Pharmacol. 2010;637:16–21. doi: 10.1016/j.ejphar.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 166.Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72:335–45. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dimri M, Bommi PV, Sahasrabuddhe AA, Khandekar JD, Dimri GP. Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells. Carcinogenesis. 2010;31:489–95. doi: 10.1093/carcin/bgp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang S, Zhu Y, He H, Liu J, Xu L, Zhang H, et al. Sorafenib suppresses growth and survival of hepatoma cells by accelerating degradation of enhancer of zeste homolog 2. Cancer Sci. 2013;104:750–9. doi: 10.1111/cas.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Avan A, Crea F, Paolicchi E, Funel N, Galvani E, Marquez VE, et al. Molecular mechanisms involved in the synergistic interaction of the EZH2 inhibitor 3-deazaneplanocin A with gemcitabine in pancreatic cancer cells. Mol Cancer Ther. 2012;11:1735–46. doi: 10.1158/1535-7163.MCT-12-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fiskus W, Rao R, Balusu R, Ganguly S, Tao J, Sotomayor E, et al. Superior efficacy of a combined epigenetic therapy against human mantle cell lymphoma cells. Clin Cancer Res. 2012;18:6227–38. doi: 10.1158/1078-0432.CCR-12-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hayden A, Johnson PW, Packham G, Crabb SJ. S-adenosylhomocysteine hydrolase inhibition by 3-deazaneplanocin A analogues induces anti-cancer effects in breast cancer cell lines and synergy with both histone deacetylase and HER2 inhibition. Breast Cancer Res Treat. 2011;127:109–19. doi: 10.1007/s10549-010-0982-0. [DOI] [PubMed] [Google Scholar]
- 172.Sun F, Chan E, Wu Z, Yang X, Marquez VE, Yu Q. Combinatorial pharmacologic approaches target EZH2-mediated gene repression in breast cancer cells. Mol Cancer Ther. 2009;8:3191–202. doi: 10.1158/1535-7163.MCT-09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lv Y, Yuan C, Xiao X, Wang X, Ji X, Yu H, et al. The expression and significance of the enhancer of zeste homolog 2 in lung adenocarcinoma. Oncol Rep. 2012;28:147–54. doi: 10.3892/or.2012.1787. [DOI] [PubMed] [Google Scholar]
- 174.Hu S, Yu L, Li Z, Shen Y, Wang J, Cai J, et al. Overexpression of EZH2 contributes to acquired cisplatin resistance in ovarian cancer cells in vivo and in vivo. Cancer Biol Ther. 2010;10:788–95. doi: 10.4161/cbt.10.8.12913. [DOI] [PubMed] [Google Scholar]