Abstract
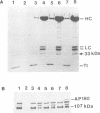
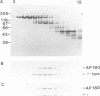
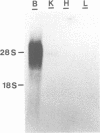
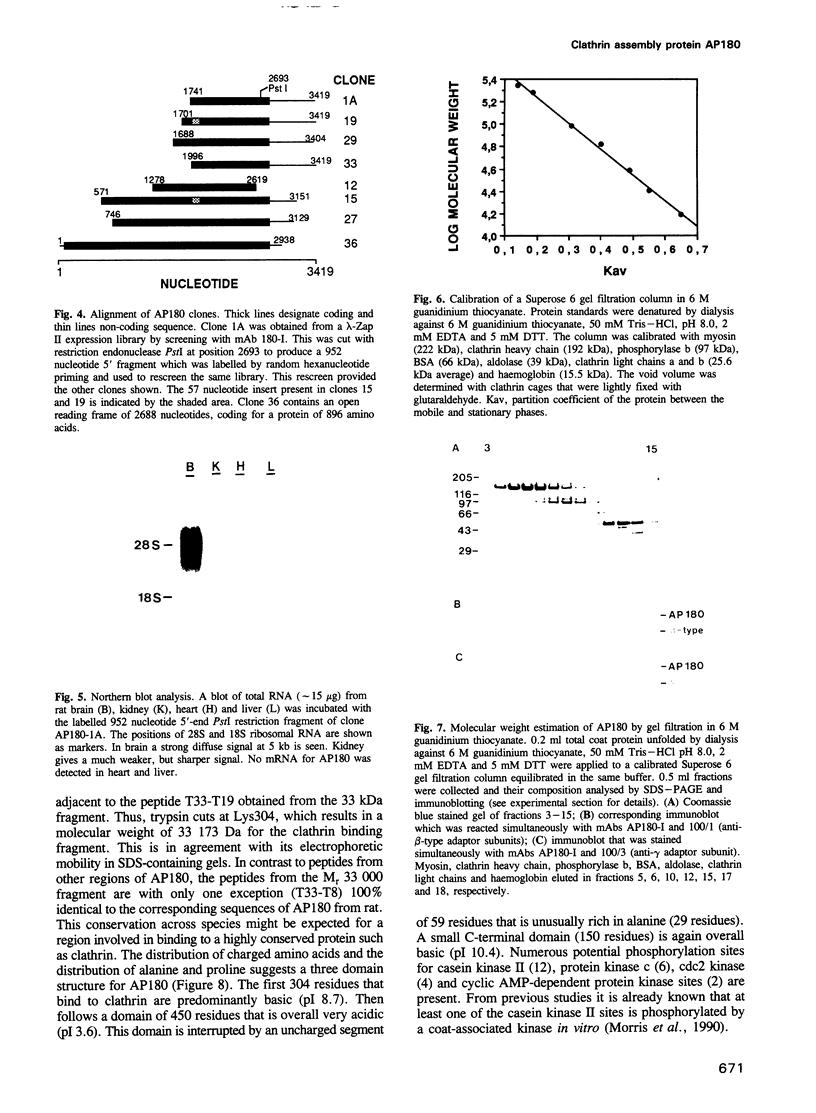
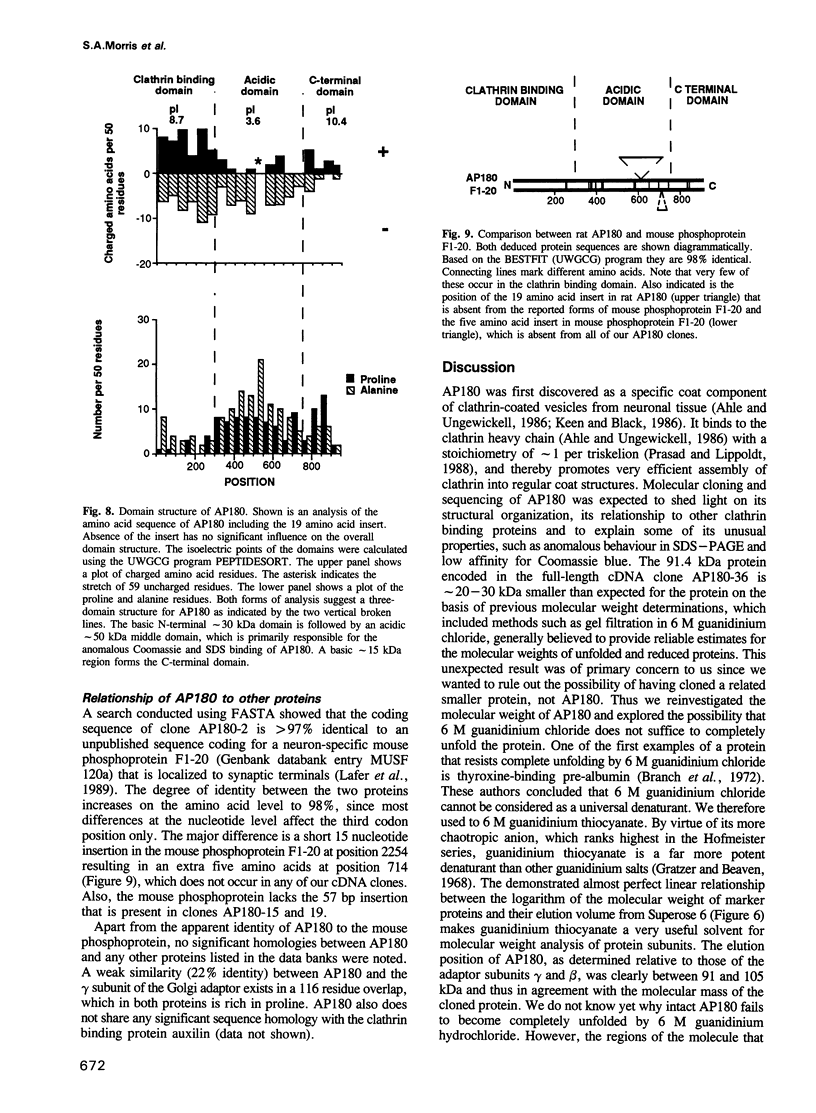
Binding of AP180 to clathrin triskelia induces their assembly into 60-70 nm coats. The largest rat brain cDNA clone isolated predicts a molecular weight of 91,430 for AP180. Two cDNA clones have an additional small 57 bp insert. The deduced molecular weight agrees with gel filtration results provided the more chaotropic denaturant 6 M guanidinium thiocyanate is substituted for the weaker guanidinium chloride. The sequence and the proteolytic cleavage pattern suggest a three domain structure. The N-terminal 300 residues (pI 8.7) harbour a clathrin binding site. An acidic middle domain (pI 3.6, 450 residues), interrupted by an uncharged alanine rich segment of 59 residues, appears to be responsible for the anomalous physical properties of AP180. The C-terminal domain (166 residues) has a pI of 10.4. AP180 mRNA is restricted to neuronal sources. AP180 shows no significant homology to known clathrin binding proteins, but is nearly identical to a mouse phosphoprotein (F1-20). This protein, localized to synaptic termini, has so far been of unknown function.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahle S., Ungewickell E. Auxilin, a newly identified clathrin-associated protein in coated vesicles from bovine brain. J Cell Biol. 1990 Jul;111(1):19–29. doi: 10.1083/jcb.111.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahle S., Ungewickell E. Purification and properties of a new clathrin assembly protein. EMBO J. 1986 Dec 1;5(12):3143–3149. doi: 10.1002/j.1460-2075.1986.tb04621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauw G., Van Damme J., Puype M., Vandekerckhove J., Gesser B., Ratz G. P., Lauridsen J. B., Celis J. E. Protein-electroblotting and -microsequencing strategies in generating protein data bases from two-dimensional gels. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7701–7705. doi: 10.1073/pnas.86.20.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch W. T., Robbins J., Edelhoch H. Thyroxine-binding prealbumin. Conformation in urea and guanidine. Arch Biochem Biophys. 1972 Sep;152(1):144–151. doi: 10.1016/0003-9861(72)90202-0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M. Living with clathrin: its role in intracellular membrane traffic. Science. 1988 Dec 9;242(4884):1396–1402. doi: 10.1126/science.2904698. [DOI] [PubMed] [Google Scholar]
- Campbell C., Squicciarini J., Shia M., Pilch P. F., Fine R. E. Identification of a protein kinase as an intrinsic component of rat liver coated vesicles. Biochemistry. 1984 Sep 11;23(19):4420–4426. doi: 10.1021/bi00314a028. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Gratzer W. B., Beaven G. H. Effect of protein denaturation on micelle stability. J Phys Chem. 1969 Jul;73(7):2270–2273. doi: 10.1021/j100727a028. [DOI] [PubMed] [Google Scholar]
- Keen J. H., Black M. M. The phosphorylation of coated membrane proteins in intact neurons. J Cell Biol. 1986 Apr;102(4):1325–1333. doi: 10.1083/jcb.102.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen J. H. Clathrin and associated assembly and disassembly proteins. Annu Rev Biochem. 1990;59:415–438. doi: 10.1146/annurev.bi.59.070190.002215. [DOI] [PubMed] [Google Scholar]
- Keen J. H. Clathrin assembly proteins: affinity purification and a model for coat assembly. J Cell Biol. 1987 Nov;105(5):1989–1998. doi: 10.1083/jcb.105.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T., Nathanson K. L., Matsui W., Vaisberg A., Chow E. P., Burne C., Keen J. H., Davis A. E. Structural and functional division into two domains of the large (100- to 115-kDa) chains of the clathrin-associated protein complex AP-2. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2612–2616. doi: 10.1073/pnas.86.8.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtz D. S., Puszkin S. A neuronal protein (NP185) associated with clathrin-coated vesicles. Characterization of NP185 with monoclonal antibodies. J Biol Chem. 1988 May 25;263(15):7418–7425. [PubMed] [Google Scholar]
- Kohtz D. S., Puszkin S. Phosphorylation of tubulin by casein kinase II regulates its binding to a neuronal protein (NP 185) associated with brain coated vesicles. J Neurochem. 1989 Jan;52(1):285–295. doi: 10.1111/j.1471-4159.1989.tb10929.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindner R., Ungewickell E. Clathrin-associated proteins of bovine brain coated vesicles. An analysis of their number and assembly-promoting activity. J Biol Chem. 1992 Aug 15;267(23):16567–16573. [PubMed] [Google Scholar]
- Lindner R., Ungewickell E. Light-chain-independent binding of adaptors, AP180, and auxilin to clathrin. Biochemistry. 1991 Sep 17;30(37):9097–9101. doi: 10.1021/bi00101a027. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Ahle S., Ungewickell E. Clathrin-coated vesicles. Curr Opin Cell Biol. 1989 Aug;1(4):684–690. doi: 10.1016/0955-0674(89)90034-3. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Mann A., Ungewickell E. Analysis of 100-180-kDa phosphoproteins in clathrin-coated vesicles from bovine brain. J Biol Chem. 1990 Feb 25;265(6):3354–3357. [PubMed] [Google Scholar]
- Murphy J. E., Keen J. H. Recognition sites for clathrin-associated proteins AP-2 and AP-3 on clathrin triskelia. J Biol Chem. 1992 May 25;267(15):10850–10855. [PubMed] [Google Scholar]
- Murphy J. E., Pleasure I. T., Puszkin S., Prasad K., Keen J. H. Clathrin assembly protein AP-3. The identity of the 155K protein, AP 180, and NP185 and demonstration of a clathrin binding domain. J Biol Chem. 1991 Mar 5;266(7):4401–4408. [PubMed] [Google Scholar]
- Pearse B. M. Receptors compete for adaptors found in plasma membrane coated pits. EMBO J. 1988 Nov;7(11):3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K., Lippoldt R. E. Molecular characterization of the AP180 coated vesicle assembly protein. Biochemistry. 1988 Aug 9;27(16):6098–6104. doi: 10.1021/bi00416a040. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. Cloning and expression of gamma-adaptin, a component of clathrin-coated vesicles associated with the Golgi apparatus. J Cell Biol. 1990 Dec;111(6 Pt 1):2319–2326. doi: 10.1083/jcb.111.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B., Hanson V., Perry D., Puszkin S. Neuronal specific protein NP185 is enriched in nerve endings: binding characteristics for clathrin light chains, synaptic vesicles, and synaptosomal plasma membrane. J Neurosci Res. 1991 Aug;29(4):461–473. doi: 10.1002/jnr.490290406. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Oestergaard L. Identification of the clathrin assembly protein AP180 in crude calf brain extracts by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1989 Jun;179(2):352–356. doi: 10.1016/0003-2697(89)90143-7. [DOI] [PubMed] [Google Scholar]