Abstract
Purpose.
To evaluate the relationship between androgen levels and subjective and objective measures of dry eye syndrome (DES).
Methods.
A total of 263 male patients from the Miami Veterans Affairs Medical Center eye clinic aged ≥50 were recruited for this prospective cross-sectional study. Patients completed Dry Eye Questionnaire 5, underwent tear film evaluation, and had serum androgen levels measured. The correlations between androgen levels, DES composite scores, DES symptoms, and global, lipid, and aqueous tear film parameters were evaluated.
Results.
Two hundred sixty-three patients with a mean age of 69 (50–95) were examined. There was no linear association between composite DES scores (generated using latent class analysis) and androgen levels. However, eyes with high DES scores (0.95–1.0) had higher levels of sex hormone-binding globulin (P = 0.03) and lower levels of dehydroepiandrosterone sulfate (DHEAS) (P = 0.02), androstenedione (A) (P = 0.02), and androstane-3α,17β-diol glucuronide (P = 0.03) compared to eyes with intermediate (0.05–0.95) or low (0–0.05) scores. There were no strong correlations between tear film measures and androgen levels. Regarding global parameters, a weak inverse correlation was found between corneal staining and A (r = −0.17, P = 0.009). For lipid parameters, a weak correlation existed between tear breakup time (TBUT) and A (r = 0.15, P = 0.02). When considering aqueous and lipid deficiency independently, the association between TBUT and A existed only with aqueous tear deficiency (r = 0.66, P = 0.002). Regarding aqueous parameters, a weak correlation existed between Schirmer test and DHEAS (r = 0.13, P = 0.047) and A (r = 0.21, P = 0.001).
Conclusions.
There was a weak correlation between higher levels of androstenedione and healthier global, lipid, and aqueous tear film parameters.
Keywords: dry eye syndrome, androgens, meibomian gland
We found weak correlations between lower levels of the androgen androstenedione and global, lipid, and aqueous tear film abnormalities in an older male veteran population.
Introduction
Dry eye syndrome (DES) is one of the most common diagnoses presenting to ophthalmologists. Dry eye syndrome describes a group of tear film disorders that cause irritation and ocular surface damage. The most common subtypes of DES include aqueous and lipid deficiency, although most patients with DES have abnormalities in both tear components.1 Nearly 7 million Americans aged 40 and older suffer from DES,2–4 which decreases mobility, increases stress, affects work productivity, and overall decreases quality of life.5–7 Each year, the United States expends $55.4 billion in DES treatment (societal cost around $11,000 per patient).8 Treatments for DES are costly because they typically target and only partially alleviate symptoms and do not cure the underlying pathology.2
Given its prevalence, morbidity and cost, it is clear that more effective prevention and treatments are needed to address the underlying pathogenesis of DES. Known risk factors for DES include increasing age and female sex.9,10 One potential mechanism underlying both is hormonal changes that occur with menopause. Androgens may be involved, as lower testosterone (T) levels have been associated with poorer tear function11 and lower meibomian gland (MG) secretion volume and orifice diameter (Suzuki TY, et al. IOVS 2007;48:ARVO E-Abstract 434; Suzuki TY, et al. IOVS 2008;49:ARVO E-Abstract 92).
Androgens are steroid hormones that are produced by the adrenals (dehydroepiandrosterone sulfate [DHEAS], dehydroepiandrosterone [DHEA], and androstenedione [A]), gonads (T), and peripheral tissues (dihydrotestosterone [DHT]). The two most important endogenous androgens that manifest both androgenic and anabolic activities are T and DHT (DHT being the most potent androgen). Dihydrotestosterone is converted to several metabolites, one of which has been shown to reflect 5α-reductase activity and peripheral androgen action, androstane-3α,17β-diol glucuronide (3α-diolG).12,13
Sebaceous glands, including MGs, contain 5α-reductase mRNA.14 Furthermore, epithelial acinar cell nuclei of human MGs contain androgen receptor protein and mRNA for 5α-reductase, type 1 and 2.14 As such, there is evidence that androgens are involved in MG health. Androgens (T, DHT) bind to androgen receptors15 and act via a nuclear receptor-mediated mechanism to regulate MG structure and function.14,16
In clinical studies of both sexes, MG dysfunction and lipid tear deficiency have been found in various androgen-depleted states.17 Sixty percent of male patients on antiandrogen therapy (due to prostate cancer or prostate hyperplasia) were found to have MG dysfunction compared to 25% of age-matched controls (P < 0.05).18 These patients also had significantly altered meibum profiles, decreased tear breakup time (TBUT), and increased DES prevalence.18 Nine patients with chronic androgen insensitivity syndrome (CAIS), another androgen-depleted state, had a 2-fold increase in dry eye symptoms compared to controls (n = 21).19 The same study also found that CAIS patients had a reduced tear meniscus and increased eyelid erythema, telangiectasia, and keratinization, as well as a higher frequency of MG orifice metaplasia (all P < 0.001).19 Finally, patients with Sjögren's syndrome (SS), a disease whose hallmark is DES, were also found to be androgen deficient compared to age-matched controls.20 Another study found a higher incidence of histologic MG destruction in SS patients (11/19 eyes) than in age-matched non-SS patients with aqueous tear deficiency (5/27 eyes, P < 0.05).21
While CAIS and SS are relatively rare androgen-deficient diseases, andropause is a more common process that begins to occur in men at age 40.22 Andropause refers to the symptoms associated with a gradual decline of androgen levels due to aging, a relationship that is well supported by studies implicating the following androgens: DHEA, DHEAS, A, T, and 3α-diolG.23–27 In addition to decreased androgen levels, aging is also correlated with abnormal lipid secretions and MG dysfunction.28,29
Despite these findings, a knowledge gap remains regarding the relationship between andropause and tear film function. Specifically, the effect of androgen levels on tear film parameters in men has not been examined. Studying this relationship is important, as DES appears to be more common in men than formerly thought. We have shown that 19% of US male veterans have DES.10,30 We hypothesize that andropause is a risk factor for lipid deficiency DES in the male population. The purpose of this study was to test this hypothesis by evaluating the relationship between androgen levels and subjective and objective measures of DES in an older male population.
Methods
Study Population
The prospective examination of this study's patients was reviewed and approved by the Miami Veterans Affairs Medical Center (VAMC) Institutional Review Board. Informed consent was acquired from all patients, and the study was conducted in accordance with the principles of the Declaration of Helsinki. The Miami VAMC prospectively recruited patients irrespective of tear film function status. Ineligible patients included those who were female; were younger than age 50; had abnormal eyelid, conjunctival, or corneal anatomy; wore contact lenses; used any ocular medication (with the exception of artificial tears/topical cyclosporine); had a history of cataract surgery within the last 3 months; or had human immunodeficiency virus, sarcoidosis, graft-versus-host disease, or a collagen vascular disease.
Data Collection
Each patient provided demographic information, past medical history, and other medical information. Each patient self-administered the Dry Eye Questionnaire 5 (DEQ5), a five-item dry eye questionnaire that includes patient responses regarding discomfort, dryness, and tearing without considering visual function. The ocular surface examination consisted of tear osmolarity (TearLAB, San Diego, CA, USA) (obtained once in each eye), TBUT (measured twice in each eye and averaged), corneal staining (punctuate epithelial erosions [PEE]; range, 0–5),31 Schirmer strips with anesthesia, and morphologic and qualitative eyelid and MG information. Morphologic parameters collected included degree of eyelid vascularity (0 = none; 1 = mild engorgement; 2 = moderate engorgement; 3 = severe engorgement)32 and the degree of inferior eyelid meibomian orifice plugging (0 = none; 1 = less than 1/3 lid involvement; 2 = between 1/3 and 2/3 lid involvement; 3 = greater than 2/3 lid involvement). Meibomian gland secretion quality was rated on a scale of 0 to 4 (0 = clear; 1 = cloudy; 2 = granular; 3 = toothpaste; 4 = no meibum extracted).33 All data were recorded and organized into a standardized database. Aqueous tear deficiency (ATD) was defined by a Schirmer value < 5, and lipid tear deficiency (LTD) was defined by either MG quality score or lid plugging score > 1 in the more severe eye.34
Blood Collection
All blood was collected between the hours of 7:30 and 9:00 AM. Blood tubes were centrifuged for 10 minutes at 3600g. The serum was then pipetted into sterile containers and frozen at −80°C for subsequent analysis.
Assay Methods
Androstenedione, DHT, and T were measured by radioimmunoassay (RIA) methods that were developed and validated at the Reproductive Endocrine Research Laboratory directed by one of the authors (FZS), as previously described.13,35,36 The androgens were first extracted from 0.5 mL serum using ethyl acetate: hexane (1:1) and then separated by Celite column partition chromatography using 0%, 10%, and 40% toluene in isooctane, respectively. Assay specificity was achieved by extraction/chromatographic purification and use of highly specific antiserum in conjunction with the appropriate iodinated radioligand in the RIA. The assay sensitivities were 0.03, 0.005, and 0.015 ng/mL for the A, DHT, and T RIAs, respectively. The interassay coefficients of variation (CVs) ranged from 8% to 13% in the three RIAs.
Bioavailable T (BioT) was calculated using the measured total T and sex hormone-binding globulin (SHBG) concentrations, as well as an average assumed concentration for albumin.37,38 This method has been found to have high validity.39 3α-diolG was quantified by direct RIA using a commercial kit obtained from Diagnostic Systems Laboratories (presently Beckman-Coulter, Minneapolis, MN, USA), which was validated extensively.40 The assay sensitivity was 0.5 ng/mL, and the interassay CV averaged 8.5%. DHEAS and SHBG were measured by a solid-phase, two-site chemiluminescent immunometric assay on the Immulite 2000 analyzer (Siemens Healthcare Diagnostics, Deerfield, IL, USA). The assay sensitivities were 3 μg/dL and 1 nmol/L, respectively, and the interassay CV was <10% for both assays.
Main Outcome Measures
The main outcome measures were the correlation between androgen levels; DES composite scores (generated by latent class analysis [LCA]); DES symptoms; and global, lipid, and aqueous tear film parameters.
Statistical Analysis
All statistical analyses were performed using SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) statistical package. Tear film parameters were characterized by descriptive statistics. Latent class analysis was used to generate a composite score for DES using all subjective and objective measures.41 To improve the model, all variables that were not normally distributed, such as tear osmolarity, TBUT, and Schirmer test, were transformed to normality. Latent class analysis provided us with (1) a dichotomous assessment as to whether the patient had DES (yes/no) and (2) a disease severity score ranging from 0 (no disease) to 1 (maximal disease). For the dichotomous assessment, patients with a posterior probability score greater than 0.5 were defined as diseased (42%) while those with scores less than 0.5 were defined as nondiseased (58%).41 The LCA composite score has the advantage of assessing patients with respect to both subjective symptoms and objectively measured tear film parameters. However, we also used DEQ5 alone as a measure of the disease severity; specifically, patients with DEQ5 ≥ 12 were considered to have severe ocular surface symptoms.42
Logistic regression was applied to evaluate the ability of androgen levels to predict DES as a dichotomous variable (LCA composite score ≥ 0.5 and DEQ5 ≥ 12). The associations between androgen levels and global, lipid, and aqueous tear film parameters were evaluated using Pearson and Spearman correlation tests for continuous and ordinal variables. Furthermore, after noting significant rank correlations between overall DES scores and androgen levels, the LCA DES scores were trichotomized (0 = 0–0.05, 1 = 0.95–1.0, 0.5 = 0.05–0.95). One-way analysis of variance (ANOVA) and post hoc least significant difference (LSD) multiple comparisons were used to evaluate the relationship between androgen levels and severity of DES. A P value less than 0.05 was considered statistically significant. For all analyses, the more severe value of the two eyes was used in the model. A moderate correlation was considered one with r2 ≥ 10%, that is, |r| ≥ 0.31. Statistically significant correlations with |r| < 0.31 were considered weak correlations. We did not adjust any correlations or comparison of means for patient age, an intervening variable, because this could have adjusted away any associations between the androgen and DES parameters.
Results
Study Population
Two hundred sixty-three male patients were included in this study. Table 1 contains demographic and clinical characteristics of our study population. Mean age was 69 (standard deviation [SD] ± 9). Sixty-nine percent of our patients were white non-Hispanic; 63% were in good or excellent health.
Table 1.
Demographic Information of Study Population
|
Patients, N = 263 |
|
| Age, mean (SD) [range] | 68.6 (9.1) [49.9–94.9] |
| Race/ethnicity, n (%) | |
| White non-Hispanic | 182 (69.2%) |
| White, Hispanic | 74 (28.1%) |
| Black, non-Hispanic | 1 (0.4%) |
| Black, Hispanic | 0 (0%) |
| Asian or Pacific Islander | 5 (1.9%) |
| Other | 1 (0.4%) |
| Smoking status, n (%) | |
| Never | 43 (16.4%) |
| Former | 148 (56.5%) |
| Current | 71 (27.1%) |
| Health status, n (%) | |
| Excellent | 19 (7.3%) |
| Good | 145 (55.6%) |
| Fair | 78 (29.9%) |
| Poor | 19 (7.3%) |
Dry Eye Syndrome and Androgen Levels
Using logistic and linear regression analyses we did not find significant associations between the frequency and severity of DES (composite scores generated by LCA) and androgen levels. However, after significant Spearman rank correlations between these scores and androgen levels were noted, LCA DES scores were trichotomized (minimal: 0–0.05, n = 81 [33%]; intermediate: 0.05–0.95, n = 107 [44%]; and severe: 0.95–1.0, n = 56 [23%]) (Table 2). With ANOVA, we found that patients with higher DES scores had higher SHBG (P = 0.03) and lower DHEAS (P = 0.02), A (P = 0.02), and 3α-diolG (P = 0.03). We did not find a significant difference between levels of BioT in patients with severe DES compared to controls.
Table 2.
Associations Between Dry Eye Syndrome Composite Scores (Generated by Latent Class Analysis) and Androgen Levels
|
Androgen |
LCA DES |
N |
Mean |
SD |
Significance,P |
| SHBG, ng/mL | Minimal | 81 | 12.8 | 8.24 | 0.03 |
| Intermediate | 107 | 16.2 | 10.7 | ||
| Severe | 56 | 16.6 | 9.81 | ||
| Total | 244 | 15.2 | 9.84 | ||
| DHEAS, ng/mL | Minimal | 81 | 792 | 694 | 0.02 |
| Intermediate | 105 | 663 | 459 | ||
| Severe | 56 | 525 | 449 | ||
| Total | 242 | 674 | 553 | ||
| Androstenedione, ng/mL | Minimal | 78 | 0.796 | 0.358 | 0.019 |
| Intermediate | 106 | 0.733 | 0.326 | ||
| Severe | 56 | 0.637 | 0.245 | ||
| Total | 240 | 0.731 | 0.324 | ||
| DHT, ng/mL | Minimal | 78 | 0.51 | 0.321 | 0.21 |
| Intermediate | 106 | 0.575 | 0.364 | ||
| Severe | 56 | 0.486 | 0.283 | ||
| Total | 240 | 0.533 | 0.334 | ||
| T, ng/mL | Minimal | 81 | 4.35 | 3.59 | 0.34 |
| Intermediate | 107 | 4.86 | 2.91 | ||
| Severe | 56 | 4.21 | 2.23 | ||
| Total | 244 | 4.54 | 3.02 | ||
| BioT, ng/mL | Minimal | 78 | 2.36 | 1.76 | 0.192 |
| Intermediate | 106 | 2.41 | 1.35 | ||
| Severe | 56 | 2 | 0.749 | ||
| Total | 240 | 2.3 | 1.4 | ||
| 3α-diolG, ng/mL | Minimal | 78 | 7.05 | 5.32 | 0.029 |
| Intermediate | 106 | 8.2 | 6.91 | ||
| Severe | 56 | 5.62 | 4.2 | ||
| Total | 240 | 7.23 | 5.93 |
Trichotomization of LCA DES scores: minimal, 0 to 0.05; intermediate, 0.05 to 0.95; severe, 0.95 to 1.0. Most severe eye measurements used for all analyses.
Ocular Surface Symptoms and Androgen Levels
We did not find a statistically significant relationship between DES symptoms (as measured by the DEQ5) and androgen levels (Fig. 1). This analysis was done using both logistic regression analysis to evaluate the effect of androgens on severe symptoms (DEQ5 ≥ 12) and linear regression analysis to evaluate symptoms on a continuous scale.
Figure 1.
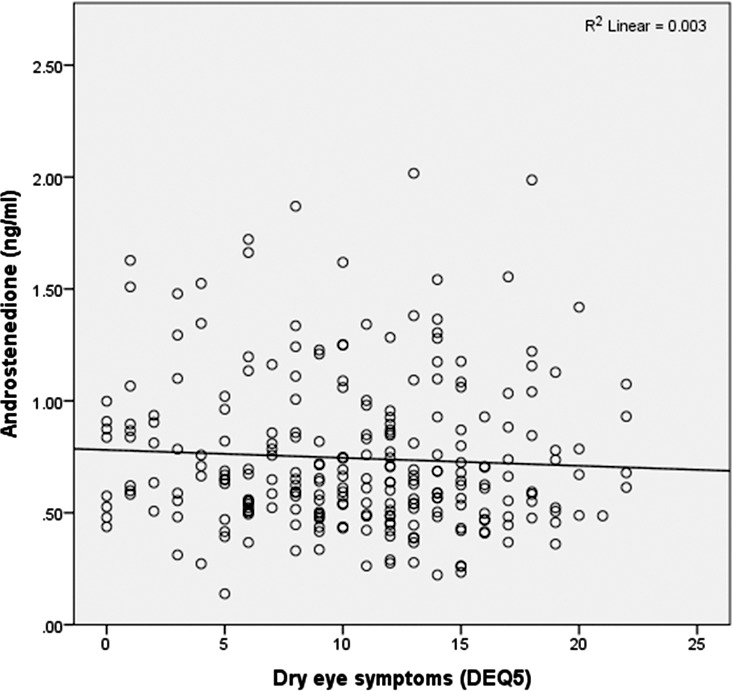
Androstenedione levels and dry eye syndrome (measured by DEQ5) show no significant correlation.
Global, Lipid, and Aqueous Tear Parameters and Androgen Levels
A weak inverse correlation was found between corneal staining and A (r = −0.17, P = 0.009); that is, lower levels of A were associated with increased staining (Table 3). A weak correlation was also found between TBUT, lid vascularity, and A (r = 0.15, P = 0.02 and r = −0.15, P = 0.02, respectively), with lower A levels associated with worse lipid parameters (Fig. 2). In addition, a weak correlation was found between the Schirmer test and two androgens: DHEAS (r = 0.13, P = 0.047) and A (r = 0.21, P = 0.001); that is, higher levels of DHEAS and A were associated with higher Schirmer values (Fig. 3).
Table 3.
Associations Between Global, Lipid, and Aqueous Tear Film Parameters and Androgen Levels
|
Tear Film Parameter |
Androgen |
N |
Coefficient | Significance |
|
Pearson (Spearman) |
Pearson (Spearman) |
|||
| Global parameters | ||||
| Tear osmolarity | SHBG | 254 | 0.04 (0.04) | 0.55 (0.55) |
| DHEAS | 220 | 0.003 (0.04) | 0.97 (0.53) | |
| Androstenedione | 249 | 0.03 (0.06) | 0.63 (0.35) | |
| DHT | 249 | 0.07 (0.07) | 0.29 (0.31) | |
| Testosterone | 254 | 0.07 (0.03) | 0.29 (0.68) | |
| 3α-diolG | 249 | 0.07 (0.10) | 0.29 (0.13) | |
| Difference in tear osmolarity between eyes | SHBG | 253 | −0.01 (0.02) | 0.89 (0.82) |
| DHEAS | 219 | 0.06 (0.13) | 0.38 (0.06) | |
| Androstenedione | 248 | 0.03 (0.06)) | 0.69 (0.37) | |
| DHT | 248 | −0.02 (−0.01) | 0.71 (0.94) | |
| Testosterone | 253 | −0.02 (−0.002) | 0.73 (0.97) | |
| 3α-diolG | 248 | 0.07 (0.06) | 0.24 (0.35) | |
| Corneal staining | SHBG | 261 | 0.01 (0.07) | 0.12 (0.27) |
| DHEAS | 224 | −0.09 (−0.08) | 0.18 (0.27) | |
| Androstenedione | 249 | −0.17 (−0.17) | 0.009 (0.009) | |
| DHT | 249 | −0.02 (0.01) | 0.74 (0.84) | |
| Testosterone | 261 | −0.02 (−0.01) | 0.81 (0.83) | |
| 3α-diolG | 249 | −0.07 (−0.05) | 0.30 (0.48) | |
| Meibomian gland parameters | ||||
| Tear breakup time | SHBG | 262 | −0.17 (−0.16) | 0.06 (0.008) |
| DHEAS | 225 | 0.04 (0.03) | 0.52 (0.62) | |
| Androstenedione | 250 | 0.15 (0.14) | 0.02 (0.02) | |
| DHT | 250 | −0.01 (−0.01) | 0.97 (0.94) | |
| Testosterone | 262 | 0.00 (−0.06) | 0.10 (0.37) | |
| 3α-diolG | 250 | −0.03 (−0.02) | 0.63 (0.72) | |
| Meibum quality | SHBG | 260 | −0.01 (0.03) | 0.89 (0.64) |
| DHEAS | 223 | −0.12 (−0.11) | 0.07 (0.10) | |
| Androstenedione | 249 | 0.11 (0.09) | 0.09 (0.18) | |
| DHT | 249 | 0.10 (0.08) | 0.12 (0.24) | |
| Testosterone | 260 | 0.05 (0.04) | 0.41 (0.48) | |
| 3α-diolG | 249 | 0.04 (0.04) | 0.54 (0.52) | |
| Lid vascularity | SHBG | 260 | 0.01 (0.08) | 0.91 (0.22) |
| DHEAS | 225 | −0.05 (−0.09) | 0.45 (0.17) | |
| Androstenedione | 250 | −0.15 (−0.13) | 0.02 (0.03) | |
| DHT | 250 | −0.03 (0.03) | 0.67 (0.69) | |
| Testosterone | 260 | −0.05 (0.02) | 0.40 (0.81) | |
| 3α-diolG | 250 | 0.01 (0.01) | 0.84 (0.89) | |
| Meibomian gland orifice plugging | SHBG | 262 | −0.08 (0.05) | 0.22 (0.41) |
| DHEAS | 225 | 0.02 (0.05) | 0.82 (0.49) | |
| Androstenedione | 250 | 0.10 (0.08) | 0.13 (0.23) | |
| DHT | 250 | −0.07 (−0.07) | 0.25 (0.26) | |
| Testosterone | 262 | 0.01 (0.01) | 0.83 (0.94) | |
| 3α-diolG | 250 | 0.07 (0.10) | 0.30 (0.11) | |
| Aqueous parameters | ||||
| Schirmer test | SHBG | 262 | −0.10 (−0.09) | 0.11 (.16) |
| DHEAS | 225 | 0.13 (0.08) | 0.047 (0.24) | |
| Androstenedione | 250 | 0.21 (0.15) | 0.001 (0.02) | |
| DHT | 250 | 0.08 (0.08) | 0.19 (0.22) | |
| Testosterone | 262 | 0.12 (0.09) | 0.06 (0.15) | |
| 3α-diolG | 250 | 0.00 (0.02) | 1.00 (0.76) |
Most severe eye measurements used for all tear film parameters.
Figure 2.
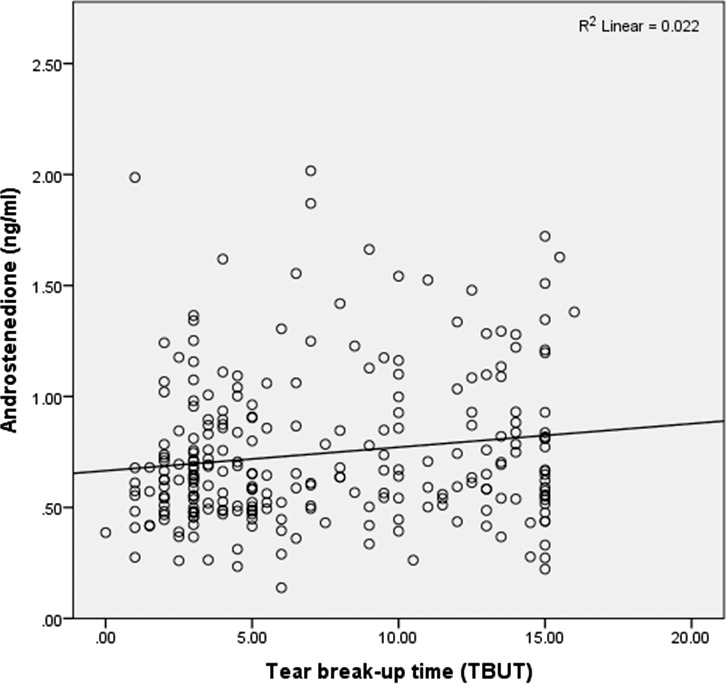
Androstenedione levels and tear breakup time show a positive, weak correlation.
Figure 3.
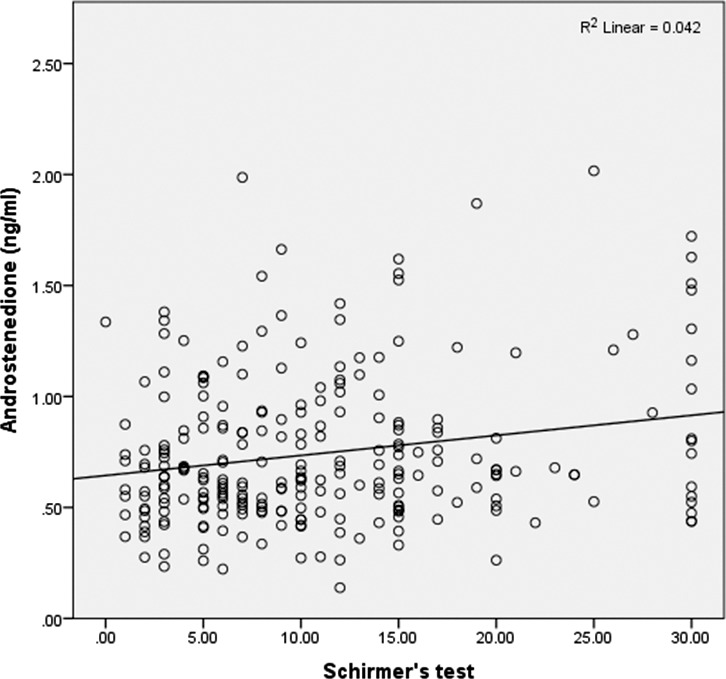
Androstenedione levels and Schirmer test show a positive, weak correlation.
To evaluate whether correlations between A and the above-noted ocular parameters were different in ATD versus LTD patients, the analysis was repeated within each group. We found that the relationship between A and TBUT was being driven by an association in the ATD group (r = 0.66, P = 0.002). In fact, when analyzed separately, the association disappeared in the LTD group (P > 0.15). None of the other variables studied displayed this differential relationship. In a separate analysis, we found that including a benign prostatic hypertrophy diagnosis and/or treatment in the model did not affect the relationship between A and TBUT.
Discussion
Our goal was to evaluate the association between androgen levels and symptoms and signs of DES in an older male population. We found that patients with severe DES had increased levels of SHBG and decreased levels of A compared to those with minimal or no DES. Furthermore, several weak associations were seen between worse global, lipid, and aqueous tear parameters and lower A levels. Interestingly, we found that this association was mostly driven by the presence of ATD, perhaps because of a synergistic relationship between low androgens and low tear production on TBUT. Of note, a similar relationship between A and DES was previously described in SS patients (n = 23), a group known to have low tear production. In that study, a moderate association was reported between low A and symptoms (rs = −0.55, P < 0.05).43 Similar to the lack of correlation between DES symptoms and signs reported in a previous study,34 we did not find a relationship between symptoms and androgen levels in our study.
With regard to androgens, it remains controversial which measured androgens most closely reflect the total androgen pool and therefore should be studied in DES. Tamer et al.44 found significantly lower levels of BioT (P = 0.002), DHEA (P = 0.009), and DHEAS (P = 0.001) in a group of nonautoimmune DES males with MG dysfunction (n = 32) compared to nonautoimmune DES males without MG dysfunction (n = 32) and age-matched controls (n = 32). Our study, however, did not replicate these findings. Similar to our study, the investigation by Tamer et al.44 did not show significant differences in total T between the groups. This has biologic plausibility, as 50% of the total T and DHT in males is peripherally produced by adrenal precursors like DHEA and DHEAS.44–46 Intracellular levels of T and DHT therefore do not parallel serum levels of T and DHT.24 While T and DHT are more potent androgens, other androgens likely provide a stronger correlation with total androgen pool and therefore diseases such as MG dysfunction.
Conjugated DHT metabolites (such as 3α-diolG) represent the total peripheral production and metabolism of androgens and may therefore be more reliable measures of the total androgen pool.24,45,46 One study measuring 3α-diolG reported lower levels of this androgen in women with SS.47 Our data on 3α-diolG are more difficult to interpret as we found a U-shaped curve for this androgen, with highest 3α-diolG levels seen in those with intermediate disease.
It is biologically plausible that androgens may affect MG function and DES in humans, as several animal models have demonstrated an effect of androgen replacement on MG output. In MGs of orchiectomized rabbits, topical T administration affected the expression of 58 mRNAs48 while topical or systemic administration of 19-nortestosterone normalized lipids patterns.49 Also, systemic T administration affected the expression of over 1000 genes in MGs of both orchiectomized50 and ovariectomized51 mice.
Similarly, androgens may also affect lacrimal gland function. Androgen binding sites have been found in lacrimal tissues of male and female rats, and androgen receptor mRNA has been found in the lacrimal glands of various species, including humans.52 Furthermore, T-containing pellets enhanced lacrimal gland function, and systemic administration suppressed lacrimal gland inflammation in mouse models of SS.53,54 Dihydrotestosterone administration also affected lacrimal gland health with improved fluid secretion in ovariectomized rabbits.55
The unanswered question is whether there is a role for local and/or systemic androgen replacement in men with low androgens and unhealthy tear parameters. Unfortunately, sparse data are available to answer this question. One study treated male and female LTD patients with either 0.03% T (n = 46) or vehicle (n = 42) and found that after 6 months, those with T treatment had a higher percentage of normal-viscosity MG secretions (65% vs. 36%, P = 0.045) and decreased ocular discomfort (30% vs. 8%; P = 0.06) (Schiffman RM, et al. IOVS 2006;47:ARVO E-Abstract 5608). While not peer reviewed, a news report highlighted Allan Panzer's success using topical T to treat hundreds of male and female DES patients.56 Results of systemic treatment are less clear; one study in females with SS (n = 23) failed to show a benefit of oral DHEA treatment on ocular sicca symptoms,43 while a separate study in females with LTD and low T (n = 14) found that a transdermal androgen patch improved TBUT and Schirmer tests (P < 0.03) and Ocular Surface Disease Index symptoms scores (P < 0.01).57 Considering the various results of these studies with variable methods of androgen administration, it is clear that more clinical trials are needed to study the effects of topical and/or systemic androgens in the treatment of DES in the aging male population.
As with all studies, our findings must be interpreted in light of our study limitations. First, our study population consisted of older male veterans seeking treatment for ocular disorders, and as such, it is possible that our findings may not generalize to other US male populations. Second, there are potential confounders (such as dietary information and environmental exposures) that were not captured. Third, our findings are specific to the metrics tested, and it is possible that different scales (e.g., Ocular Surface Disease Index) or measurements (other conjugated metabolites such as androsterone glucuronide) may have displayed different relationships. Finally, it is important to remember that as with all statistical methodologies, LCA is based on assumptions that need to be considered when interpreting the results. For example, LCA makes the assumption of local independence (i.e., once a certain class is assigned, the measurements within that class are assumed statistically independent). However, the effect of this latter assumption was evaluated in our original paper.41 As the correlations among the measured parameters were low, assignment to latent classes had little effect on the observed correlations (data not shown).
To conclude, we found that individuals with lower levels of A had less healthy tear film parameters, and that this finding was more prominent in the ATD group. No meaningful correlations between androgen levels and DES symptoms were found. More research is needed on the role of androgens in tear function in males, as this avenue may be of potential preventive and therapeutic use in DES.
Acknowledgments
Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development's Career Development Award CDA-2-024-10S (AG); National Institutes of Health Center Core Grant P30EY014801; Research to Prevent Blindness unrestricted grant; Department of Defense (DOD Grants W81XWH-09-1-0675 and W81XWH-13-1-0048 ONOVA) (institutional).
Disclosure: P.M. Azcarate, None; V.D. Venincasa, None; W. Feuer, None; F. Stanczyk, None; A.V. Schally, None; A. Galor, None
References
- 1. Foulks GN, Nichols KK, Bron AJ, Holland EJ, McDonald MB, Nelson JD. Improving awareness, identification, and management of meibomian gland dysfunction. Ophthalmology. 2012; 119: S1–S12 [DOI] [PubMed] [Google Scholar]
- 2. Fiscella RG. Understanding dry eye disease: a managed care perspective. Am J Manag Care. 2011; 17 (suppl 16): S432–S439 [PubMed] [Google Scholar]
- 3. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003; 136: 318–326 [DOI] [PubMed] [Google Scholar]
- 4. Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000; 118: 1264–1268 [DOI] [PubMed] [Google Scholar]
- 5. Mertzanis P, Abetz L, Rajagopalan K, et al. The relative burden of dry eye in patients' lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005; 46: 46–50 [DOI] [PubMed] [Google Scholar]
- 6. Patel VD, Watanabe JH, Strauss JA, Dubey AT. Work productivity loss in patients with dry eye disease: an online survey. Curr Med Res Opin. 2011; 27: 1041–1048 [DOI] [PubMed] [Google Scholar]
- 7. Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003; 110: 1412–1419 [DOI] [PubMed] [Google Scholar]
- 8. Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011; 30: 379–387 [DOI] [PubMed] [Google Scholar]
- 9. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 93–107 [DOI] [PubMed] [Google Scholar]
- 10. Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011; 152: 377–384, e372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mathers WD, Stovall D, Lane JA, Zimmerman MB, Johnson S. Menopause and tear function: the influence of prolactin and sex hormones on human tear production. Cornea. 1998; 17: 353–358 [DOI] [PubMed] [Google Scholar]
- 12. Stanczyk FZ, Azen CG, Pike MC. Effect of finasteride on serum levels of androstenedione, testosterone and their 5alpha-reduced metabolites in men at risk for prostate cancer. J Steroid Biochem Mol Biol. 2013; 138C: 10–16 [DOI] [PubMed] [Google Scholar]
- 13. Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985; 43: 74–78 [PubMed] [Google Scholar]
- 14. Rocha EM, Wickham LA, da Silveira LA, et al. Identification of androgen receptor protein and 5alpha-reductase mRNA in human ocular tissues. Br J Ophthalmol. 2000; 84: 76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bremner W, Matsumoto A. Testicular disorders. In: Williams Textbook of Endocrinology. Philadelphia: Elsevier/Saunders; 2011: 706–710 [Google Scholar]
- 16. Sullivan DA, Sullivan BD, Evans JE, et al. Androgen deficiency, meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci. 2002; 966: 211–222 [DOI] [PubMed] [Google Scholar]
- 17. Foster CS. Dry eye syndrome. Medscape. http://emedicine.medscape.com/article/1210417-overview#showall. Updated February 20, 2014. Accessed October 10, 2013 [Google Scholar]
- 18. Krenzer KL, Dana MR, Ullman MD, et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. J Clin Endocrinol Metab. 2000; 85: 4874–4882 [DOI] [PubMed] [Google Scholar]
- 19. Cermak JM, Krenzer KL, Sullivan RM, Dana MR, Sullivan DA. Is complete androgen insensitivity syndrome associated with alterations in the meibomian gland and ocular surface? Cornea. 2003; 22: 516–521 [DOI] [PubMed] [Google Scholar]
- 20. Sullivan DA, Belanger A, Cermak JM, et al. Are women with Sjogren's syndrome androgen-deficient? J Rheumatol. 2003; 30: 2413–2419 [PubMed] [Google Scholar]
- 21. Shimazaki J, Goto E, Ono M, Shimmura S, Tsubota K. Meibomian gland dysfunction in patients with Sjogren syndrome. Ophthalmology. 1998; 105: 1485–1488 [DOI] [PubMed] [Google Scholar]
- 22. Lamberts S. Endocrinology and aging. In: Williams Textbook of Endocrinology. Philadelphia: Elsevier/Saunders; 2011: 1225–1229 18606617 [Google Scholar]
- 23. Chen CY, Lee CP, Chen Y, Jiang JR, Chu CL, Chen CL. The correlation between emotional distress and aging males' symptoms at a psychiatric outpatient clinic: sexual dysfunction as a distinguishing characteristic between andropause and anxiety/depression in aging men. Clin Interv Aging. 2013; 8: 635–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997; 82: 2396–2402 [DOI] [PubMed] [Google Scholar]
- 25. Molina PE. Male reproductive system. In: Molina PE. ed Endocrine Physiology. New York: McGraw-Hill; 2013. [Google Scholar]
- 26. Morales A. The andropause: bare facts for urologists. BJU Int. 2003; 91: 311–313 [DOI] [PubMed] [Google Scholar]
- 27. Morales A. Andropause (or symptomatic late-onset hypogonadism): facts, fiction and controversies. Aging Male. 2004; 7: 297–303 [DOI] [PubMed] [Google Scholar]
- 28. Ding J, Sullivan DA. Aging and dry eye disease. Exp Gerontol. 2012; 47: 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006; 124: 1286–1292 [DOI] [PubMed] [Google Scholar]
- 30. Galor A, Feuer W, Lee DJ, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative database. Am J Ophthalmol. 2012; 154: 340–346, e342 [DOI] [PubMed] [Google Scholar]
- 31. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003; 22: 640–650 [DOI] [PubMed] [Google Scholar]
- 32. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003; 1: 107–126 [DOI] [PubMed] [Google Scholar]
- 33. Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011; 52: 2006–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galor A, Feuer W, Lee DJ, Florez H, Venincasa VD, Perez VL. Ocular surface parameters in older male veterans. Invest Ophthalmol Vis Sci. 2013; 54: 1426–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goebelsmann U, Horton R, Mestman JH, et al. Male pseudohermaphroditism due to testicular 17-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 1973; 36: 867–879 [DOI] [PubMed] [Google Scholar]
- 36. Goebelsmann U, Bernstein GS, Gale JA, et al. Serum gonadotropin, testosterone, estradiol and estrone levels prior to and following bilateral vasectomy. In: Lepow IH, Crozier R. eds Vasectomy: Immunologic and Pathophysiologic Effects in Animals and Man. New York: Academic Press; 1979: 165–181 [Google Scholar]
- 37. Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982; 16: 801–810 [DOI] [PubMed] [Google Scholar]
- 38. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999; 84: 3666–3672 [DOI] [PubMed] [Google Scholar]
- 39. Rinaldi S, Geay A, Dechaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002; 11: 1065–1071 [PubMed] [Google Scholar]
- 40. Hsing AW, Stanczyk FZ, Belanger A, et al. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2007; 16: 1004–1008 [DOI] [PubMed] [Google Scholar]
- 41. See C, Bilonick RA, Feuer W, Galor A. Comparison of two methods for composite score generation in dry eye syndrome. Invest Ophthalmol Vis Sci. 2013; 54: 6280–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010; 33: 55–60 [DOI] [PubMed] [Google Scholar]
- 43. Forsblad-d'Elia H, Carlsten H, Labrie F, Konttinen YT, Ohlsson C. Low serum levels of sex steroids are associated with disease characteristics in primary Sjogren's syndrome; supplementation with dehydroepiandrosterone restores the concentrations. J Clin Endocrinol Metab. 2009; 94: 2044–2051 [DOI] [PubMed] [Google Scholar]
- 44. Tamer C, Oksuz H, Sogut S. Androgen status of the nonautoimmune dry eye subtypes. Ophthalmic Res. 2006; 38: 280–286 [DOI] [PubMed] [Google Scholar]
- 45. Labrie F, Luu-The V, Labrie C, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003; 24: 152–182 [DOI] [PubMed] [Google Scholar]
- 46. Labrie F, Belanger A, Simard J, Van L-T, Labrie C. DHEA. and peripheral androgen and estrogen formation: intracinology. Ann N Y Acad Sci. 1995; 774: 16–28 [DOI] [PubMed] [Google Scholar]
- 47. Sullivan DA, Belanger A, Cermak JM, et al. Are women with Sjogren's syndrome androgen-deficient? J Rheumatol. 2003; 30: 2413–2419 [PubMed] [Google Scholar]
- 48. Steagall RJ, Yamagami H, Wickham LA, Sullivan DA. Androgen control of gene expression in the rabbit meibomian gland. Adv Exp Med Biol. 2002; 506: 465–476 [DOI] [PubMed] [Google Scholar]
- 49. Sullivan DA, Sullivan BD, Ullman MD, et al. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci. 2000; 41: 3732–3742 [PubMed] [Google Scholar]
- 50. Schirra F, Suzuki T, Richards SM, et al. Androgen control of gene expression in the mouse meibomian gland. Invest Ophthalmol Vis Sci. 2005; 46: 3666–3675 [DOI] [PubMed] [Google Scholar]
- 51. Sullivan DA, Jensen RV, Suzuki T, Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis. 2009; 15: 1553–1572 [PMC free article] [PubMed] [Google Scholar]
- 52. Sullivan DA, Edwards JA, Wickham LA, et al. Identification and endocrine control of sex steroid binding sites in the lacrimal gland. Curr Eye Res. 1996; 15: 279–291 [DOI] [PubMed] [Google Scholar]
- 53. Sullivan DA, Edwards JA. Androgen stimulation of lacrimal gland function in mouse models of Sjogren's syndrome. J Steroid Biochem Mol Biol. 1997; 60: 237–245 [DOI] [PubMed] [Google Scholar]
- 54. Rocha FJ, Sato EH, Sullivan BD, Sullivan DA. Comparative efficacy of androgen analogues in suppressing lacrimal gland inflammation in a mouse model (MRL/lpr) of Sjogren's syndrome. Adv Exp Med Biol. 1994; 350: 697–700 [DOI] [PubMed] [Google Scholar]
- 55. Azzarolo AM, Mircheff AK, Kaswan RL, et al. Androgen support of lacrimal gland function. Endocrine. 1997; 6: 39–45 [DOI] [PubMed] [Google Scholar]
- 56. Myers C. Test for dry eyes can lead to relief. http://abclocal.go.com/ktrk/story?section=news/health&id=8682565. Published May 30, 2012. Accessed March 15, 2014 [Google Scholar]
- 57. Nanavaty MA, Long M, Malhotra R. Transdermal androgen patches in evaporative dry eye syndrome with androgen deficiency: a pilot study. Br J Ophthalmol. 2014; 98: 567–569 [DOI] [PubMed] [Google Scholar]


