Abstract
Purpose.
Crowding describes the increased difficulty in identifying a target object when it is surrounded by nearby objects (flankers). A recent study investigated the effect of age on visual crowding and found equivocal results: Although crowded visual acuity was worse in older participants, crowding expressed as a ratio did not change with age. However, the spatial extent of crowding is a better index of crowding effects and remains unknown. In the present study, we used established psychophysical methods to characterize the effect of age on visual crowding (magnitude and extent) in a letter recognition task.
Methods.
Letter recognition thresholds were determined for three different flanker separations in 54 adults (aged 18–76 years) with normal vision. Additionally, the spatial extent of crowding was established by measuring spacing thresholds: the flanker-to-target separation required to produce a given reduction in performance. Uncrowded visual acuity, crowded visual acuity, and spacing thresholds were expressed as a function of age, avoiding arbitrary categorization of young and old participants.
Results.
Our results showed that uncrowded and crowded visual acuities do not change significantly as a function of age. Furthermore, spacing thresholds did not change with age and approximated Bouma's law (half eccentricity).
Conclusions.
These data show that crowding in adults is unaffected by senescence and provide additional evidence for distinct neural mechanisms mediating surround suppression and visual crowding, since the former shows a significant age effect. Finally, our data suggest that the well-documented age-related decline in peripheral reading ability is not due to age-related changes in visual crowding.
Keywords: crowding, aging, visual acuity, critical spacing, reading
Crowding for a peripheral letter recognition task was measured as a function of age. Both the magnitude and spatial extent of crowding did not change with age. The results have important implications for the visual rehabilitation of patients with central vision loss.
Introduction
The perceptual consequences of aging are becoming increasingly more relevant, given the growing number of people living longer lives. A range of visual functions deteriorate as a part of the normal aging process. For example, visual acuity,1 contrast sensitivity,2 motion perception,3 contour integration,4 shape perception,5 and visual processing speed6 all become worse with age. These changes arise due to a combination of optical and neural changes that occur throughout life as we age (for reviews see Refs. 7–9). However, some functions do not change with age, including blur adaptation,10 spatial interval discrimination,11 spatial summation,12 and orientation discrimination at high contrasts.13 Here we investigate the effect of age on visual crowding in adults.
Crowding describes the deleterious influence of neighboring objects (flankers) on the recognition of a target.14,15 The effects of visual crowding are relatively small at the fovea and increase with eccentricity.16 Therefore, the issue of whether age affects visual crowding is an important one with respect to performing peripheral visual tasks. This is particularly relevant for people with ocular pathology that results in central vision loss. Age-related macular degeneration is a good example and remains the most common cause of irreversible blindness in the western world.17 Individuals with central vision loss rely exclusively on their peripheral vision and the majority develop a surrogate fixation locus, referred to as a preferred retinal locus (PRL), to carry out everyday tasks such as reading. Peripheral reading speed becomes slower with age18 and is inherently linked to crowding.19 Is a change in crowding with age responsible for slowing peripheral reading speed?
The amount of crowding exerted by flankers is dependent on their proximity to the target. The closer flankers are to the target, the greater the degree of crowding exerted.16 Away from the fovea, the spatial extent of crowding is a fixed proportion of the eccentricity of the target object, a relationship commonly referred to as Bouma's law.16 This proportion is usually 0.5 when measured as the center-to-center distance between flankers and the target along the radial meridian connecting the target and fovea.16,20 It has been suggested that the observed spatial extent of crowding represents the neuroanatomical constraint for object recognition in visual cortex; to be recognized, objects must be separated by 1 mm in the tangential direction and 6 mm apart in the radial direction in primary visual cortex.21 Crowding is spatially heterogeneous throughout the visual field. Objects that are positioned more peripherally have a stronger crowding effect than equally spaced, less peripheral, objects.22 This is referred to as an inward-outward anisotropy. Additionally, objects positioned radially from the target have a stronger crowding effect compared with objects located tangentially to the target, referred to as radial-tangential anisotropy.23 The nature of the surrounding objects also influences crowding: Objects that are more similar to the target generally exert a larger crowding effect.24–26
Crowding can be quantified as a ratio between crowded acuity and isolated acuity. A recent study27 compared peripheral crowding using this metric between young and old groups of participants. The study measured participants' ability to detect the gap in a Landolt C, which was either at the top or bottom of the target, and was presented at 3 and 6° left and right of fixation along the horizontal midline. The degree of crowding was measured by assessing performance on the task with and without vertical bar flankers adjacent to the Landolt C target. The width of the flankers and the flanker-to-target separation was equal to the stroke width of the target (which was one fifth of the width of the letter). Crowding expressed as a ratio between crowded and uncrowded visual acuity did not change with age. However, the study found that absolute isolated and crowded acuity was worse in the older group.
The current study investigates the influence of age on visual crowding for a letter recognition task, where target and flankers are letters—a task more closely related to peripheral reading. Crowded visual acuity was measured using three fixed flanker separations. Additionally, the effect of age on critical spacing was measured directly by altering the flanker separation while keeping letter size constant. Crowding was assessed along a large age continuum, avoiding arbitrary classification of young and old participants. The data provide strong evidence that the magnitude and extent of crowding in adults are not affected significantly by senescence. This has important implications for the rehabilitation of peripheral reading ability in patients with central vision loss and provides additional evidence for distinct neural mechanisms mediating surround suppression and visual crowding.
Methods
Participants
Fifty-four participants with normal or corrected-to-normal vision took part in the study (18 to 76 years; mean 37 ± 20 years, 43 females, 11 males). All participants scored within the normal range on the Mini-Mental State Examination28 (mean score = 29.5), indicating they did not have any cognitive impairment. All participants were optically corrected for the appropriate viewing distance. Informed consent was obtained from the participants after explanation of the nature of the study. The experimental procedures adhered to the tenets of the Declaration of Helsinki and were approved by a local ethics committee at the school of psychology, The University of Nottingham.
Apparatus
Stimuli were generated on a personal computer using custom software29 written in Python (Python Software Foundation, Wilmington, DE, USA) and presented on a gamma-corrected CRT monitor (Belinea 108035; [Maxdata, Marl, Germany]) with a refresh rate of 85 Hz, resolution of 1024 × 768 pixels, pixel size of 0.40 mm, and mean luminance of 45 cd/m−2. A forehead and chin rest were used to hold participants' heads in position and maintain a constant viewing distance of 57 cm. Testing was carried out in a darkened room. All participants underwent a practice run (30 trials). Any participants who were unable to maintain fixation during the practice run were excluded from the study.
Stimuli
A target letter was presented 10° above a fixation point. For measurement of uncrowded acuity, a single target letter was presented in isolation. For measurements of crowded acuity and spacing thresholds, an array of five letters were presented, configured in a cross shape, with the target letter in the center and four outer letters along the cardinal axes (see Fig. 1). Letters were presented on a mean-luminance gray background (90 cd/m−2). Target and flankers were selected randomly from the following list of 10 letters: C, D, H, K, N, O, R, S, V, Z. All letters were rendered in upper case Arial font.
Figure 1.

Example of stimuli used to measure crowding. Participants were required to identify the central (target) letter (N in this example). In the crowded acuity tasks, flanking letters were separated from the target letter by fixed proportions of 1.7, 2.0, and 2.6 letter heights. The size of the letters varied on each trial. Uncrowded acuity was measured with a single target letter presented in isolation. In the spacing threshold task, letter size remained fixed while the separation of the flanking letters and the target letter varied.
Procedure
One eye was randomly selected for testing and the other eye occluded using an eye patch. Each participant was tested on three tasks: uncrowded visual acuity, crowded visual acuity and a task in which the interletter separation was manipulated.
Crowded visual acuity was measured by changing the size of the target letter using a one-up, three-down adaptive staircase while keeping the flanker-to-target separation a constant proportion of the letter size. Initial letter size was 4° (above the peripheral isolated acuity threshold for all participants). Crowded visual acuity was measured with three different fixed separation conditions: 1.7, 2.0, and 2.6 multiples of letter size (center-to-center distance divided by letter height). Uncrowded visual acuity was measured in the same way but without flanking letters.
Critical spacing thresholds were measured by varying the flanker-to-target letter separation using a one-up, three-down adaptive staircase while letter size remained fixed. Initial flanker-to-target letter separation was set to three letter heights. The letter size used was equivalent to the acuity threshold from the crowded visual acuity task with the largest flanker-to-target separation (2.6 letter heights), which did not change with age. Crowded visual acuity with a 2.6-letter height flanker separation was always measured prior to spacing thresholds. The order of the other tasks was otherwise randomized for each participant.
For all tasks and conditions, stimulus duration was 153 ms (13 frames) and participants were required to identify the target letter and input their responses directly using a keyboard. Correct responses were indicated by a high-pitched tone and incorrect responses by a low-pitched tone. All staircases terminated after 100 trials.
Data Analysis
Thresholds were calculated as the mean of the last six reversals of each staircase. For post hoc comparisons, ANOVAs and Tukey's honestly significant difference tests were used to assess the statistical significance of threshold differences between tasks and conditions. Acuity thresholds were quantified in terms of letter height in degrees of visual angle. Critical spacing thresholds were quantified in terms of the center-to-center letter spacing in degrees of visual angle.
For each participant, crowding ratios for each of the three flanker separations were calculated as the ratio of crowded acuity threshold to uncrowded acuity threshold.
In addition to critical spacing that was measured directly from the spacing task, critical spacing was also determined indirectly from the results of the crowded acuity task. Since flanker-to-target separation was quantified in terms of proportion of letter size, critical spacing could be calculated using Equation 1:
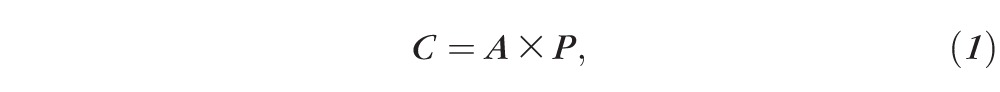 |
where C is the critical spacing in degrees, A is the acuity in degrees, and P is the proportional flanker-to-target separation in multiples of letter size.
Two participants did not complete the crowded acuity task with the 2.6-letter height separation (and thus also the spacing task) and one participant did not complete the crowded acuity task with the 2.0-letter height separation due to illness or withdrawal from the study.
Results
Uncrowded visual acuity was measured with isolated target letters and provided a measure of a participant's peripheral visual acuity without the deleterious effects of crowding. Figure 2 shows uncrowded letter acuity thresholds as a function of age. The slope of the linear regression line fitted through the data did not differ significantly from zero (P = 0.26). Therefore, isolated visual acuity did not change significantly with age.
Figure 2.
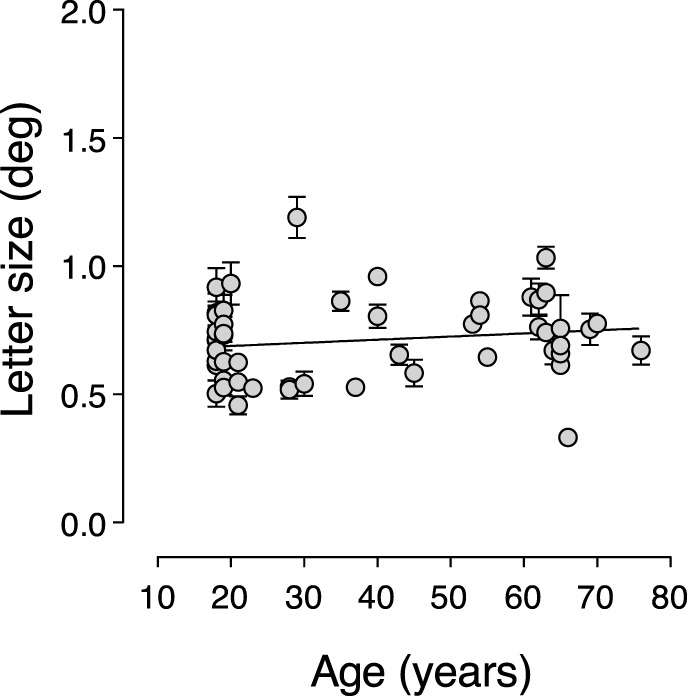
Isolated letter acuity (letter size) as a function of age. The slope of the linear regression curve was 0.001 and did not differ significantly from zero. Isolated letter acuity did not vary significantly with age. Error bars represent SEM.
Crowded letter acuity thresholds were determined for the same participants. Flanking letters were spaced 1.7-, 2.0-, and 2.6-letter heights away from the target letter. Figure 3 shows individual crowded visual acuity for each of the three letter separations, as a function of age. The slopes of the linear regression lines are 7 × 10−3, 3 × 10−3, and 2 × 10−3 for letter separations of 1.7, 2.0, and 2.6 letter widths, respectively. The slope of the linear regression line fitted through the data for each fixed separation did not significantly differ from zero (1.7 [P = 0.15], 2.0 [P = 0.41], and 2.6 [P = 0.51] letter height separations). Therefore, like isolated acuity, there was no statistically significant effect of age on crowded acuity.
Figure 3.
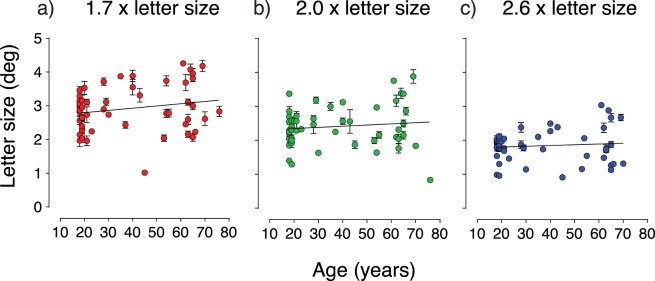
Crowded letter acuity as a function of age when the target-to-flanker separation was a multiple of (a) 1.7, (b) 2.0, and (c) 2.6 times isolated letter acuity. Thresholds were higher when flankers were closer to the target but did not change significantly with age, for each of the target-to-flanker separations. Error bars represent SEM.
It is well established that acuity thresholds deteriorate with decreasing flanker separation. Figure 3 illustrates this, showing closer flanking letters elicit higher letter recognition thresholds. Mean (±SD) letter recognition thresholds were 2.9 (±0.68), 2.4 (±0.60), and 1.8° (±0.47) for flanker-to-target separations of 1.7, 2.0, and 2.6 letter heights, respectively. A one-way repeated measures ANOVA was conducted to compare the effect of acuity condition (uncrowded acuity and crowded acuity with three different separations) on acuity threshold. Mauchly's test indicated that the assumption of sphericity had been violated (χ2[22.9], P < 0.0001); therefore, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = 0.77). There was a significant effect of test condition, (F[2.314, 115.7] = 396.7, P < 0.0001) with post hoc comparisons indicating significant differences between all conditions (all P values < 0.0001).
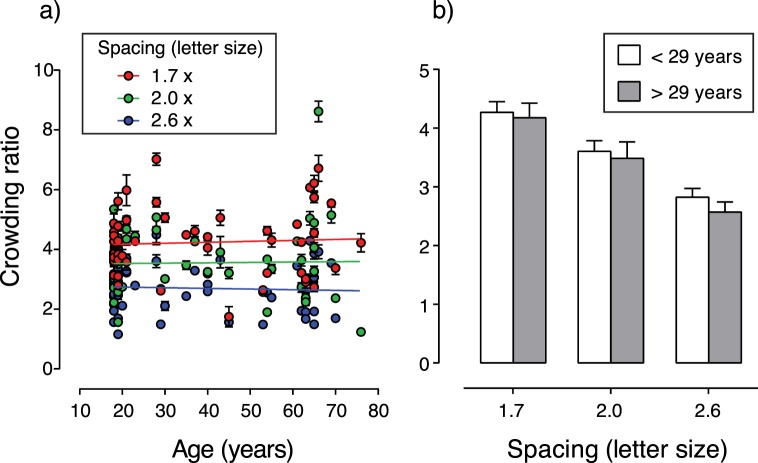
Crowding is often expressed as a ratio. A crowding ratio of 1 indicates no effect of crowding (i.e., crowded and uncrowded acuity are equal). The larger the crowding ratio, the greater the influence of crowding. Figure 4a shows crowding ratios calculated for each individual at each separation level, plotted as a function of age. Again, the slope of the linear regression lines fitted through the data do not differ significantly from zero (P = 0.67, 0.88, and 0.71 for the 1.7, 2.0, and 2.6 letter height separations, respectively). This would of course be expected, given that the slopes of the linear regression lines fitted to the crowded acuity data do not differ significantly from 0.
Figure 4.
Crowding ratios calculated for each separation level of the crowded acuity test. (a) Individual crowding ratios for each separation level, as a function of age. The slope of the linear regression lines plotted through the data did not differ significantly from zero. (b) Mean crowding ratio for each separation level for younger (<29 years) and older (>29 years) participants. The crowding ratio decreased with increasing flanker-to-target separation. There was no statistical difference in the crowding ratio between the younger and older groups at each separation. Error bars represent SEM.
It is common for studies investigating the effects of age on visual function to analyze differences between groups of old and young participants.4,12,13,27 Figure 4b shows the mean crowding ratio for each separation when data are divided for participants who are younger or older than the median age (29 years). A two-way ANOVA (repeated measures for separation) found a main effect of separation (F[2, 98] = 123.8, P < 0.0001). However, no effect of age group was found (F[1, 49] = 0.03, P = 0.85). There was no significant interaction effect between age group and separation (F[2, 98] = 0.98 , P = 0.38). Post hoc analysis revealed a significant difference between the mean crowding ratio for each separation and other separations (all P < 0.0001). The same results are also found when participants are divided according to the mid-point of the age range (47 years): there is a main effect of separation (F[2, 98] = 114.9, P < 0.0001), no effect of age group (F[1, 49] = 0.19, P = 0.67), and no significant interaction effect between age group and separation (F[2, 98] = 1.32, P = 0.27). Therefore, crowding expressed as a crowding ratio increased with decreasing flanker separation but did not change significantly with age, regardless of whether it was analyzed as a function of age or as a difference between those participants who were younger or older than some arbitrary age whereby participants might be considered to change from being “young” to “old” (e.g., the median age or the midpoint of the age range).
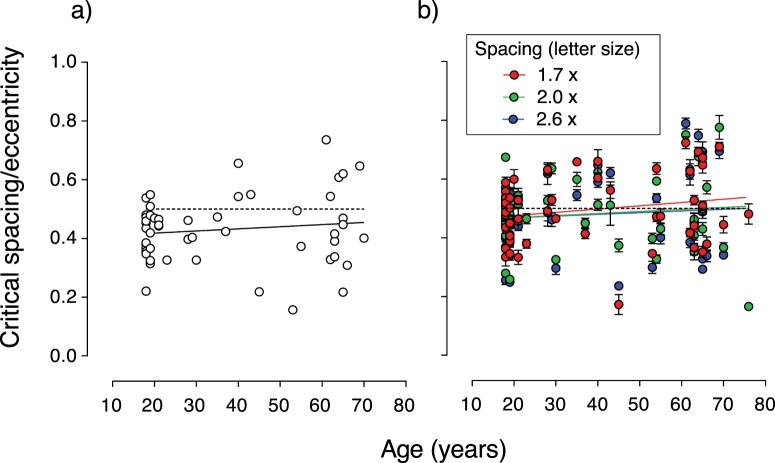
Critical spacing thresholds were measured in the same participants by varying the separation of the flankers from the central target letter while keeping letter size constant. Figure 5a shows the ratio of critical spacing, measured with the spacing task, to eccentricity for each individual plotted as a function of age. The slope of the linear regression line fitted through the data is 7 × 10−4 and did not differ significantly from zero (P = 0.38). The mean critical spacing to eccentricity ratio was 0.43 (±0.11).
Figure 5.
The effect of age on critical spacing/eccentricity. Critical spacing to eccentricity ratio for individuals plotted as a function of age. (a) Critical spacing measured directly with the spacing task, where flanker-to-target separation was varied while letter size remained constant. (b) Critical spacing determined from results of the crowded acuity task using Equation 1. There was no significant effect of age on the critical spacing-to-eccentricity ratio, which was in the region of 0.5 and thus agrees with Bouma's law (dashed lines). Error bars represent SEM.
Although the crowded acuity conditions did not measure critical spacing directly, it is possible to determine the critical spacing for each individual from their acuity threshold on the crowded acuity task. Since flanker-to-target separation was quantified as a constant proportion of letter size, the absolute critical spacing is the product of the separation and the acuity threshold for that separation (Equation 1). Critical spacing thresholds for each of the three spacing levels on the crowded acuity test were calculated for each individual and are shown in Figure 5b. The mean critical spacing to eccentricity ratio, with critical spacing determined from the crowded acuity task, was 0.49 (±0.11) for the 1.7-letter separation, 0.48 (±0.12) for the 2.0-letter separation and 0.48 (±0.12) for the 2.6-letter separation. The effect of separation on critical spacing calculated from crowded visual acuity thresholds was compared with a one-way repeated measures ANOVA. There was no significant difference in the critical spacing calculated from each of the separation conditions of the crowded acuity task (F[2, 100] = 1.20, P = 0.31). The mean critical spacing, calculated from all three separations of the crowded visual acuity test, was calculated for each individual and correlates strongly with the critical spacing measured with the spacing task: r(50) = 0.76, P = < 0.0001.
The slopes of the linear regression lines fitted through the spacing data derived from the crowded acuity test did not differ significantly from zero (0.06, 0.05, and 0.32 for the 1.7-, 2.0-, and 2.6-letter separations, respectively). Therefore, the critical spacing to threshold ratio measured directly as a spacing threshold, or calculated indirectly from visual acuity, did not change significantly with age. Furthermore, Bouma's law did not change with age.
Discussion
The amount of crowding differs between healthy adults with normal vision and individuals with amblyopia,30 schizophrenia,31 and dyslexia.32 Children experience greater crowding until adolescence.33 Recent studies have shown that crowding changes following practice34–37 and following loss of central vision.38 Our results indicate that crowding for a peripheral letter recognition task is not affected by senescence in adults. The data are compelling and show no statistically significant relationship between crowding and age, when quantified as a crowding ratio or as the spatial extent of crowding (critical spacing). Critical spacing was measured directly and the results were consolidated by data derived from flanked letter acuity thresholds. Additionally, crowded and isolated visual acuity for peripheral letter recognition did not change significantly as a function of age.
A previous study27 found no effect of age on visual crowding when it was quantified in terms of a ratio between crowded and isolated acuity. However, it found that both isolated and crowded acuity were worse in the older group. In contrast, we found no effect of age on crowding, regardless of the way it was quantified: either in terms of crowded letter acuity, a crowding ratio, or as the spatial extent of crowding. There are a number of differences between the two studies that could explain these contrasting findings. First, the stimuli, task, and task requirements used in each study were different. The previous study used a Landolt C target and required participants to detect the position of the gap, which was oriented either at the top or bottom (two-alternate forced choice task). The present study used a letter recognition task, where the target letter had to be identified from a set of 10 possible letters, chosen because it is more closely related to peripheral reading ability. It has been argued that crowding only occurs for identification and not for detection tasks,39–42 and that the reduced ability in detecting the gap in a Landolt C target flanked by bars may not measure crowding at all.39,43
Second, some older participants in the Scialfa et al.,27 study had visual health problems. For example, one participant had glaucoma, one had loss of peripheral vision, and five had cataracts. Although post hoc analysis revealed no difference in the crowding ratio between older participants with and without self-reported vision problems, it does not remove the possibility that those with visual problems had higher isolated and crowded peripheral visual acuity thresholds, which might have influenced the differences found between the young and older groups.27
Third, the targets were presented at different eccentricities in the two studies. Scialfa et al.27 presented stimuli at 6 or 9° from fixation along the horizontal midline, while we presented targets 10° above fixation. This location was chosen because the majority of central scotomas in patients with AMD are 20° or less in diameter44; therefore, individuals with AMD are likely to use PRLs located 10° or more away from the fovea. Because the target was randomly presented at either 6 or 9° left or right of fixation in the Scialfa et al.27 study, participants did not know where the target was going to appear. Target recognition in the periphery is highly dependent on the deployment of attention.45 Randomly presenting the target at different locations introduces spatial uncertainty and is likely to change the attentional demands of the task. Visual attention gets worse with age46 and older individuals, who perform more poorly at tasks requiring visual attention,47–49 are likely to perform worse when the location of a target is uncertain. This is connected to the useful field of view, the area of visual field that an individual can rapidly and accurately process visual information, which has been shown to reduce with age50 and may explain the higher acuity thresholds found in the older group by Scialfa et al.27 The present study eliminates this influence by ensuring the eccentricity that the target is presented at remains fixed (at 10° above fixation).
The differences found between the present study and that by Scialfa et al.27 are analogous to those found between studies investigating visual crowding in autism spectrum disorder.51–53 For example, one study53 that used stimuli similar to those used by Scialfa et al.27 found people with autism experienced less crowding than people without autism. In contrast, another study, which measured the spatial extent of crowding, found no difference in crowding between people with autism and normal controls.51
Crowding shares a number of characteristics with surround suppression, whereby a high contrast surround reduces the perceived contrast of a center stimulus, suggesting that the two phenomena may share the same neural mechanisms. For example, both crowding and surround suppression show radial-tangential anisotropy,23,54 tuning for orientation,41,55 and spatial frequency.20,55 Additionally, their effects scale with eccentricity16,23,54 and do not depend on stimulus size.54,56 However, unlike crowding, surround suppression does not show inward-outward anisotropy57 (but see also Ref. 58), and occurs only when the contrast of the surround is greater than the target contrast.59 There is evidence that surround suppression changes with age, though this has been reported as either an increase60 or decrease61 in the effects. Our finding that crowding does not change as a function of age adds further evidence to support the idea that crowding and surround suppression are mediated by distinct mechanisms.
The speed of peripheral reading reduces with age.18 This could be due to a number of factors including changes in temporal processing or crowding. Temporal processing has been shown to influence reading speed62 and evidence suggests temporal reading speed deteriorates with age.46,49 Our results indicate that, although crowding also determines reading speed,19 it is probably not the cause of the age-related decline in the speed of peripheral reading.
Conclusions
Visual acuity and both the magnitude and spatial extent of crowding for a letter recognition task in the periphery do not change with age in adults. This provides further evidence that crowding and surround suppression are mediated by different underlying mechanisms and has important implications for studies investigating peripheral reading ability in elderly individuals, including those with central vision loss.
Acknowledgments
The authors would like to thank two anonymous reviewers for their helpful comments.
Supported by an Age UK Scholarship (AJB); a National Institute of Health Research (NIHR) Postdoctoral Fellowship (ATA); and a Wellcome Trust Career Development Fellowship (BSW).
This article presents independent research funded by the NIHR. The authors alone are responsible for the content and writing of the paper.
Disclosure: A.T. Astle, None; A.J. Blighe, None; B.S. Webb, None; P.V. McGraw, None
References
- 1. Elliott DB, Yang KC, Whitaker D. Visual acuity changes throughout adulthood in normal, healthy eyes: seeing beyond 6/6. Optom Vis Sci. 1995; 72: 186–191 [DOI] [PubMed] [Google Scholar]
- 2. Sloane ME, Owsley C, Jackson CA. Aging and luminance-adaptation effects on spatial contrast sensitivity. J Opt Am A. 1988; 5: 2181–2190 [DOI] [PubMed] [Google Scholar]
- 3. Ball K, Sekuler R. Improving visual perception in older observers. J Gerontol. 1986; 41: 176–182 [DOI] [PubMed] [Google Scholar]
- 4. Roudaia E, Bennett PJ, Sekuler AB. The effect of aging on contour integration. Vision Res. 2008; 48: 2767–2774 [DOI] [PubMed] [Google Scholar]
- 5. McKendrick AM, Weymouth AE, Battista J. The effect of normal aging on closed contour shape discrimination. J Vis. 2010; 10: 1.1–1.9 [DOI] [PubMed] [Google Scholar]
- 6. Kline DW, Birren JE. Age differences in backward dichoptic masking. Exp Aging Res. 1975; 1: 17–25 [DOI] [PubMed] [Google Scholar]
- 7. Andersen GJ. Aging and vision: changes in function and performance from optics to perception. Wiley Interdiscip Rev Cogn Sci. 2012; 3: 403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Owsley C. Aging and vision. Vision Res. 2011; 51: 1610–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faubert J. Visual perception and aging. Can J Exp Psychol. 2002; 56: 164–176 [DOI] [PubMed] [Google Scholar]
- 10. Elliott SL, Hardy JL, Webster MA, Werner JS. Aging and blur adaptation. J Vis. 2007; 7: 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latham K, Barrett BT. No effect of age on spatial interval discrimination as a function of eccentricity or separation. Curr Eye Res. 1998; 17: 1010–1017 [DOI] [PubMed] [Google Scholar]
- 12. Brown B, Peterken C, Bowman KJ, Crassini B. Spatial summation in young and elderly observers. Ophthalmic Physiol Opt. 1989; 9: 310–313 [DOI] [PubMed] [Google Scholar]
- 13. Betts LR, Sekuler AB, Bennett PJ. The effects of aging on orientation discrimination. Vision Res. 2007; 47: 1769–1780 [DOI] [PubMed] [Google Scholar]
- 14. Flom MC, Weymouth FW, Kahneman D. Visual Resolution and Contour Interaction. J Opt Soc Am. 1963; 53: 1026–1032 [DOI] [PubMed] [Google Scholar]
- 15. Stuart JA, Burian HM. A study of separation difficulty. Its relationship to visual acuity in normal and amblyopic eyes. Am J Ophthalmol. 1962; 53: 471–477 [PubMed] [Google Scholar]
- 16. Bouma H. Interaction effects in parafoveal letter recognition. Nature. 1970; 226: 177–178 [DOI] [PubMed] [Google Scholar]
- 17. Bunce C, Wormald R. Leading causes of certification for blindness and partial sight in England and Wales. BMC Public Health. 2006; 6: 58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu D, Cheung S-H, Chung STL, Legge GE. Age effects on reading speed and visual span in peripheral vision. J Vis. 2006; 1001–1001 [Google Scholar]
- 19. Pelli DG, Tillman KA, Freeman J, Su M, Berger TD, Majaj NJ. Crowding and eccentricity determine reading rate. J Vis. 2007; 7: 20.1–20.36 [DOI] [PubMed] [Google Scholar]
- 20. Chung STL, Levi DM, Legge GE. Spatial-frequency and contrast properties of crowding. Vision Res. 2001; 41: 1833–1850 [DOI] [PubMed] [Google Scholar]
- 21. Pelli DG. Crowding: A cortical constraint on object recognition. Curr Opin Neurobiol. 2008; 18: 445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bouma H. Visual interference in the parafoveal recognition of initial and final letters of words. Vision Res. 1973; 13: 767–782 [DOI] [PubMed] [Google Scholar]
- 23. Toet A, Levi DM. The two-dimensional shape of spatial interaction zones in the parafovea. Vision Res. 1992; 32: 1349–1357 [DOI] [PubMed] [Google Scholar]
- 24. Kooi FL, Toet A, Tripathy SP, Levi DM. The effect of similarity and duration on spatial interaction in peripheral vision. Spat Vis. 1994; 8: 255–279 [DOI] [PubMed] [Google Scholar]
- 25. Sayim B, Westheimer G, Herzog MH. Contrast polarity, chromaticity, and stereoscopic depth modulate contextual interactions in vernier acuity. J Vis. 2008; 8 (8): 12.1–12.9 [DOI] [PubMed] [Google Scholar]
- 26. Bernard JB, Chung STL. The dependence of crowding on flanker complexity and target–flanker similarity. J Vis. 2011; 11 (8): 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scialfa CT, Cordazzo S, Bubric K, Aging Lyon J. and visual crowding. J Gerontol B Psychol Sci Soc Sci. 2013; 68: 522–528 [DOI] [PubMed] [Google Scholar]
- 28. Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12: 189–198 [DOI] [PubMed] [Google Scholar]
- 29. Peirce JW. PsychoPy—Psychophysics software in Python. J Neurosci Methods. 2007; 162: 8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Res. 1985; 25: 979–991 [DOI] [PubMed] [Google Scholar]
- 31. Kraehenmann R, Vollenweider FX, Seifritz E, Kometer M. Crowding deficits in the visual periphery of schizophrenia patients. PLoS One. 2012; 7: e45884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martelli M, Di Filippo G, Spinelli D, Zoccolotti P. Crowding, reading, and developmental dyslexia. J Vis. 2009; 9 (4): 14.1–14.18 [DOI] [PubMed] [Google Scholar]
- 33. Huurneman B, Boonstra FN, Cox RF, Cillessen AH, van Rens G. A systematic review on ‘foveal crowding' in visually impaired children and perceptual learning as a method to reduce crowding. BMC Ophthalmol. 2012; 12: 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chung STL. Learning to identify crowded letters: does it improve reading speed? Vision Res. 2007; 47: 3150–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hussain Z, Webb BS, Astle AT, McGraw PV. Perceptual learning reduces crowding in amblyopia and in the normal periphery. J Neurosci. 2012; 32: 474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung STL, Li RW, Levi DM. Learning to identify near-acuity letters, either with or without flankers, results in improved letter size and spacing limits in adults with amblyopia. PLoS One. 2012; 7: e35829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chung STL, Truong SR. Learning to identify crowded letters: does the learning depend on the frequency of training? Vision Res. 2012; 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung STL. Cortical reorganization after long-term adaptation to retinal lesions in humans. J Neurosci. 2013; 33: 18080–18086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: distinguishing feature integration from detection. J Vis. 2004; 4: 1136–1169 [DOI] [PubMed] [Google Scholar]
- 40. Levi DM. Crowding--an essential bottleneck for object recognition: a mini-review. Vision Res. 2008; 48: 635–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levi DM, Hariharan S, Klein SA. Suppressive and facilitatory spatial interactions in peripheral vision: peripheral crowding is neither size invariant nor simple contrast masking. J Vis. 2002; 2: 167–177 [DOI] [PubMed] [Google Scholar]
- 42. Livne T, Sagi D. Configuration influence on crowding. J Vis. 2007; 7: 4.1–4.12 [DOI] [PubMed] [Google Scholar]
- 43. Flom M. Contour interaction and the crowding effect. Probl Optom. 1991; 3: 237–257 [Google Scholar]
- 44. Cheung S, Legge GE. Functional and cortical adaptations to central vision loss. Vis Neurosci. 2005; 22: 187–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Talgar CP, Pelli DG, Carrasco M. Covert attention enhances letter identification without affecting channel tuning. J Vis. 2004; 4: 22–31 [DOI] [PubMed] [Google Scholar]
- 46. Madden DJ. Adult age differences in the time course of visual attention. J Gerontol. 1990; 45: P9–P16 [DOI] [PubMed] [Google Scholar]
- 47. Steinman SB, Steinman BA, Trick GL, Lehmkuhle S. A sensory explanation for visual attention deficits in the elderly. Optom Vis Sci. 1994; 71: 743–749 [DOI] [PubMed] [Google Scholar]
- 48. Plude DJ, Hoyer WJ. Age and the selectivity of visual information processing. Psychol Aging. 1986; 1: 4–10 [DOI] [PubMed] [Google Scholar]
- 49. Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993; 34: 3110–3123 [PubMed] [Google Scholar]
- 50. Ball KK, Beard BL, Roenker DL, Miller RL, Griggs DS. Age and visual search: expanding the useful field of view. J Opt Soc Am A. 1988; 5: 2210–2219 [DOI] [PubMed] [Google Scholar]
- 51. Constable PA, Solomon JA, Gaigg SB. Crowding and visual search in high functioning adults with autism spectrum disorder. Clin Optom. 2010; 2: 93–103 [Google Scholar]
- 52. Baldassi S, Pei F, Megna N, et al. Search superiority in autism within, but not outside the crowding regime. Vision Res. 2009; 49: 2151–2156 [DOI] [PubMed] [Google Scholar]
- 53. Kéïta L, Mottron L, Bertone A. Far visual acuity is unremarkable in autism: do we need to focus on crowding? Autism Res. 2010; 3: 333–341 [DOI] [PubMed] [Google Scholar]
- 54. Petrov Y, McKee SP. The effect of spatial configuration on surround suppression of contrast sensitivity. J Vis. 2006; 6: 224–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petrov Y, Carandini M, McKee S. Two distinct mechanisms of suppression in human vision. J Neurosci. 2005; 25: 8704–8707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Strasburger H, Harvey LO, Rentschler I. Contrast thresholds for identification of numeric characters in direct and eccentric view. Percept Psychophys. 1991; 49: 495–508 [DOI] [PubMed] [Google Scholar]
- 57. Petrov Y, Popple AV, McKee SP. Crowding and surround suppression: not to be confused. J Vis. 2007; 7: 12.1–12.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van den Berg R, Roerdink JB, Cornelissen FW. On the generality of crowding: Visual crowding in size, saturation, and hue compared to orientation. J Vis. 2007; 7: 14.1–14.11 [DOI] [PubMed] [Google Scholar]
- 59. Chubb C, Sperling G, Solomon JA. Texture interactions determine perceived contrast. Pro Natl Acad Sci U S A. 1989; 86: 9631–9635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Karas R, McKendrick AM. Aging alters surround modulation of perceived contrast. J Vis. 2009; 9: 11.1–11.9 [DOI] [PubMed] [Google Scholar]
- 61. Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging reduces center-surround antagonism in visual motion processing. Neuron. 2005; 45: 361–366 [DOI] [PubMed] [Google Scholar]
- 62. Chung STL, Mansfield JS, Legge GE. Psychophysics of reading. XVIII. The effect of print size on reading speed in normal peripheral vision. Vision Res. 1998; 38: 2949–2962 [DOI] [PubMed] [Google Scholar]