Abstract
Introduction
Fine needle aspiration (FNA) is a safe and quick method of diagnosing superficial lumps, which aids preoperative planning. However, FNA of the parotid gland has not gained the widespread acceptance noted in other head and neck lumps. The aim of this study was to determine the ability of FNA of the parotid gland to differentiate benign and malignant disease, and to determine the impact on surgical outcome.
Methods
A retrospective analysis of 201 consecutive parotid operations with preoperative FNA in a large district hospital in the UK was performed. The diagnostic characteristics were calculated for benign and malignant disease, and the impact on surgical procedure was determined.
Results
In identifying benign disease, FNA has a sensitivity of 85% and a specificity of 76%. In detecting malignant disease, FNA has a sensitivity and specificity of 52% and 92% respectively. A false positive on FNA was associated with a higher incidence of neck dissection.
Conclusions
FNA is a useful diagnostic test. However, owing to low sensitivity, it is necessary to interpret it in the context of all other clinical information.
Keywords: Parotid neoplasm, Fine needle aspiration, Otolaryngology
There is a wide range of parotid gland pathologies that are not readily differentiated by either clinical or radiological means. The role of fine needle aspiration (FNA) remains controversial in the preoperative investigation of parotid lesions. 1,2 FNA of the parotid gland is a safe, reliable, rapid and cost effective procedure that has the potential to differentiate between non-neoplastic and neoplastic disease, and furthermore between benign and malignant neoplasia. 3,4 FNA offers the surgeon the ability to risk-stratify patients, to counsel them appropriately and to avoid surgery in those cases where it is not appropriate or unnecessary. Moreover, should a benign process be suspected on FNA, the facial nerve can be preserved safely. 5,6
FNA is used to identify neoplasia, which is the process of abnormal cell division. This may be either benign or malignant, which relates to the ability of the abnormal cells to spread to secondary sites.
The sensitivity and specificity of parotid FNA in distinguishing neoplastic from non-neoplastic (eg infection) disease is reported to be between 79% and 100%, and between 71% and 100% respectively. 4,7,8 The sensitivity and specificity of FNA in distinguishing between benign and malignant neoplasia is between 33% and 100%, and between 67% and 100% respectively. 7,9,10 Sensitivity is generally lower and more variable than specificity. 1,11,12,13
Variability in the diagnostic accuracy varies with operator experience and geographical location owing to differences in referral patterns as well as in the prevalence of benign and malignant disease. 1,14 However, concerns regarding the diagnostic accuracy of FNA, the high prevalence of benign versus malignant lesions and the similarity in operative management irrespective of the FNA diagnosis has meant that it has not gained the acceptance noted in other head and neck lumps. 15 Opponents argue that FNA is unnecessary in the majority of cases owing to an unacceptably high rate of false negatives and low sensitivity, and that the anatomical location of the lesion is more influential on the choice of operation than the FNA diagnosis. 11,13,16,17
Owing to the heterogeneity of study populations and the variation in reported accuracy, it is essential to explore the relationship between FNA and histological findings further. This study aimed to determine the diagnostic ability of FNA to differentiate between malignant and benign neoplasia in parotid lesions in the UK, and to determine the influence on the choice of surgical procedure.
Methods
A retrospective review of case notes of all parotid surgery (parotid lumpectomy, superficial/partial parotidectomy, deep lobe parotidectomy, total parotidectomy) in our centre between 1996 and 2011 was undertaken where both a preoperative FNA and operative specimen histology was available. Details on age, sex, presenting symptoms, preoperative radiology, clinician performing the FNA (surgeon or pathologist), number of attempts needed to get an adequate sample from FNA, FNA diagnosis, use of ultrasonography guidance for FNA, operation performed, histological diagnosis, complications and need for revision surgery were collected.
In our centre, all parotid lumps are investigated with FNA. All FNA was carried out using a 25-gauge needle and standard aspiration technique by either a surgeon or a pathologist.The time between FNA and gaining histology was less than 12 weeks. Histology was evaluated with knowledge of the FNA.
The ability of FNA to specifically diagnose a benign process or a malignant process was evaluated in terms of sensitivity (ability to identify positive results), specificity (ability to identify negative results), positive predictive value (the proportion of positive test results that are indeed positive according to a gold standard), negative predictive value (the proportion of negative test results that are indeed negative according to a gold standard) and accuracy (overall measure of a test’s diagnostic ability). A receiver operating characteristic (ROC) curve was plotted. Categorical data were compared using Fisher’s exact test unless otherwise stated. A p-value of <0.05 was considered statistically significant. If the pathologist was unable to give a tissue diagnosis, the result was considered inconclusive.Statistical analysis was carried out using SPSS® version 20 (SPSS, Chicago, IL, US).
Results
Overall, 231 consecutive parotid operations were performed in our centre. Referrals for parotid lesions were shared between otolaryngologists (55.3% of cases) and maxillofacial surgeons (44.7% of cases).All patients presented with a lump. In addition, 21 (10.4%) presented with pain and 5 (2.5%) with palsy. Pain was not associated with increased prevalence of malignant disease (p=0.17). Presentation with palsy was strongly associated with malignant disease in 100% of cases (p<0.0001).
Figure 1.
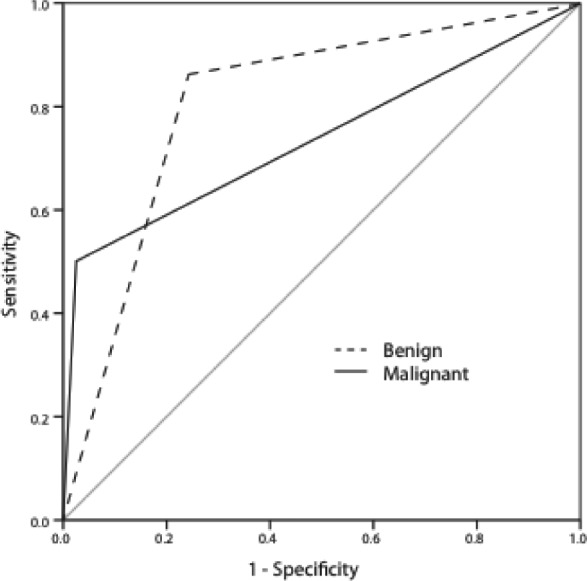
Receiver operating characteristic curves for the use of fine needle aspiration (FNA) to determine benign and malignant disease. For detecting benign disease, FNA has an area under the curve (AUC) of 0.80 (95% confidence interval [CI]: 0.71–0.89). For detecting malignant disease, FNA has an AUC of 0.75 (95% CI: 0.63–0.87) and is therefore a useful diagnostic test. The AUC is a measure of the diagnostic ability of a test. A result of 0.5 represents a test with no greater power than that of chance. Between 0.6 and 0.7 represents good diagnostic ability and >0.7 represents strong diagnostic ability.
Complications of FNA
Of the 231 patients with parotid operations, 211 (91.7%) had an FNA. The only reported complications were two cases (0.5%) of minor and self-limiting haematoma. This confirms parotid FNA as an extremely safe procedure. Inadequate sample necessitated a second FNA in seven (3.0%) cases. In one of these, no successful sample was obtained despite two further attempts.
Radiology
Ultrasonography, computed tomography and magnetic resonance imaging was used in 52%, 33%and 2% of cases respectively (Table 1). Radiology was useful in determining the anatomical location of the lesion and identifying cystic lesions. However, a specific diagnosis was given rarely, suggesting radiology is used primarily for operative planning rather than for diagnosis.
Table 2.
Diagnosis by fine needle aspiration (FNA)
| FNA result | Frequency |
|---|---|
| Pleomorphic adenoma | 98 (48.8%) |
| Warthin’s tumour | 31 (15.4%) |
| Inconclusive | 28 (13.9%) |
| Carcinoma | 18 (9.0%) |
| Epidermal cyst | 7 (3.5%) |
| Cystic/fluid filled | 5 (2.5%) |
| Lymphoma | 4 (2.0%) |
| Infection/inflammation | 3 (1.5%) |
| Metastasis | 2 (1.0%) |
| No abnormalities detected | 2 (1.0%) |
| Salivary tumour | 1 (0.5%) |
| Spindle cell | 1 (0.5%) |
| Other | 1 (0.5%) |
Table 1.
The use of radiology prior to parotid surgery
| Radiology | Cases |
|---|---|
| Ultrasonography | 105 (52.2%) |
| Computed tomography | 67 (33.3%) |
| Magnetic resonance imaging | 4 (2.0%) |
Comparison of FNA and histology
Both FNA and histology were recorded for 201 cases (87.3%) of parotid surgery (Tables 3 and 4). The most common diagnosis for FNA and histology was pleomorphic adenoma and Warthin’s tumour. Carcinoma was underdiagnosed by FNA compared with histology (9.0% vs 13.4%). Histology showed benign neoplasia to be five times more common than malignant neoplasia.
Table 3.
Diagnosis by histology
| Histological diagnosis | Frequency |
|---|---|
| Pleomorphic adenoma | 104 (51.7%) |
| Warthin’s tumour | 46 (22.9%) |
| Carcinoma | 27 (13.4%) |
| Other | 4 (2.0%) |
| Benign squamous cyst | 3 (1.5%) |
| Lymphoma | 3 (1.5%) |
| Metastasis | 3 (1.5%) |
| Non-specific inflammation | 3 (1.5%) |
| Inconclusive | 2 (1.0%) |
| Lymphoepithelial cyst | 2 (1.0%) |
| Basal cell adenoma | 1 (0.5%) |
| Lipoma | 1 (0.5%) |
| Salivary gland retention duct | 1 (0.5%) |
| No abnormalities detected | 1 (0.5%) |
Table 4.
Carcinoma subtype on fine needle aspiration (FNA) and histology
| FNA | Histology | |
|---|---|---|
| Acinic cell carcinoma | 4 (2.0%) | 7 (3.5%) |
| Adenocarcinoma | 1 (0.5%) | 6 (3.0%) |
| Squamous cell carcinoma | 4 (2.0%) | 5 (2.5%) |
| Mucoepidermoid carcinoma | – | 5 (2.5%) |
| Adenoid cell carcinoma | – | 2 (1.0%) |
| Oncocytic carcinoma | 1 (0.5%) | 1 (0.5%) |
| Myoepithelial carcinoma | – | 1 (0.5%) |
| Pleomorphic ex-carcinoma | – | 1 (0.5%) |
| Poor differentiation | 3 (1.5%) | 0 (0.0%) |
| Total | 13 (6.5%) | 28 (13.9%) |
An exact tissue diagnosis was given in 50% of carcinomas and in 74% of benign cases. Notably, Warthin’s tumour was only identified correctly in 30/46 (65%)cases. Pleomorphic adenoma was identified correctly in 90/104 (87%) cases.
In line with previous reports, the sensitivity of detecting malignant disease was lower than the specificity. Similarly, the ability of FNA to exclude malignant disease (negative predictive value) was stronger than its ability to confirm it (positive predictive value). The overall diagnostic accuracy of FNA was higher for detecting malignant disease than for benign disease. For both benign and malignant disease, the ROC area under the curve reported FNA as a useful diagnostic tool with values of 0.75 and 0.80 respectively (Table 5).
Table 5.
Fine needle aspiration diagnostic characteristics for determining benign disease
| Benign | Malignant | |
|---|---|---|
| Sensitivity | 0.85 | 0.52 |
| Specificity | 0.76 | 0.98 |
| Positive predictive value | 0.95 | 0.78 |
| Negative predictive value | 0.49 | 0.93 |
| Diagnostic accuracy | 0.83 | 0.92 |
| ROC area under the curve | 0.80 (95% CI: 0.71–0.89) | 0.75 (95% CI: 0.63–0.87) |
ROC = receiver operating characteristic; CI = confidence interval
Influence of FNA on operative management
To determine the influence of FNA results on surgical management, the proportion of neck dissections, two-stage operations and total parotidectomies were calculated for those with a correct and incorrect FNA diagnosis of malignant disease (Table 6).Neck dissection was performed in 6.5% of cases: in 9/27 (29.6%) malignant cases and in 4/174 (2.3%) benign cases (Table 7). The facial nerve was sacrificed in 2/27 (7.4%) cases of carcinoma and in none of the benign lesions (p=0.01). In these cases of carcinoma, the diagnosis was given correctly by FNA.
Table 6.
Operations performed for parotid lumps
| Operation | Frequency |
|---|---|
| Superficial/partial parotidectomy | 148 (73.6%) |
| Deep lobe parotidectomy | 16 (8.0%) |
| Total parotidectomy | 14 (7.0%) |
| Lumpectomy | 13 (6.5%) |
| Subtotal parotidectomy | 5 (2.5%) |
Table 7.
Influence of fine needle aspiration (FNA) on choice of operation
| Histology | FNA | Two-stage operations | p-value* | Neck dissection | p-value* | Total parotidectomy | p-value* | |
|---|---|---|---|---|---|---|---|---|
| Benign | Malignant | FP | 1/4 (25%) | – | 2/4 (50%) | – | 0/3 (0%) | – |
| Benign | Benign | TN | 3/170 (2%) | 0.09 | 2/170 (1%) | <0.01 | 3/169 (2%) | 0.94 |
| Malignant | Malignant | TP | 3/14 (21%) | – | 7/14 (50%) | – | 8/14 (57%) | – |
| Malignant | Benign | FN | 3/13 (23%) | 1.00 | 2/13 (15%) | 0.07 | 3/12 (25%) | 0.10 |
FP = false positive; TN = true negative; TP = true positive; FN = false negative
Comparing proportion between positive and negative FNA result, Fisher’s exact test
A false positive (FNA diagnosis of malignant disease in benign disease) is associated with a higher incidence of neck dissection (p<0.01). In this situation, it may be that FNA falsely informed the surgeon to perform an overly aggressive operation. For malignant disease, the influence of negative FNA in malignant disease (false negative) did not quite meet statistical significance. This may suggest that there are other signs (eg intraoperative appearance, clinical signs) that suggested malignancy despite a negative FNA result.
FNA result in cases of carcinoma
FNA identified malignant disease correctly in 51.9% of cases. The second most common result in those with a carcinoma diagnosis was inconclusive (25.9%) (Table 8). This may relate to the often atypical appearance of carcinoma cells and, furthermore, the innate difficulty in diagnosing carcinoma, which is conclusively defined histologically as breach of the basement membrane. 18
Table 8.
Fine needle aspiration result in those with histology diagnosis of carcinoma
| Result | Frequency |
|---|---|
| Carcinoma | 14 (51.9%) |
| Inconclusive | 7 (25.9%) |
| Infection/inflammation | 2 (7.4%) |
| Pleomorphic adenoma | 2 (7.4%) |
| Metastasis | 1 (3.7%) |
| Salivary tumour | 1 (3.7%) |
Histology result in inconclusive FNA
Of the inconclusive FNA results, Warthin’s tumour and carcinoma were the most common (Table 9). This may reflect what are often subtle changes in this benign lymphoma that frequently appears similar to normal lymphoid tissue, which unlike other salivary glands, is present in the parotid gland.
Table 9.
Histological diagnosis in those with an inconclusive fine needle aspiration
| Result | Frequency |
|---|---|
| Warthin’s tumour | 12 (41.4%) |
| Carcinoma | 7 (24.1%) |
| Pleomorphic adenoma | 4 (13.8%) |
| Other | 2 (10.3%) |
| Benign squamous cyst | 1 (3.4%) |
| Non-specific inflammation | 1 (3.4%) |
| Inconclusive | 1 (3.4%) |
Operator and use of ultrasonography guidance
In our cohort, 6 patients (3.0%) required more than one FNA. Table 10 shows that fewer second attempts were required by a pathologist (4 vs 2, p=0.035). There was a trend towards fewer second attempts of FNA when performed under ultrasonography guidance (4 vs 3, p=0.066).
Table 10.
Number of second attempts by profession and ultrasonography guidance
| Number of cases | Number of second attempts | p-value | ||
|---|---|---|---|---|
| Operator | Surgeons | 50 (24.9%) | 4 (8.0%) | – |
| Pathologists | 151 (75.1%) | 2 (1.3%) | 0.035 | |
| Ultrasonography guidance | No | 46 (22.9%) | 4 (8.7%) | – |
| Yes | 148 (73.6%) | 3 (2.0%) | 0.066 |
Discussion
While numerous studies have tried to determine the diagnostic characteristics of FNA in parotid surgery, the use of FNA continues to be controversial. It is useful to further explore the diagnostic ability of FNA because it has the potential to influence surgical management and the underlying prevalence of disease differs between populations.
A primary function of FNA prior to parotid surgery is to differentiate benign from malignant disease. In detecting malignant disease, FNA has a relatively low specificity. Just over half of cases of malignant disease are detected by FNA. However, where malignancy is reported by FNA, it is highly likely to be so.
Warthin’s tumour, carcinoma and pleomorphic adenoma made up the majority of inconclusive FNA results. Approximately half of the inconclusive results were attributable to carcinoma. FNA of this subgroup is challenging owing to subtle changes and a variable appearance. Furthermore, carcinoma is something that requires histology for a definitive diagnosis. Pleomorphic adenoma is identified reliably, and this may be due to its high incidence as well as a relatively well defined and consistent morphology. 19
Among patients with benign disease, neck dissection was performed more often in those with FNA reporting a malignancy rather than benign disease. Conversely, a false negative was associated with a lower incidence of neck dissection although this marginally failed to meet statistical significance. This suggests FNA has the potential to misguide the clinician and the multidisciplinary team in the choice of surgical procedure.
A second FNA was less likely to be needed if a pathologist performed the FNA, perhaps because they are more experienced in performing the procedure. The reduction in failed FNA under ultrasonography guidance narrowly missed out on statistical significance. Taken together, these data may suggest that FNA should ideally be performed by selected clinicians and possibly under ultrasonography guidance.
While this study highlights some of the shortcomings of the use of FNA, it should be noted that it is uniquely positioned to identify radiosensitive cutaneous squamous cell carcinomas and also, given a benign result on FNA, aiding the decision to opt for conservative treatment in a poor surgical candidate.
Conclusions
From these data, it is apparent that FNA is a useful clinical tool with greatest benefit when performed by experienced operators and interpreted with all other clinical information.
References
- 1.Schmidt RL, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of fine-needle aspiration cytology for parotid gland lesions. Am J Clin Pathol 2011; 136: 45–59 [DOI] [PubMed] [Google Scholar]
- 2.Stewart CJ, MacKenzie K, McGarry GW, Mowat A. Fine-needle aspiration cytology of salivary gland: a review of 341 cases. Diagn Cytopathol 2000; 22: 139–146 [DOI] [PubMed] [Google Scholar]
- 3.Berrone S, Cubetta M, Amasio Met al.Fine needle biopsy in the preoperative diagnosis of parotid tumors. Minerva Stomatol 1995; 44: 515–519 [PubMed] [Google Scholar]
- 4.Zurrida S, Alasio L, Tradati Net al.Fine-needle aspiration of parotid masses. Cancer 1993; 72: 2,306–2,311 [DOI] [PubMed] [Google Scholar]
- 5.Wong DS, Li GK. The role of fine-needle aspiration cytology in the management of parotid tumors: a critical clinical appraisal. Head Neck 2000; 22: 469–473 [DOI] [PubMed] [Google Scholar]
- 6.Salgarelli AC, Capparè P, Bellini P, Collini M. Usefulness of fine-needle aspiration in parotid diagnostics. Oral Maxillofac Surg 2009; 13: 185–190 [DOI] [PubMed] [Google Scholar]
- 7.Atula T, Greénman R, Laippala P, Klemi PJ. Fine-needle aspiration biopsy in the diagnosis of parotid gland lesions: evaluation of 438 biopsies. Diagn Cytopathol 1996; 15: 185–190 [DOI] [PubMed] [Google Scholar]
- 8.Behzatoğlu K, Bahadir B, Kaplan HHet al.Fine needle aspiration biopsy of the parotid gland. Diagnostic problems and 2 uncommon cases. Acta Cytol 2004; 48: 149–154 [DOI] [PubMed] [Google Scholar]
- 9.Al Khafaji BM, Nestok BR, Katz RL. Fine-needle aspiration of 154 parotid masses with histologic correlation. Cancer 1998; 84: 153–159 [PubMed] [Google Scholar]
- 10.Que Hee CG, Perry CF. Fine-needle aspiration cytology of parotid tumours: is it useful? ANZ J Surg 2001; 71: 345–348 [PubMed] [Google Scholar]
- 11.Tandon S, Shahab R, Benton JIet al.Fine-needle aspiration cytology in a regional head and neck cancer center: comparison with a systematic review and meta-analysis. Head Neck 2008; 30: 1,246–1,252 [DOI] [PubMed] [Google Scholar]
- 12.Layfield LJ, Gopez EV. Histologic and fine-needle aspiration cytologic features of polycystic disease of the parotid glands: case report and review of the literature. Diagn Cytopathol 2002; 26: 324–328 [DOI] [PubMed] [Google Scholar]
- 13.Hughes JH, Volk EE, Wilbur DC. Pitfalls in salivary gland fine-needle aspiration cytology: lessons from the College of American Pathologists Interlaboratory Comparison Program in Nongynecologic Cytology. Arch Pathol Lab Med 2005; 129: 26–31 [DOI] [PubMed] [Google Scholar]
- 14.Gal R. Fine needle aspiration of the salivary glands: a review. Oper Tech Otolaryngol Head Neck Surg 1996; 7: 323–326 [Google Scholar]
- 15.Peng Y, Wang HH. A meta-analysis of comparing fine-needle aspiration and frozen section for evaluating thyroid nodules. Diagn Cytopathol 2008; 36: 916–920 [DOI] [PubMed] [Google Scholar]
- 16.McGurk M, Hussain K. Role of fine needle aspiration cytology in the management of the discrete parotid lump. Ann R Coll Surg Engl 1997; 79: 198–202 [PMC free article] [PubMed] [Google Scholar]
- 17.Tew S, Poole AG, Philips J. Fine-needle aspiration biopsy of parotid lesions: comparison with frozen section. ANZ J Surg 1997; 67: 438–441 [DOI] [PubMed] [Google Scholar]
- 18.Bosman FT, Havenith M, Cleutjens JP. Basement membranes in cancer. Ultrastruct Pathol 1985; 8: 291–304 [DOI] [PubMed] [Google Scholar]
- 19.Stanley MW. Selected problems in fine needle aspiration of head and neck masses. Mod Pathol 2002; 15: 342–350 [DOI] [PubMed] [Google Scholar]


