Abstract
Introduction
Growth factors such as keratinocyte growth factor-2 (KGF-2) and transforming growth factor-beta (TGF-β) are important immunoregulatory and epithelial growth factors. They are also potential therapeutic proteins for inflammatory bowel disease. However, owing to protein instability in the upper gastrointestinal tract, it is difficult to achieve therapeutic levels of these proteins in the injured colon when given orally. Furthermore, the short half-life necessitates repeated dosage with large amounts of the growth factor, which may have dangerous side effects, hence the importance of temporal and spatial control of growth factor delivery.
Methods
The human commensal gut bacterium, Bacteroides ovatus, was genetically engineered to produce human KGF-2 or TGF-β 1 (BO-KGF or BO-TGF) in a regulated manner in response to the dietary polysaccharide, xylan. The successful application of BO-KGF or BO-TGF in the prevention of dextran sodium sulphate induced murine colitis is presented here.
Results
This novel drug delivery system had a significant prophylactic effect, limiting the development of intestinal inflammation both clinically and histopathologically. The ability to regulate heterologous protein production by B ovatus using xylan is both unique and an important safety feature of this drug delivery system.
Conclusions
The use of genetically engineered B ovatus for the controlled and localised delivery of epithelial growth promoting and immunomodulatory proteins has potential clinical applications for the treatment of various diseases targeting the colon.
Keywords: Bacteroides ovatus, Xylan, Inflammatory bowel disease
Inflammatory bowel disease (IBD) is an idiopathic chronic inflammation of the gut mucosa. The highest incidence and prevalence for both Crohn’s disease and ulcerative colitis have been reported from Northern Europe, the UK and North America. 1 Owing to the possible role of indigenous microbiota in the pathogenesis of IBD, 2 the external manipulation of its composition using probiotic organisms may be a promising therapeutic. Data from previous studies suggest that probiotic administration helps to restore microbial homoeostasis in the gut, down-regulating intestinal inflammation. 3
Genetically modified probiotics
The exploitation of microbes is no longer limited to their role as live probiotics. Commensal and food-grade bacteria can be engineered for the delivery of anti-inflammatory cytokines or other biologically active molecules to the gut. Lactococcus lactis was engineered to co-express tetanus toxin and either murine interleukin-2 (IL-2) or IL-6 4 for vaccination. Schotte et al took this step further and designed the L lactis system that secretes biologically active IL-10 and proposed this as a possible way of delivering IL-10 to the inflamed bowel in IBD. 5 The efficacy of L lactis secreting IL-10 has been demonstrated in a murine model of IBD 6 and in patients with Crohn’s disease. 7 This trial demonstrated the feasibility of using genetically modified bacteria for mucosal delivery of therapeutic proteins in humans. However, it is too early to draw any conclusions about its clinical efficacy as there were insufficient clinical data.
The L lactis system has significant drawbacks. First, despite being designed in a way so that the transgenic bacteria cannot live in the absence of thymidine, transgenic L lactis can still survive in thymidine deficient media for more than 72 hours. 8 A second safety issue is that expression of IL-10 is driven by the constitutive lactococcal P1 promoter. 5 Lack of regulation of the production of therapeutic molecules can cause unwanted side effects. Third, L lactis is a non-colonising bacterium. Repeated dosing is therefore required to achieve therapeutic levels of the secreted cytokines.
Other than IL-10, keratinocyte growth factor-2 (KGF-2) and transforming growth factor-β (TGF-β) are well studied for their role in promoting healing of injured intestinal epithelium. 9 Systemic administration of these cytokines may not be the optimal way of delivery because of the short half-life and the risk of dangerous side effects. The oral route is probably more appropriate. However, protein stability makes the oral route not ideal as these proteins have to pass through the extreme conditions of the stomach. The short in vivo half-lives, the physical and chemical instability, and the low oral bioavailability of proteins currently necessitate their administration by frequent injections of protein solutions.
Bacteroides system
To overcome the drawbacks of the L lactis system and exploit the potential therapeutic effects of KGF and TGF, we have used Bacteroides ovatus, a colonic commensal anaerobic Gram-negative bacterium, as a potentially improved genetically modified probiotic. The delivery of protein by B ovatus can be controlled by the xylan induction system. 10 Furthermore, this system is naturally contained because of the anaerobic nature of B ovatus. Our hypothesis is that recombinant strains of B ovatus producing biologically active KGF (BO-KGF) or TGF (BO-TGF) under the control of hemicellulose xylan can produce therapeutic and prophylactic effects on acute colitis (Fig 1). This hypothesis was tested in a murine model of colitis.
Figure 1.
Diagrammatic representation of the proposed Bacteroides ovatus xylan-controlled protein delivery system. B ovatus is a predominant commensal colonic microbiota with the ability to colonise the bowel. Repeated administration is therefore not needed. B ovatus can ferment the xylan. The xylanase promoter is cloned upstream of the recombinant mature protein gene (kgf or tgf) and drives the transcription of the gene only in the presence of xylan. Once secreted, the recombinant protein will induce healing of the injured bowel mucosa. After healing is completed, xylan supplementation is removed from the diet and the protein production is halted. In addition, the anaerobic nature of B ovatus is a natural biosafety and containment system that will prevent spread to the environment.
Methods
The coding region of KGF-2 and TGF-β 1 was polymerase chain reaction (PCR) amplified and cloned downstream of xylanase promoter sequence and Bacteroides fragilis enterotoxin signals. The resultant sequence was cloned into B ovatus genomic deoxyribonucleic acid (DNA) by conjugation using a suitable vector as demonstrated previously. 11–13 Transconjugates were detected by performing colony PCR using recombinant genes. Reverse transcription PCR was performed to detect recombinant and native gene transcripts as follows: B ovatus was grown anaerobically on a repulsive guidance molecule and haemin medium supplemented with 0.1% (weight/volume [w/v]) glucose and 0.05% (w/v) xylan. Xylan was added to the medium 2–3 hours before ribonucleic acid (RNA) preparation. RNA was prepared using a Total RNA Isolation kit (Promega, Southampton, UK). RNA was transcribed into complementary DNA (cDNA) using Moloney murine leukaemia virus reverse transcriptase (NEB, Hitchin, UK). PCR was performed using 2µ1 cDNA. (Primers sequences are available on request.)
Animal experiments
This study was carried out under project licence PPL 40/2784 issued by the Home Office in conjunction with the Animals (Scientific Procedures) Act 1986. Six eight-week-old male C57BL/6 mice were fed standard laboratory chow and tap water. Twenty-four hours prior to treatment, the mice were fed a xylan-reduced diet (SDS, Witham, UK). Acute colitis was induced in the mice by addition of 2.5% (w/v) dextran sodium sulphate (DSS) (molecular weight 35–45kDa; MP Biomedicals, Cambridge, UK) to the drinking water. Bacterial treatment and xylan (30mg/ml) were started at the same point as DSS administration and continued for five days. Bacteria (2 × 10 8 colony forming units in 0.2ml phosphate buffered saline) were administered by orogastric gavage.
Seven groups of mice (with eight mice in each group) were colonised on alternate days until the end of the experiment. Two groups received BO-KGF (with [+X] and without [−X] xylan), two groups received BO-TGF (+X and −X) and one group received wild type B ovatus V975 (BO-V975) (+X). The two control groups received phosphate buffered saline (DSS control) or no treatment (normal control). At day 5, all animals were euthanised.
Disease activity was monitored. Diarrhoea was scored as 0, with mucus covered stool as 1, loose stools as 2 and watery diarrhoea as 4. Blood in stool was scored as 0, with occult bleeding as 1, slight bleeding as 2 and gross bleeding as 4. No weight reduction was scored as 0, with 0–5% as 1, 5–10% as 2, 10–12.5% as 3, 12.5–15% as 4 and >15% as 5. The disease activity index was calculated to clinically assess colitis; it was the sum of the three scores. At autopsy (on day 5), the colonic length was measured from the ileocaecal junction to the anal verge and the colon was sampled at 1cm, 2cm and 3cm from the anal verge. Tissue samples were fixed in formalin. Paraffin embedded tissue sections were stained with haematoxylin and eosin, and examined microscopically.
Statistical analysis
Statistical significance was determined using Student’s t-test or the Mann–Whitney test where appropriate. An associated p-value of <0.05 was considered significant.
Results
A strain of B ovatus capable of producing and secreting human KGF-2 or TGF-β 1 in a xylan-inducible manner was constructed by DNA engineering technology. 12,13 The presence of recombinant genes in the transconjugates was confirmed by colony PCR using primers specific for the cloned genes (Fig 2A). To confirm that the recombinant strains of B ovatus (BO-KGF and BO-TGF) transcribed the cloned gene from the xylanase promoter, endpoint reverse transcription PCR was performed. In the absence of xylan, hardly any visible PCR products could be detected. After two hours of xylan exposure, strong transcript products of the corresponding gene were detected (Fig 2B).
Figure 2.
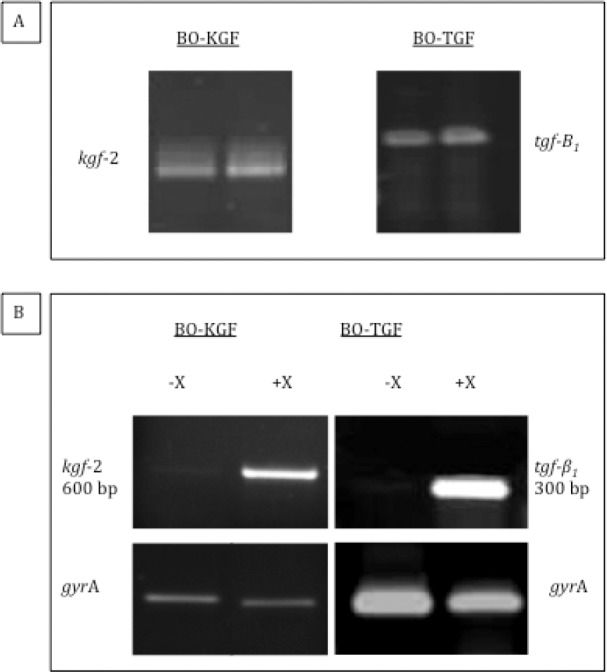
Agarose gel electrophoresis of Bacteroides ovatus strains expressing human keratinocyte growth factor-2 (BO-KGF) and transforming growth factor-beta (BO-TGF) colony polymerase chain reaction (PCR) and reverse transcription PCR: colony PCR of BO-KGF/BO-TGF transconjugates (A) and increased expression of KGF-2 and TGF-β 1 messenger ribonucleic acid in response to xylan (B). gyrA was used as a positive control.
Following normalisation of the amount of cDNA, it is estimated that there was a 1,000-fold increase in the transcript level after xylan exposure. Recombinant cytokine was detected in the colon of C57BL/6 mice maintained on a xylan-containing diet after oral administration of BO-KGF or BO-TGF at levels significantly higher than in mice given recombinant strains but maintained on a xylan-reduced diet (p<0.05), as well as higher than in non-treated mice and in mice administered wild type BO-V975 maintained on a xylan-containing diet (p<0.05). These results are consistent with the ability of BO-KGF and BO-TGF to produce KGF-2 and TGF-β 1 respectively in vivo in a xylan-controlled manner as previously shown. 12,13
Prophylactic administration of xylan to BO-KGF or BO-TGF treated mice resulted in significant improvement of colitis, reducing weight loss, reducing rectal bleeding, improving stool consistency and significantly improving the disease activity index score (p<0.05) (Fig 3). The colons in the BO-KGF and xylan treated group (mean: 7.2cm ± 0.08cm) were significantly longer than mice treated with BO-KGF alone (mean: 6.2cm ±0.2cm) (14%, p<0.001), than mice treated with BO-V975+X (mean: 6.6cm ± 0.2cm) (8%, p<0.01) and than the non-treated DSS control group (mean: 6.2cm ± 0.1cm) (14%, p<0.001). Similar results were achieved with BO-TGF and xylan treated animals (Fig 4). Histological analysis showed that treatment with BO-KGF or BO-TGF and xylan-reduced the depth and extent of crypt epithelium damage, acute and chronic inflammatory infiltrate, and goblet cells depletion compared with non-treated DSS controls and mice treated with BO-KGF or BO-TGF alone (Fig 5).
Figure 3.

Effect of Bacteroides ovatus strains expressing human keratinocyte growth factor-2 (BO-KGF) or transforming growth factor-beta (BO-TGF) and xylan on disease activity index (DAI) scores of dextran sodium sulphate (DSS) induced colitis
Figure 4.
Prophylactic effect of treatment with Bacteroides ovatus strains expressing human keratinocyte growth factor-2 (BO-KGF) or transforming growth factor-beta (BO-TGF) and xylan on colon shortening associated with dextran sodium sulphate (DSS) induced colitis: Mean colonic length ± standard error of the mean (8 mice in each group) (A); Representative macroscopic appearance of colons. Colons treated with BO-KGF or BO-TGF but without xylan had shorter colons and grossly appeared more inflamed than colons from animals treated with recombinant B ovatus and xylan.
Figure 5.
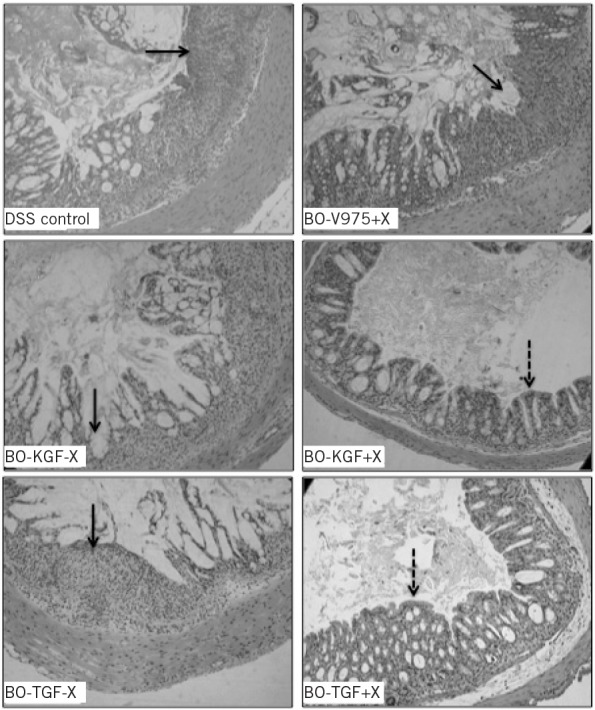
Histopathology of prophylactic effect of treatment of Bacteroides ovatus strains expressing human keratinocyte growth factor-2 (BO-KGF) or transforming growth factor-beta (BO-TGF) and xylan on the development of dextran sodium sulphate (DSS) colitis: Haematoxylin and eosin stained sections of colons from animals treated with BO-KGF or BO-TGF with xylan (+X) or without xylan (−X) at the inception of DSS exposure. Control groups had DSS alone (DSS control) or DSS treated with B ovatus V975 and xylan (BO-V975+X). Areas of crypt damage and inflammatory infiltrate are indicated by solid arrows. Less inflammatory cell infiltrate and crypt damage seen in the BO-KGF or BO-TGF and xylan-treated mice are shown by interrupted arrows.
Discussion
Administration of xylan to BO-KGF or BO-TGF treated mice resulted in significant improvement of colitis, reducing weight loss, improving stool consistency, reducing rectal bleeding, accelerating the healing of damaged colonic epithelium, and reducing inflammatory cell and neutrophil infiltration. The use of genetically engineered B ovatus for the controlled and localised delivery of epithelial growth promoting and immunomodulatory proteins has potential clinical applications for the treatment of various diseases targeting the colon, including IBD. Regulation of heterologous protein production by recombinant B ovatus using xylan is a novel and important safety feature of this drug delivery system. The recent identification of the B ovatus xylan operon promoter 11 enabled human cytokine producing strains to be generated.
The Bacteroides are dominant among commensal anaerobes and have been identified in the mucin layer coating the colonic mucosa, 14 making them ideal for therapeutic protein delivery directly to the injured epithelium. The ability of B ovatus to use xylan as its sole energy source 15,16 and the inability of the human gut to digest xylan 17 probably contributes to their predominance in the colonic microbiota. It is likely that BO-KGF and BO-TGF delivers growth factors directly to the epithelium via diffusion or by receptors expressed on the basolateral surface of epithelial cells 18,19 made accessible as a result of compromised barrier function in the inflamed colon.
An added advantage of using B ovatus as a drug delivery vehicle is its anaerobic nature that provides an important inbuilt biosafety feature that is an attribute lacking in other recombinant bacteria currently in use, including L lactis. 8 Furthermore, the concern of gene transfer and containment that is important in recombinant bacterial expression systems relying on extrachromosomal plasmids for expression of heterologous genes is much less of an issue with recombinant Bacteroides, in which the growth factor gene is integrated into the bacterial chromosome. 11
KGF-2 and TGF-β are potent epithelial growth factors able to ameliorate colitis and promote healing of intestinal ulcerations when given systemically. 20,21 However, owing to protein instability in the acid and protease-rich environment of the upper gastrointestinal tract, it is difficult to achieve therapeutic levels of these growth factors in the injured colon when given orally. In addition, bolus administration does not keep the protein localised, necessitating repeated dosing with large amounts of the growth factor that may have dangerous side effects such as vascularisation of non-target tissues or growth of tumours. 22 Furthermore, active TGF-β has a short half-life (2–3 minutes), 23 making it necessary for recombinant cytokine to be given frequently to replenish the level in the circulation every few minutes. The short biological half-life, lack of tissue selectivity and the risk of carcinogenesis demand, therefore, temporal and spatial control of growth factor delivery.
Conclusions
This study has demonstrated a novel mucosal drug delivery system. B ovatus has been used to secrete biologically active growth factor under the control of plant polysaccharide xylan. It provides new insights into the efficacy of KGF-2 and TGF-β in suppressing acute mucosal inflammation, and efforts should now be directed to evaluate the possible beneficial effect in inflamed human colons.
By introducing various other genes into the Bacteroides genome, it is possible to produce different strains of recombinant B ovatus producing factors or cytokines to attack different illnesses. For example, our group is now working on strains that produce endostatin (antiangiogenic factor) to treat colorectal cancer. A strain that can produce pancreatic enzymes will be novel for treatment of patients with chronic pancreatitis, as would a strain that produces absorbable insulin to treat diabetes in humans. If successful, future management of these disorders may be in the form of a xylan-rich diet to activate the engineered Bacteroides in the patient’s colon when the disease is active.
Acknowledgements
The Medical Research Council and Royal College of Surgeons of England research fellowship kindly supported this work. Further support was received from Techtran Ltd.
References
- 1.Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004; 126: 1,504–1,517 [DOI] [PubMed] [Google Scholar]
- 2.Darfeuille-Michaud A, Boudeau J, Bulois Pet al.High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004; 127: 412–421 [DOI] [PubMed] [Google Scholar]
- 3.Bai AP, Ouyang Q. Probiotics and inflammatory bowel diseases. Postgrad Med J 2006; 82: 376–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steidler L, Robinson K, Chamberlain Let al.Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect Immun 1998; 66: 3,183–3,189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schotte L, Steidler L, Vandekerckhove J, Remaut E. Secretion of biologically active murine interleukin-10 by Lactococcus lactis. Enzyme Microb Technol 2000; 27: 761–765 [DOI] [PubMed] [Google Scholar]
- 6.Steidler L, Hans W, Schotte Let al.Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000; 289: 1,352–1,355 [DOI] [PubMed] [Google Scholar]
- 7.Braat H, Rottiers P, Hommes DWet al.A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol 2006; 4: 754–759 [DOI] [PubMed] [Google Scholar]
- 8.Steidler L, Neirynck S, Huyghebaert Net al.Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol 2003; 21: 785–789 [DOI] [PubMed] [Google Scholar]
- 9.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol 2008; 14: 348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrar MD, Whitehead TR, Lan Jet al.Engineering of the gut commensal bacterium Bacteroides ovatus to produce and secrete biologically active murine interleukin-2 in response to xylan. J Appl Microbiol 2005; 98: 1,191–1,197 [DOI] [PubMed] [Google Scholar]
- 11.Hamady ZZ, Farrar M, Whitehead TRet al.Identification and use of the putative Bacteroides ovatus xylanase promoter for the inducible production of recombinant human proteins. Microbiology 2008; 154: 3,165–3,174 [DOI] [PubMed] [Google Scholar]
- 12.Hamady ZZ, Scott N, Farrar MDet al.Xylan-regulated delivery of human keratinocyte growth factor-2 to the inflamed colon by the human anaerobic commensal bacterium Bacteroides ovatus. Gut 2010; 59: 461–469 [DOI] [PubMed] [Google Scholar]
- 13.Hamady ZZ, Scott N, Farrar MDet al.Treatment of colitis with a commensal gut bacterium engineered to secrete human TGF-β 1 under the control of dietary xylan 1. Inflamm Bowel Dis 2011; 17: 1,925–1,935 [DOI] [PubMed] [Google Scholar]
- 14.Croucher SC, Houston AP, Bayliss CE, Turner RJ. Bacterial populations associated with different regions of the human colon wall. Appl Environ Microbiol 1983; 45: 1,025–1,033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salyers AA, Gherardini F, O’Brien M. Utilization of xylan by two species of human colonic Bacteroides. Appl Environ Microbiol 1981; 41: 1,065–1,068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitehead TR, Hespell RB. The genes for three xylan-degrading activities from Bacteroides ovatus are clustered in a 3.8-kilobase region. J Bacteriol 1990; 172: 2,408–2,412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings JH, Macfarlane GT, Englyst HN. Prebiotic digestion and fermentation. Am J Clin Nutr 2001; 73: 415S–420S [DOI] [PubMed] [Google Scholar]
- 18.Visco V, Belleudi F, Marchese Cet al.Differential response to keratinocyte growth factor receptor and epidermal growth factor receptor ligands of proliferating and differentiating intestinal epithelial cells. J Cell Physiol 2004; 200: 31–44 [DOI] [PubMed] [Google Scholar]
- 19.Murphy SJ, Doré JJ, Edens Met al.Differential trafficking of transforming growth factor-beta receptors and ligand in polarized epithelial cells. Mol Biol Cell 2004; 15: 2,853–2,862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neurath MF, Fuss I, Kelsall BLet al.Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med 1996; 183: 2,605–2,616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han DS, Li F, Holt Let al.Keratinocyte growth factor-2 (FGF-10) promotes healing of experimental small intestinal ulceration in rats. Am J Physiol Gastrointest Liver Physiol 2000; 279: G1011–G1022 [DOI] [PubMed] [Google Scholar]
- 22.Epstein SE, Fuchs S, Zhou YFet al.Therapeutic interventions for enhancing collateral development by administration of growth factors: basic principles, early results and potential hazards. Cardiovasc Res 2001; 49: 532–542 [DOI] [PubMed] [Google Scholar]
- 23.Wakefield LM, Winokur TS, Hollands RSet al.Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest 1990; 86: 1,976–1,984 [DOI] [PMC free article] [PubMed] [Google Scholar]




