Abstract
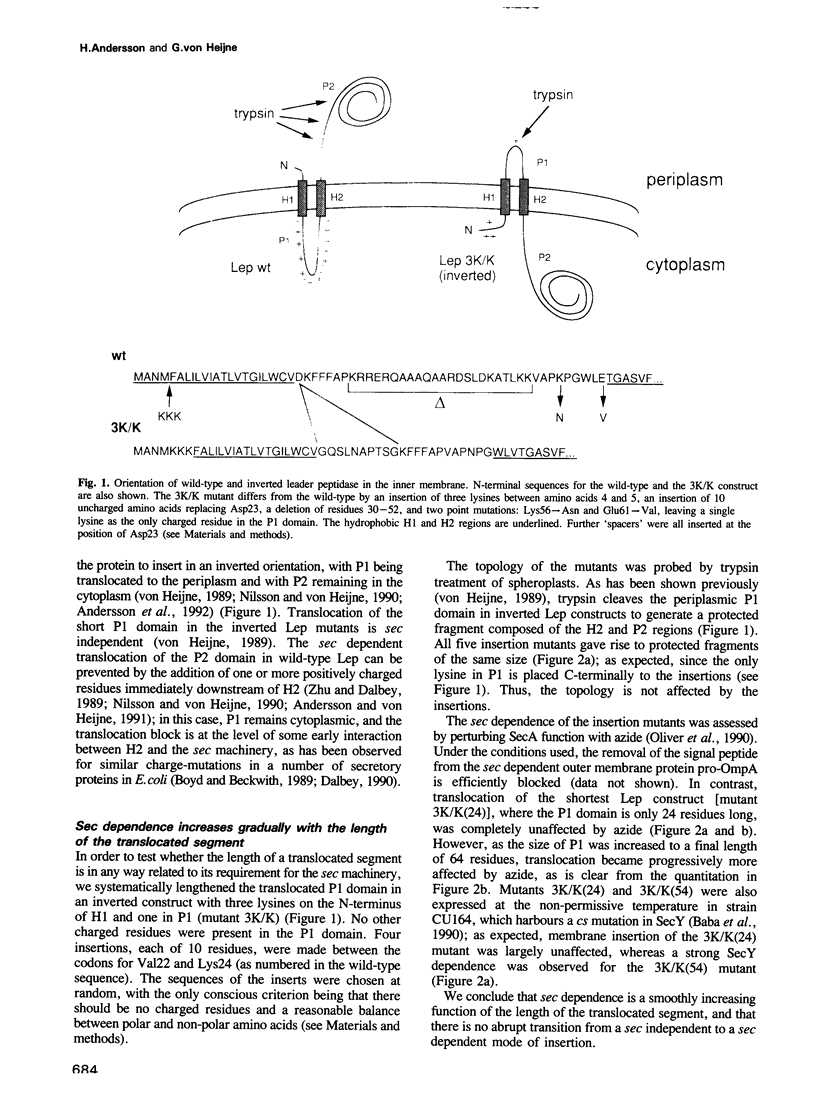
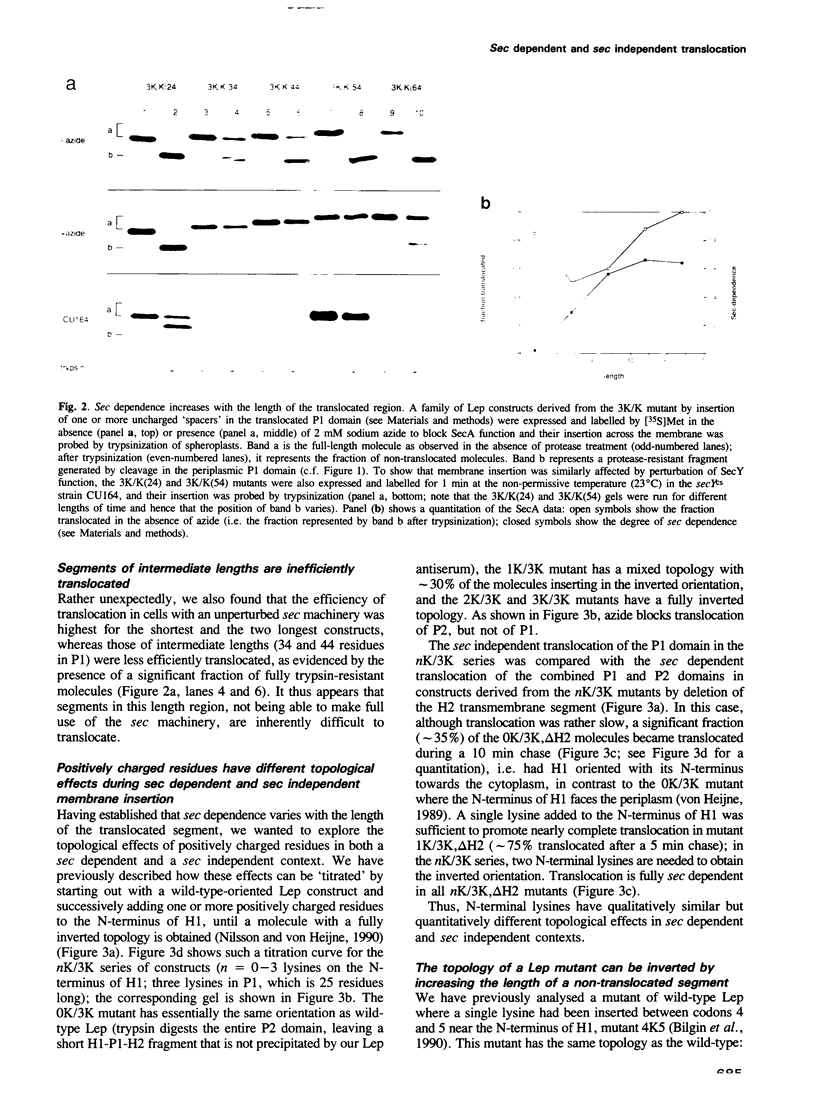
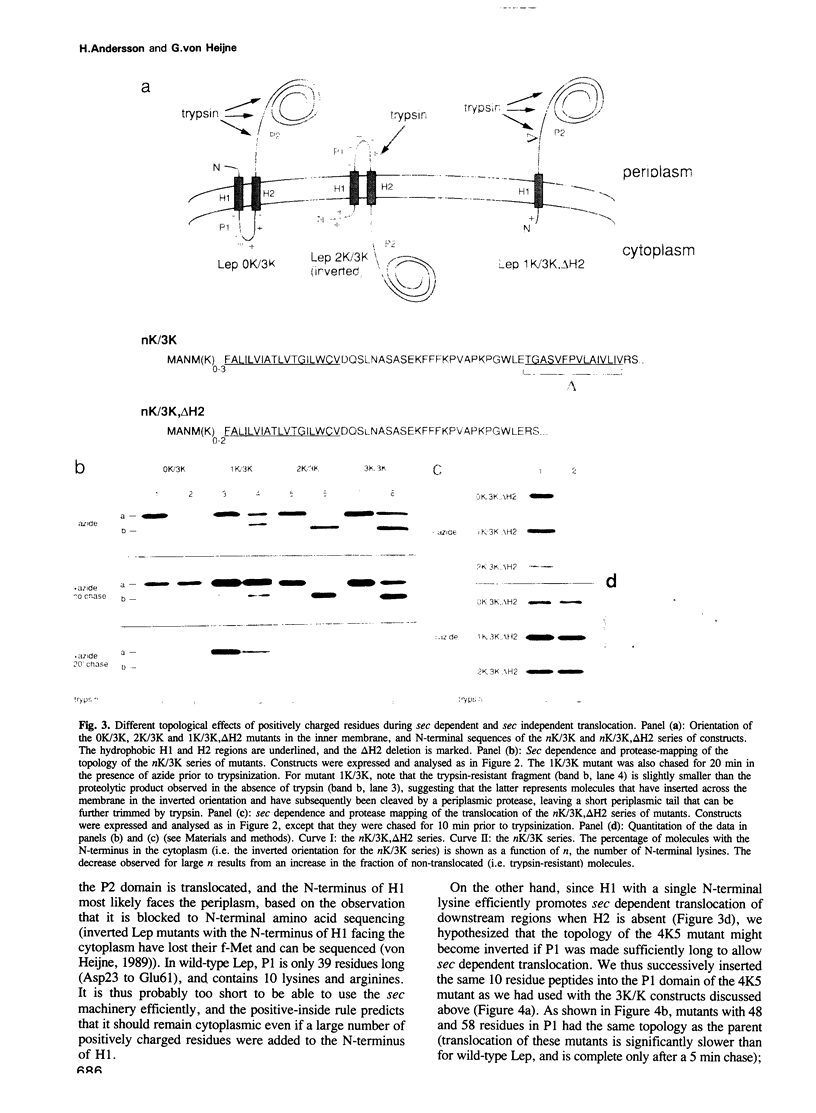
Translocation of proteins across the inner membrane of Escherichia coli normally requires the participation of the sec machinery. A number of proteins are known, however, where translocation can proceed unhindered even when the function of either SecA or SecY, central components of the sec machinery, is blocked. We now show that there is a linear correlation between the length of a translocated region and its degree of dependence on SecA and SecY for lengths between 25 and 55 residues. We also find that positively charged residues have distinctly different topological effects during SecA dependent and SecA independent membrane protein insertion, and that a short cytoplasmic segment in Lep can be converted to a translocated segment (with a concomitant inversion of the original topology of the whole molecule) by increasing its length into the SecA/Y dependent realm.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Ito K. Export of Escherichia coli alkaline phosphatase attached to an integral membrane protein, SecY. J Biol Chem. 1989 Jan 5;264(1):437–442. [PubMed] [Google Scholar]
- Andersson H., Bakker E., von Heijne G. Different positively charged amino acids have similar effects on the topology of a polytopic transmembrane protein in Escherichia coli. J Biol Chem. 1992 Jan 25;267(3):1491–1495. [PubMed] [Google Scholar]
- Andersson H., von Heijne G. A 30-residue-long "export initiation domain" adjacent to the signal sequence is critical for protein translocation across the inner membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9751–9754. doi: 10.1073/pnas.88.21.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T., Jacq A., Brickman E., Beckwith J., Taura T., Ueguchi C., Akiyama Y., Ito K. Characterization of cold-sensitive secY mutants of Escherichia coli. J Bacteriol. 1990 Dec;172(12):7005–7010. doi: 10.1128/jb.172.12.7005-7010.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieker-Brady K., Silhavy T. J. Suppressor analysis suggests a multistep, cyclic mechanism for protein secretion in Escherichia coli. EMBO J. 1992 Sep;11(9):3165–3174. doi: 10.1002/j.1460-2075.1992.tb05393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgin N., Lee J. I., Zhu H. Y., Dalbey R., von Heijne G. Mapping of catalytically important domains in Escherichia coli leader peptidase. EMBO J. 1990 Sep;9(9):2717–2722. doi: 10.1002/j.1460-2075.1990.tb07458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Beckwith J. Positively charged amino acid residues can act as topogenic determinants in membrane proteins. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9446–9450. doi: 10.1073/pnas.86.23.9446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobet W. W., Mollay C., Müller G., Zimmermann R. Export of honeybee prepromelittin in Escherichia coli depends on the membrane potential but does not depend on proteins secA and secY. J Biol Chem. 1989 Jun 15;264(17):10169–10176. [PubMed] [Google Scholar]
- Collier D. N., Strobel S. M., Bassford P. J., Jr SecB-independent export of Escherichia coli ribose-binding protein (RBP): some comparisons with export of maltose-binding protein (MBP) and studies with RBP-MBP hybrid proteins. J Bacteriol. 1990 Dec;172(12):6875–6884. doi: 10.1128/jb.172.12.6875-6884.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey R. E. Leader peptidase. Mol Microbiol. 1991 Dec;5(12):2855–2860. doi: 10.1111/j.1365-2958.1991.tb01844.x. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E. Positively charged residues are important determinants of membrane protein topology. Trends Biochem Sci. 1990 Jul;15(7):253–257. doi: 10.1016/0968-0004(90)90047-f. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. Leader peptidase of Escherichia coli: critical role of a small domain in membrane assembly. Science. 1987 Feb 13;235(4790):783–787. doi: 10.1126/science.3544218. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. The role of the polar, carboxyl-terminal domain of Escherichia coli leader peptidase in its translocation across the plasma membrane. J Biol Chem. 1986 Oct 15;261(29):13844–13849. [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Degen M., Henning U. A lower size limit exists for export of fragments of an outer membrane protein (OmpA) of Escherichia coli K-12. J Mol Biol. 1989 Feb 20;205(4):771–775. doi: 10.1016/0022-2836(89)90321-5. [DOI] [PubMed] [Google Scholar]
- Gebert J. F., Overhoff B., Manson M. D., Boos W. The Tsr chemosensory transducer of Escherichia coli assembles into the cytoplasmic membrane via a SecA-dependent process. J Biol Chem. 1988 Nov 15;263(32):16652–16660. [PubMed] [Google Scholar]
- Hartl F. U., Lecker S., Schiebel E., Hendrick J. P., Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990 Oct 19;63(2):269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Accumulation of prolipoprotein in Escherichia coli mutants defective in protein secretion. J Bacteriol. 1985 Mar;161(3):949–954. doi: 10.1128/jb.161.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986 Nov;5(11):3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt D. W., Gierasch L. M. Hydrophobic content and lipid interactions of wild-type and mutant OmpA signal peptides correlate with their in vivo function. Biochemistry. 1991 Oct 22;30(42):10155–10163. doi: 10.1021/bi00106a012. [DOI] [PubMed] [Google Scholar]
- Johnston S., Lee J. H., Ray D. S. High-level expression of M13 gene II protein from an inducible polycistronic messenger RNA. Gene. 1985;34(2-3):137–145. doi: 10.1016/0378-1119(85)90121-0. [DOI] [PubMed] [Google Scholar]
- Kato M., Tokuda H., Mizushima S. In vitro translocation of secretory proteins possessing no charges at the mature domain takes place efficiently in a protonmotive force-dependent manner. J Biol Chem. 1992 Jan 5;267(1):413–418. [PubMed] [Google Scholar]
- Kuhn A. Alterations in the extracellular domain of M13 procoat protein make its membrane insertion dependent on secA and secY. Eur J Biochem. 1988 Nov 1;177(2):267–271. doi: 10.1111/j.1432-1033.1988.tb14372.x. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Zhu H. Y., Dalbey R. E. Efficient translocation of positively charged residues of M13 procoat protein across the membrane excludes electrophoresis as the primary force for membrane insertion. EMBO J. 1990 Aug;9(8):2385–2389. doi: 10.1002/j.1460-2075.1990.tb07413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch Y. M., Tachias K., Moomaw C. R., Dreyfus L. A., Urban R., Slaughter C., Whipp S. Secretion of methanol-insoluble heat-stable enterotoxin (STB): energy- and secA-dependent conversion of pre-STB to an intermediate indistinguishable from the extracellular toxin. J Bacteriol. 1990 May;172(5):2427–2432. doi: 10.1128/jb.172.5.2427-2432.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws J. K., Dalbey R. E. Positive charges in the cytoplasmic domain of Escherichia coli leader peptidase prevent an apolar domain from functioning as a signal. EMBO J. 1989 Jul;8(7):2095–2099. doi: 10.1002/j.1460-2075.1989.tb03618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. I., Kuhn A., Dalbey R. E. Distinct domains of an oligotopic membrane protein are Sec-dependent and Sec-independent for membrane insertion. J Biol Chem. 1992 Jan 15;267(2):938–943. [PubMed] [Google Scholar]
- Lill R., Dowhan W., Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990 Jan 26;60(2):271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- McGovern K., Beckwith J. Membrane insertion of the Escherichia coli MalF protein in cells with impaired secretion machinery. J Biol Chem. 1991 Nov 5;266(31):20870–20876. [PubMed] [Google Scholar]
- Moore K. E., Miura S. A small hydrophobic domain anchors leader peptidase to the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1987 Jun 25;262(18):8806–8813. [PubMed] [Google Scholar]
- Nilsson I., von Heijne G. Fine-tuning the topology of a polytopic membrane protein: role of positively and negatively charged amino acids. Cell. 1990 Sep 21;62(6):1135–1141. doi: 10.1016/0092-8674(90)90390-z. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Cabelli R. J., Dolan K. M., Jarosik G. P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Schiebel E., Driessen A. J., Hartl F. U., Wickner W. Delta mu H+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991 Mar 8;64(5):927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- Tian G., Wu H. C., Ray P. H., Tai P. C. Temperature-dependent insertion of prolipoprotein into Escherichia coli membrane vesicles and requirements for ATP, soluble factors, and functional SecY protein for the overall translocation process. J Bacteriol. 1989 Apr;171(4):1987–1997. doi: 10.1128/jb.171.4.1987-1997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W. Bacterial leader peptidase, a membrane protein without a leader peptide, uses the same export pathway as pre-secretory proteins. Cell. 1984 Apr;36(4):1067–1072. doi: 10.1016/0092-8674(84)90056-4. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W., Goodman J. M. Sequence of the leader peptidase gene of Escherichia coli and the orientation of leader peptidase in the bacterial envelope. J Biol Chem. 1983 Oct 10;258(19):12073–12080. [PubMed] [Google Scholar]
- Zhu H. Y., Dalbey R. E. Both a short hydrophobic domain and a carboxyl-terminal hydrophilic region are important for signal function in the Escherichia coli leader peptidase. J Biol Chem. 1989 Jul 15;264(20):11833–11838. [PubMed] [Google Scholar]
- von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989 Oct 5;341(6241):456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988 Jul 1;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Manoil C. Membrane proteins: from sequence to structure. Protein Eng. 1990 Dec;4(2):109–112. doi: 10.1093/protein/4.2.109. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992 May 20;225(2):487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Wickner W., Dalbey R. E. The cytoplasmic domain of Escherichia coli leader peptidase is a "translocation poison" sequence. Proc Natl Acad Sci U S A. 1988 May;85(10):3363–3366. doi: 10.1073/pnas.85.10.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]