Abstract
Purpose
Non-metastatic colorectal cancer patients with diabetes have poor overall survival than those without diabetes. However, the effect of hyperglycemia on survival after diagnosis of metastatic colorectal cancer (CRC) has not been assessed. Therefore, we assessed the impact of hyperglycemia on the survival and infection-related adverse events (AEs) in patients with metastatic CRC.
Materials and Methods
We reviewed the records of 206 patients with newly diagnosed metastatic CRC who were treated with palliative chemotherapy from March 2000 to December 2012 at Chungbuk National University Hospital. The mean glucose level of each patient was calculated using all available glucose results.
Results
The mean glucose levels ranged between 76.8 and 303.5 mg/dL, and patients were categorized into quartiles in accordance to their mean glucose level: group 1 (< 106.7 mg/dL), group 2 (106.7-117.2 mg/dL), group 3 (117.3-142.6 mg/dL), and group 4 (> 142.6 mg/dL). The median overall survival for patients in groups 1, 2, 3, and 4 were 22.6, 20.1, 18.9, and 17.9 months, respectively; however, this difference was not statistically significant (p=0.643). Compared with patients in group 1, those in groups 2, 3, and 4 were at a higher risk of infection-related AEs, according to a multivariate analysis (p=0.002).
Conclusion
Hyperglycemia was not associated with shorter survival; however, it was associated with infection-related AEs in patients with newly diagnosed metastatic CRC receiving palliative chemotherapy.
Keywords: Colorectal neoplasms, Hyperglycemia, Infection, Survival
Introduction
The prevalence of diabetes mellitus (DM) and cancer has steadily increased as people live longer [1,2]. Indeed, many patients with pre-existing DM may develop cancer, and cancer patients often develop DM. Epidemiologic evidence suggests that individuals with DM are at a significantly higher risk of many forms of cancer, such as liver, pancreatic, colorectal, breast, renal, bladder, and endometrial cancer [3]. A 10-year prospective cohort study of 1,298,385 Koreans, who received health insurance from the National Health Insurance Corporation, showed that an elevated fasting serum glucose level and a diagnosis of DM are independent risk factors for several major cancers, and the risk tended to increase as the fasting serum glucose level increased [4]. The association between DM and colorectal cancer (CRC) has been extensively studied. Convincing evidence from previous studies, including a large meta-analysis, suggested that individuals with DM are at a higher risk of developing CRC [5]. Evidence also suggests that DM is associated with an increased risk of CRC mortality, and diabetic patients with CRC have poorer overall survival than non-diabetic patients with CRC [6-8]. A recent study from the Cancer Prevention Study-II (CPS-II) Nutrition Cohort showed that patients with non-metastatic CRC and type 2 DM have a higher risk of death from all causes, including CRC and cardiovascular disease, than patients with non-metastatic CRC and without type 2 DM [7].
DM and hyperglycemia are not only common in nonmetastatic CRC patients, but also in metastatic CRC patients. Moreover, patients with metastatic CRC are at a higher risk of hyperglycemia because their supportive treatment routinely includes high-dose glucocorticoids, such as dexamethasone (used as an antiemetic) and megestrol acetate (used as an appetite stimulant), which increase the plasma glucose level. Furthermore, hyperglycemia is associated with infectious complications in immunocompromised cancer patients, which may delay and decrease the efficacy of treatment and thus directly affect survival [9]. Although there are several reports that describe the association between diabetes and an outcome of patients with non-metastatic CRC, the impact of hyperglycemia on the outcome of patients with metastatic CRC has not been evaluated.
Therefore, we assessed the impact of hyperglycemia on the survival and infection-related adverse events (AEs) of patients with metastatic CRC, receiving palliative chemotherapy.
Materials and Methods
1. Study population
A retrospective chart review was performed on all patients with newly diagnosed metastatic CRC, who presented at Chungbuk National University Hospital between March 2000 and December 2012. Patients were included in this analysis if they had a histologically confirmed adenocarcinoma, a newly diagnosed metastatic CRC, and had received palliative chemotherapy. Patients were excluded if they died at the initial hospitalization, had not received palliative chemotherapy, had a non-adenocarcinoma histology, or had a history of another malignancy.
This study was reviewed and approved by the Institutional Review Board of Chungbuk National University Hospital.
2. Data collection
The baseline clinical and pathological characteristics of the patients at the time of metastatic CRC diagnosis were reviewed, including age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), body mass index (BMI), comorbidity conditions by Charlson Comorbidity Index (CCI) scale [10], pre-existing DM and hypertension, location of the primary tumor, and stage. We also collected the medical record information on palliative treatments, including chemotherapeutic agents, number of prior palliative chemotherapies, and the use of dexamethasone or megestrol acetate.
The mean glucose level of each patient was calculated using all available values collected during the follow-up. The fasting status of the patient at the time of testing was not known. Infection-related AEs were calculated by grade in accordance to the Common Terminology Criteria for Adverse Events ver. 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf). The overall survival was defined as the time from diagnosis of metastatic CRC to death from any cause and was censored at the date of the last contact for those who remained alive.
3. Statistical analysis
The means and standard deviations (SDs) of clinical characteristics were calculated. The characteristics of patients were compared using Student’s t-test for the means and chi-square test for categorical variables.
Survival curves were estimated using the Kaplan-Meier method, which were compared using the log rank test. Infection- related AEs of patients were compared using the Fisher’s exact test. Multivariate logistic regression analysis with a backward stepwise method was used to evaluate the effects of hyperglycemia on the development of infection-related AEs. p-values of < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL).
Results
1. Baseline patient characteristics
A total of 206 patients with newly diagnosed metastatic CRC, receiving palliative chemotherapy, were included in the final analysis. The median age of patients was 61 years (range, 16 to 84 years), and 44 patients (21.4%) had DM at the time of metastatic CRC diagnosis. The mean±SD glucose level was 132.0±40.1 mg/dL and the mean±SD number of glucose measurements per patient was 20.5±13.5. The mean glucose levels ranged between 76.8 and 303.5 mg/dL (median, 117.4 mg/dL), and the patients were divided into quartiles according to their mean glucose level: group 1 (< 106.7 mg/dL), group 2 (106.7-117.2 mg/dL), group 3 (117.3-142.6 mg/dL), and group 4 (> 142.6 mg/dL). Table 1 shows the demographic and clinical characteristics of patients in each group. The mean age, comorbidity conditions by CCI scale, and incidence of DM increased from group 1 to group 4 (p=0.038, p=0.007, and p < 0.001 for trend, respectively). There were no differences in the distributions of ECOG PS, BMI, hypertension, location of primary tumor, tumor stage, use of chemotherapeutic agents, number of palliative chemotherapies, and the use of dexamethasone or megestrol acetate between the four groups.
Table 1.
Patient characteristics according to their mean glucose levels
| Quartile of mean glucose (% of patients) |
p-value | ||||
|---|---|---|---|---|---|
| Group 1 (n=52) | Group 2 (n=51) | Group 3 (n=51) | Group 4 (n=52) | ||
| Age at diagnosis metastatic CRC (yr) | 0.038 | ||||
| Mean±SD | 59.7±12.4 | 60.1±9.8 | 63.9±11.5 | 67.7±9.6 | |
| Gender | 0.038 | ||||
| Male | 61.5 | 78.4 | 56.9 | 76.9 | |
| Female | 38.5 | 21.6 | 43.1 | 23.1 | |
| ECOG performance status | 0.082 | ||||
| 0, 1 | 88.5 | 82.4 | 70.6 | 86.5 | |
| ≥ 2 | 11.5 | 17.6 | 29.4 | 13.5 | |
| BMI (kg/m2) | 0.701 | ||||
| < 25 | 76.9 | 70.6 | 66.7 | 73.1 | |
| ≥ 25 | 23.1 | 29.4 | 33.3 | 26.9 | |
| Comorbidity conditions by CCI scale | 0.007 | ||||
| 0, 1 | 98.1 | 96.1 | 86.3 | 80.8 | |
| ≥ 2 | 1.9 | 3.9 | 13.7 | 19.2 | |
| Diabetes mellitus | < 0.001 | ||||
| Yes | 0 | 0 | 17.6 | 69.2 | |
| No | 100 | 100 | 82.4 | 30.8 | |
| Hypertension | 0.076 | ||||
| Yes | 19.2 | 29.4 | 35.3 | 42.3 | |
| No | 80.8 | 70.6 | 64.7 | 57.7 | |
| Location of primary tumor | 0.382 | ||||
| Colon | 50.0 | 45.1 | 58.8 | 59.6 | |
| Rectum | 50.0 | 54.9 | 41.2 | 40.4 | |
| Stage | 0.072 | ||||
| M1a | 19.2 | 43.1 | 29.4 | 32.7 | |
| M1b | 80.8 | 56.9 | 70.6 | 67.3 | |
| No. of palliative chemotherapies | 0.598 | ||||
| 1 | 59.6 | 52.9 | 58.8 | 63.5 | |
| 2 | 21.2 | 33.3 | 29.4 | 28.8 | |
| ≥ 3 | 19.2 | 13.7 | 11.8 | 7.7 | |
| Use of dexamethasone (mean±SD) | |||||
| Dose (mg) | 278.4±317.1 | 224.9±263.7 | 246.9±284.7 | 249.5±234.4 | 0.808 |
| Duration (days) | 22.0±21.8 | 23.1±25.8 | 25.1±28.6 | 28.9±43.5 | 0.689 |
| Use of megestrol acetate (mean±SD) | |||||
| Dose (g) | 0.43±1.17 | 0.40±0.94 | 0.66±1.16 | 0.71±1.58 | 0.468 |
| Duration (days) | 17.0±28.5 | 25.4±49.9 | 33.0±54.8 | 39.0±78.1 | 0.213 |
CRC, colorectal cancer; SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; CCI, Charlson Comorbidity Index; M1a, metastasis confined to one organ or site (e.g., liver, lung, ovary, or non-regional node); M1b, metastases in more than one organ/site or the peritoneum.
2. Effect of mean glucose level on patient survival
After a median follow-up of 3.5 years, 144 deaths were identified in the cohort of 206 patients with metastatic CRC. Kaplan-Meier survival curves of patients in each of the four groups are shown in Fig. 1A. The median overall survival was 22.6 months in group 1, 20.1 in group 2, 18.9 months in group 3, and 17.9 months in group 4; however, this difference was not statistically significant (p=0.643). The estimated hazard ratios (HRs) were 1.06 for group 2 (95% confidence interval [CI], 0.68 to 1.66), 1.29 for group 3 (95% CI, 0.82 to 2.03), and 1.25 for group 4 (95% CI, 0.79 to 1.97). There was also no statistically significant difference in the overall survival between patients with pre-existing DM or those without pre-existing DM (Fig. 1B). The median overall survival among patients with pre-existing DM and without pre-existing DM was 20.5 months and 19.7 months, respectively (HR, 1.08; 95% CI, 0.73 to 1.59; p=0.707).
Fig. 1.
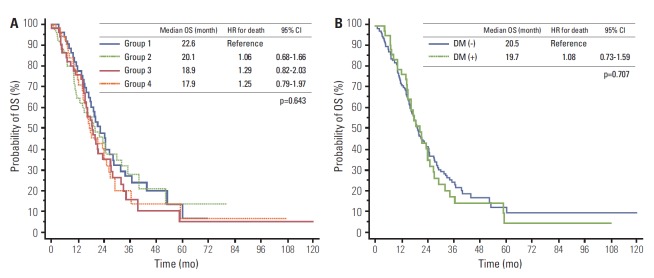
Kaplan-Meier estimates of the overall survival (OS) in metastatic colorectal cancer patients by the mean glucose level (A) and pre-existing diabetes mellitus (B). HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus.
3. Effect of mean glucose level on infection-related AEs
The incidence of infection-related AEs among patients in each of the four groups is presented in Table 2. Compared with patients in group 1, those in groups 2, 3, and 4 were at higher risk of infection-related AEs, including any grade, grade 3 or grade 4 infection, and were more susceptible to hospitalization from infection (p=0.002, p=0.003, and p=0.025 for trend, respectively). The incidence of any grade of infection- related AEs was significantly affected by comorbidity conditions in accordance to the CCI scale, including pre-existing DM, use of dexamethasone and megesterol acetate, and the mean glucose level. However, duration of dexamethasone use and the mean glucose level were the only significant factors for the incidence of infection-related AEs (p=0.008 and p=0.002, respectively). Other clinical characteristics were not significant factors for the incidence of infection- related AEs, according to a multivariate analysis (Table 3).
Table 2.
Infection-related adverse events by mean glucose level
| Quartile of mean glucose (% of patients) |
p-value | ||||
|---|---|---|---|---|---|
| Group 1 (n=52) | Group 2 (n=51) | Group 3 (n=51) | Group 4 (n=52) | ||
| Any grade | 11.5 | 33.3 | 41.2 | 42.3 | 0.002 |
| Grade 3 or 4 | 7.7 | 29.4 | 35.3 | 28.8 | 0.003 |
| Hospitalization | 7.7 | 29.4 | 25.5 | 23.1 | 0.025 |
Table 3.
Univariate and multivariate analysis of infection-related adverse events
| Any grade infection-related adverse events |
||||
|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
|||
| Patients (%) | p-value | OR (95% CI) | p-value | |
| Age at diagnosis of metastatic CRC | 0.761 | - | - | |
| < 65 (n=124) | 33.1 | |||
| ≥ 65 (n=82) | 30.5 | |||
| Gender | 0.749 | - | - | |
| Male (n=141) | 31.2 | |||
| Female (n=65) | 33.8 | |||
| ECOG performance status | 0.847 | - | - | |
| 0, 1 (n=169) | 32.5 | |||
| ≥ 2 (n=37) | 29.7 | |||
| BMI (kg/m2) | - | - | ||
| < 25 (n=148) | 31.8 | 0.890 | ||
| ≥ 25 (n=58) | 32.8 | |||
| Comorbidity conditions by CCI scale | 0.025 | 2.54 (0.96-6.77) | 0.061 | |
| 0, 1 (n=186) | 29.6 | |||
| ≥ 2 (n=20) | 55.0 | |||
| Diabetes mellitus | 0.007 | - | - | |
| Yes (n=45) | 48.9 | |||
| No (n=161) | 27.3 | |||
| Hypertension | 0.873 | - | - | |
| Yes (n=65) | 30.8 | |||
| No (n=141) | 32.6 | |||
| Location of primary tumor | 0.456 | - | - | |
| Colon (n=110) | 34.5 | |||
| Rectum (n=96) | 29.2 | |||
| Stage | 0.152 | - | - | |
| M1a (n=64) | 25.0 | |||
| M1b (n=142) | 35.2 | |||
| No. of palliative chemotherapies | 0.636 | - | - | |
| 1 (n=121) | 31.4 | |||
| 2 (n=58) | 36.2 | |||
| ≥ 3 (n=27) | 25.9 | |||
| Use of dexamethasone | ||||
| Dose | - | 0.035 | - | - |
| Duration | - | 0.003 | 1.02 (1.00-1.03) | 0.008 |
| Use of megestrol acetate | ||||
| Dose | - | 0.038 | - | - |
| Duration | - | 0.016 | - | - |
| Mean glucose level (mg/dL) | 0.002 | 0.23 (0.09-0.58) | 0.002 | |
| Group 1 (n=52) | 11.5 | |||
| Group 2 (n=51) | 33.3 | |||
| Group 3 (n=51) | 41.2 | |||
| Group 4 (n=52) | 42.3 | |||
OR, odds ratio; CI, confidence interval; CRC, colorectal cancer; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; CCI, Charlson Comorbidity Index; M1a, metastasis confined to one organ or site (e. g., liver, lung, ovary, or non-regional node); M1b, metastases in more than one organ/site or the peritoneum.
Discussion
This study showed that hyperglycemia is not associated with shorter survival in patients with newly diagnosed metastatic CRC receiving palliative chemotherapy. However, infection-related AEs increased with an increase in the mean glucose level.
Recent large cohort studies suggest that pre-existing diabetes at the time of cancer diagnosis is associated with increased all-cause mortality and cancer recurrence, especially in patients with colon and breast cancers [6-9,11,12]. Several explanations are proposed for the association between pre-existing diabetes and increased all-cause mortality in cancer patients. Firstly, cancer patients with diabetes may have increased tumor cell proliferation and metastases as a consequence of the physiological environment of hyperinsulinemia. It is possible that high insulin levels or an increase in free insulin-like growth factor levels in hyperinsulinemic states promote cancer cell and tumor growth [13,14]. Secondly, it is possible that the association between hyperglycemia and poor survival is mediated not by tumor growth, but rather by other adverse effects of hyperglycemia, such as increased rates of infection [15,16]. Thirdly, diabetes in cancer patients may indirectly impact the cancer outcome. For instance, a previous study indicated that cancer patients with pre-existing diabetes are often treated less aggressively than those without diabetes [9]. Thus, differences in cancer treatment between patients with and without diabetes may contribute to this increased mortality.
To date, several studies have examined CRC-specific mortality among diabetic and non-diabetic patients with CRC (Table 4) [6-8,17-19]. Two large cohort studies [6,7] and one retrospective study [8] reported the association between diabetes and a higher rate of overall mortality, even after adjustment for other predictors of CRC outcome. The two large cohort studies only included patients with non-metastatic CRC and excluded patients with metastatic CRC because the 5-year survival rate for patients with metastatic CRC is poor (approximately 10%), in accordance to the assumption that diabetes affect the long-term mortality in CRC patients [6,7]. One retrospective study showed that the prognostic impact of diabetes on the overall survival was particularly significant in patients with stage II colon cancer and was attenuated in both early (stage I) and metastatic (stage IV) colon cancer patients [8]. Each of the other three studies, which included patients with both non-metastatic and metastatic CRC, did not show any association between diabetes and CRC-specific survival [17-19]. A Norwegian study of 1,194 hospital-based patients with CRC reported that DM affects only the shortterm survival of patients treated with curative intent and the shorter overall survival was associated with cardiac disease and increased age [17]. A population-based study of the Connecticut Tumor Registry of 9,395 patients with CRC reported that cormorbid DM was not associated with a risk of death from CRC and was associated with statistical significance with death from all causes, excluding CRC [18]. Moreover, in a CPS-II Nutrition Cohort study, patients with CRC and type 2 DM had a higher risk of mortality than patients with CRC and without type 2 DM, with a particularly higher risk of death from cardiovascular disease [7]. In the current study, only patients with metastatic CRC were included, and the overall survival of patients with metastatic CRC was not significantly affected by hyperglycemia or DM. Therefore, the impact of hyperglycemia or DM on survival may be attenuated in patients with metastatic CRC or obscured by the greater tumor burden. Moreover, the high mortality risk in patients with non-metastatic CRC might be related to concurrent illnesses other than cancer, such as ischemic heart disease, hypertension, chronic renal failure, and stroke. Although several previous studies, using a subgroup analysis, have suggested that diabetes has minimal negative impact on the survival of patients with metastatic CRC, this is the first study to systematically evaluate the impact of hyperglycemia on the survival in only patients with metastatic CRC receiving palliative chemotherapy. Because the survival prognosis of patients with metastatic CRC is so poor, it is difficult to definitively conclude that there is no impact of hyperglycemia on the outcomes of these patients. Two previous studies report that hyperglycemia is independently associated with shorter survival in patients with newly diagnosed glioblastoma [20] or non-small cell lung cancer [21], and survival in these patients is much shorter than in patients with metastatic CRC.
Table 4.
Studies of the association between diabetes and outcomes of patients with colorectal cancer
| References | Study design | Patients | No. of patients | Survival (diabetics vs. non-diabetics) | p-value | Subgroup analysis |
|---|---|---|---|---|---|---|
| Meyerhardt et al. [6] | A cohort study within a large, randomized adjuvant chemotherapy trial | High risk stage II and III colon cancer | 3,759 | 5-yr OS; 57% vs. 66% | < 0.001 | - |
| 5-yr DFS; 48% vs. 59% | < 0.001 | |||||
| Dehal et al. [7] | The CPS-II Nutrition Cohort, a prospective study |
Non-metastatic CRC | 2,278 | 5-yr OS; 68% vs. 78% | < 0.001 | - |
| 5-yr CRC-specific OS; 82% vs. 87% | 0.04 | |||||
| 5-yr CVD-specific OS; 93% vs. 97% | < 0.001 | |||||
| Huang et al. [8] | A retrospective study | Stage I-IV colon cancer | 2,762 | 5-yr OS; 48.9% vs. 56.4% | 0.001 | Stage I (p=0.831) Stage II (p < 0.001) Stage III (p=0.049) Stage IV (p=0.508) |
| Jullumstro et al. [17] | A Norwegian retrospective study | Stage I-IV CRC | 1,194 | 5-yr CRC-specific OS; 73% vs. 79% | NS | Non-metastatic CRC (46% vs. 65%, p < 0.01) |
| Polednak [18] | A population-based study of the Connecticut Tumor Registry | Stage I-IV CRC | 9,395 | HR for death from CRC; 1.06 (95% CI, 0.94-1.20) | NS | HR for death from all causes excluding CRC; 1.84 (95% CI, 1.65-2.06) |
| Noh et al. [19] | A retrospective study | Stage I-IV CRC | 657 | 5-yr OS; 68.8% vs. 75.0% | 0.498 | - |
| Present case | A retrospective study | Metastatic CRC | 206 | Median OS; 22.6 mo vs. 20.1 mo vs. 18.9 mo vs. 17.9 mo (by mean glucose quartiles) | 0.643 | - |
| Median OS; 20.5 mo vs. 19.7 mo (by pre-existing DM) | 0.707 |
OS, overall survival; DFS, disease free survival; CPS-II, Cancer Prevention Study-II; CRC, colorectal cancer; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus.
Hyperglycemia affects all major components of the innate immunity system and impairs the body’s ability to combat infection [22]. In addition, hyperglycemia adversely affects neutrophil activity, including chemotaxis, formation of reactive oxygen species, and phagocytosis of bacteria [23]. The diabetic state increases lymphocyte apoptosis and suppresses the proliferation of T-cells, due to a lower expression of adenosine kinase [24]. Furthermore, the functions of immunoglobulins and complement are attenuated by glycosylation during hyperglycemia [25]. Finally, hyperglycemia may promote the exponential growth of bacteria and increase their virulence. In the current study, the incidence of any grade, grade 3 or 4 infections, or hospitalization for infection-related AEs increased with the mean glucose level in patients with metastatic CRC who were receiving palliative chemotherapy. These infection-related AEs may delay or result in a decrease in the intensity of chemotherapy in these patients; therefore, the results suggest the need for a regular monitoring of hyperglycemia to prevent infectionrelated AEs.
The limitations of this study include its retrospective design and the relatively small sample size, which resulted in an insufficient statistical power for detailed subgroup analyses and accurate assessments of infection-related AEs. Also, there was heterogeneity in the frequency of glucose recording and the glucose measurements available were performed at random. Fasting plasma glucose, 2-hour postprandial plasma glucose, and hemoglobin A1c levels would have provided a better index for evaluating hyperglycemia during the course of the disease. Further investigation of the proposed hypotheses in this study with a larger sample size and more refined categorization might help further elucidate the relationship between hyperglycemia, survival, and infection- related AEs in patients with metastatic CRC.
Conclusion
The present study shows that hyperglycemia was not associated with shorter survival, but it was associated with infection-related AEs in patients with newly diagnosed metastatic CRC receiving palliative chemotherapy.
Acknowledgments
This research was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (NRF-2007-0054930).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Kim SG, Choi DS. The present state of diabetes mellitus in Korea. J Korean Med Assoc. 2008;51:791–8. [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 4.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 5.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21:433–40. doi: 10.1200/JCO.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 7.Dehal AN, Newton CC, Jacobs EJ, Patel AV, Gapstur SM, Campbell PT. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:53–9. doi: 10.1200/JCO.2011.38.0303. [DOI] [PubMed] [Google Scholar]
- 8.Huang YC, Lin JK, Chen WS, Lin TC, Yang SH, Jiang JK, et al. Diabetes mellitus negatively impacts survival of patients with colon cancer, particularly in stage II disease. J Cancer Res Clin Oncol. 2011;137:211–20. doi: 10.1007/s00432-010-0879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007;120:1986–92. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and metaanalysis. JAMA. 2008;300:2754–64. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–92. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 14.Tran TT, Naigamwalla D, Oprescu AI, Lam L, McKeown-Eyssen G, Bruce WR, et al. Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology. 2006;147:1830–7. doi: 10.1210/en.2005-1012. [DOI] [PubMed] [Google Scholar]
- 15.Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–8. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 16.Turina M, Fry DE, Polk HC., Jr Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624–33. doi: 10.1097/01.ccm.0000170106.61978.d8. [DOI] [PubMed] [Google Scholar]
- 17.Jullumstro E, Kollind M, Lydersen S, Edna TH. Diabetes mellitus and outcomes of colorectal cancer. Acta Oncol. 2009;48:361–7. doi: 10.1080/02841860802637765. [DOI] [PubMed] [Google Scholar]
- 18.Polednak AP. Comorbid diabetes mellitus and risk of death after diagnosis of colorectal cancer: a population-based study. Cancer Detect Prev. 2006;30:466–72. doi: 10.1016/j.cdp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Noh GY, Hwang DY, Choi YH, Lee YY. Effect of diabetes mellitus on outcomes of colorectal cancer. J Korean Soc Coloproctol. 2010;26:424–8. doi: 10.3393/jksc.2010.26.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27:1082–6. doi: 10.1200/JCO.2008.19.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J, Chen YJ, Chang LJ. Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2012;76:242–7. doi: 10.1016/j.lungcan.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- 23.Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15:256–60. doi: 10.2337/diacare.15.2.256. [DOI] [PubMed] [Google Scholar]
- 24.Sakowicz-Burkiewicz M, Kocbuch K, Grden M, Szutowicz A, Pawelczyk T. Diabetes-induced decrease of adenosine kinase expression impairs the proliferation potential of diabetic rat T lymphocytes. Immunology. 2006;118:402–12. doi: 10.1111/j.1365-2567.2006.02380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peake PW, Charlesworth JA, Timmermans V, Gavrilovic L, Pussell B. Does non-enzymatic glycosylation affect complement function in diabetes? Diabetes Res. 1989;11:109–14. [PubMed] [Google Scholar]


