Abstract
Purpose.
Although visual impairment is a well-recognized consequence of cataract development, little is known about the ability of the melanopsin-based photosensitive retinal ganglion cells (pRGCs) to regulate sleep–wake timing in the presence of cataract. In this study, we replaced a cataractous natural crystalline lens with two different types of artificial intraocular lenses, a UV-blocking lens or a blue-filtering lens. We investigated the level of sleep disturbance before cataract surgery and any change in sleep due to improved light transmission following surgery and compared this in both types of intraocular lens.
Methods.
Quality of sleep in 961 patients undergoing cataract surgery was assessed by administering the validated self-reported Pittsburgh Sleep Quality Index (PSQI) questionnaire. The PSQI distinguishes good sleepers from poor sleepers by scoring seven different sleep components over the last month, which are combined to produce an overall score for sleep quality. Patients received either an ultraviolet-blocking (UVB) clear intraocular lens (IOL) or a blue-filtering (BF) IOL. Questionnaires were completed four times: 1 month preoperatively and again 1, 6 (UVB-IOL only), and 12 months postoperatively.
Results.
Half of the patients reported poor sleep in the presence of cataract in both the UVB-IOL (mean PSQI = 6.35; SD = 3.82) and BF-IOL (mean PSQI = 6.39; SD = 4.04) groups. Cataract removal improved overall sleep quality significantly 1 month postoperatively in the UVB-IOL (mean PSQI = 5.89; SD = 3.71) and BF-IOL (mean PSQI = 6.08; SD = 3.88) groups. Sleep latency also improved for the UVB-IOL (preoperative mean = 1.16; SD = 1.003) and BF-IOL (preoperative mean = 1.17; SD = 1.03) groups at 1 month (UVB-IOL group mean = 1.01; SD = 0.923 and BF-IOL group mean = 1.00; SD = 0.95), which was sustained at 6 months for the UVB-IOL group (mean = 1.02; SD = 0.93) and 12 months for both the UVB-IOL and BF-IOL groups (6 months: UVB-IOL group mean = 0.96; SD = 0.92 and for the BF-IOL group mean = 0.99; SD = 0.96).
Conclusions.
Overall sleep quality and sleep latency improves after removal of cataract irrespective of the type of IOL implanted. These data show that implantation of BF-IOL does not have a negative impact on the sleep–wake cycle.
Keywords: cataract, intraocular lens, circadian rhythms, sleep
The key finding of this study is the sustained improvement in sleep latency after surgery irrespective of intraocular lens type implanted. This result highlights a significant benefit of cataract surgery in addition to improvement in visual acuity.
Introduction
Development of cataract decreases transmission of light to the retina.1,2 It is one of the most common ocular pathologies (responsible for 51% of world blindness3), particularly affecting the elderly. The role of cataract in sleep disturbance is not widely appreciated. Progressive aging and opacification of the natural crystalline lens affect the spectrum of light transmitted and reduces the amount of light reaching the retina, particularly in the short wavelength range of the visible spectrum. Replacement of the aging lens with an artificial intraocular lens restores the transmission to young adult levels and increases the amount of light reaching the retina.4,5 Apart from improved visual acuity from the implanted artificial intraocular lens (IOL), the increased amount of light reaching the retina is likely to stimulate the activity of all photoreceptors, rods, cones, and the directly photosensitive retinal ganglion cells (pRGCs), which are maximally sensitive to light at ∼480 nm, corresponding to the blue part of the visual light spectrum.6–8 Importantly, these pRGCs project to multiple structures in the brain, which respond to environmental irradiance including the circadian and sleep systems, and the pupillary light reflex.9 The suprachiasmatic nuclei (SCN) of the anterior hypothalamus act as the master circadian pacemaker. They receive a direct projection from the pRGCs, and light information is used to synchronize the internal clock to the environmental day–night cycle. The SCN in turn regulates circadian biology throughout the body. Ocular light input is required in order to synchronize the body clock to the environmental day–night cycle before relaying this information to the rest of the brain and the body. If light input is reduced (as in poorly lit accommodation) or absent, as in individuals without eyes, the SCN cannot adjust to the day–night cycle and internal circadian rhythms such as the sleep–wake cycle become desynchronized with reference to external time. There is some evidence for disruption of circadian patterns of sleep due to cataract, but the extent to which the aging lens in humans might contribute to a decrease in photic input to the SCN remains unclear.10 However, only a few studies with small numbers or with study design limitations have investigated the impact of cataract removal and artificial lens replacement on sleep. Preoperative sleep quality has been assessed retrospectively in two related studies,11,12 while only prospectively in two independent studies.13,14 Asplund and Ejdervik Lindblad11 investigated changes in self-reported parameters of sleep 1 month after cataract surgery, in comparison with retrospectively recalled presurgery states in 328 patients. Then Asplund and Ejdervik Lindblad12 extended the patient cohort to 407 patients, who completed a follow-up assessment 9 months after surgery. Their combined results suggested an improvement in sleep following surgery. However, no statistical validation has been given and, consequently, a measurable difference between 1 and 9 months postoperatively cannot be assumed. No difference in actigraphically derived sleep before and after cataract surgery in 15 patients was reported by Tanaka et al.,13 which could be a result of low power due to low sample size or low specificity of their sleep questions. The most comprehensive study by Ayaki et al.14 used the self-rated Pittsburgh Sleep Quality Index (PSQI) in 155 patients undergoing cataract surgery to assess changes in subjective sleep quality over time. A total of 44% of patients reported poor sleep preoperatively, which improved significantly 2 months after surgery, but was not sustained at 7 months. Overall, these studies suggest that poor sleep is present in almost half of the patients with cataract, and implantation of an artificial lens is associated with an improvement in sleep quality that is not sustained.
There are two main classes of IOLs currently in use, which differ in their transmission properties: UV-light blocking (UVB-IOLs) and blue-light filtering (BF-IOLs) in addition to UV-light blocking. Historically, BF-IOLs were developed to improve contrast sensitivity and reduce the blue hue experienced by patients after implantation with UVB-IOLs.15 To our knowledge, there is only one cross-sectional study. This compared the sleep quality of 31 individuals with UVB-IOL and 18 individuals with BF-IOL.16 Their results showed that PSQI-derived sleep quality did not differ between UVB-IOL and BF-IOL lens types, possibly due to insufficient power to resolve a small difference. Espindle et al.17 did not assess sleep but health-related quality of life in 257 patients, who received either a UV-IOL or a BF-IOL after cataract surgery. They used a vision-specific (NEI VFQ-39) and a generic physical and mental health (SF-12) scale and reported an improvement in vision-related functions in both groups after surgery, but no difference was reported between those with bilateral UVB-IOL implants and those with bilateral BF-IOL implants.
The pRGCs are maximally sensitive to blue light; and because BF-IOLs filter blue light, concerns were raised that such IOLs may cause disruption of the sleep–wake cycle. To test this hypothesis, we assessed the impact of cataract surgery and type of implant on sleep in two large groups of patients receiving either UVB-IOL or BF-IOL. Using the PSQI questionnaire, we compared sleep quality before and after cataract surgery and between these two groups.
Methods
Study Design
This was a prospective, consecutive, dual-site study, carried out at the Oxford Eye Hospital, UK, and the Prince Charles Eye Unit at King Edward VII Hospital in Windsor, UK. Patients underwent cataract surgery as routinely carried out in those centers. The type of IOL implanted following cataract extraction was predetermined by the site at which the patients were seen. Patients undergoing unilateral cataract surgery in Oxford received the UVB-IOL lens (AcrySof SA60AT; Alcon, Fort Worth, TX, USA) and those in Windsor received the BF-IOL (AcrySof IQ SN60AT; Alcon). Neither investigators nor patients were masked to the IOL allocation.
Participants
Patients were identified from preadmission clinics for cataract surgery during a 4-year period between 2008 and 2011 and recruited into the study prior to surgery. Eligibility for cataract surgery was according to local National Health Service (NHS) guidelines and included clinically significant cataracts, usually with vision worse than 6/12 (Snellen chart). Individuals with sleep disorders; treatment with benzodiazepines; and a diagnosis of physical or psychiatric conditions, head injury, and past alcohol or drug abuse were excluded from the study. Visual acuities (VA) of individuals able to read the Snellen chart were recorded (range 6/60 worst to 6/4 best). For those unable to read the chart, the ability to count fingers, see hand movements, or perceive light (most severely affected) was recorded. Only patients undergoing cataract surgery for their first eye were included in the analysis.
At the time of recruitment, age, sex, the presence or absence of any other ocular comorbidity, and the best-corrected VA for each eye were recorded. The best VA value of the two eyes was recorded and grouped into ≤6/12 and >6/12, according to the NHS guidelines for cataract surgery, for the statistical analysis.
Patients with other eye conditions were included in the study to get a representative sample of the general population of individuals being presented for cataract extraction. The whole cohort was dichotomized into coexistent ocular pathology: present or absent. Other coexistent ocular pathology included common and rare diseases such as glaucoma, age-related macular degeneration, diabetic retinopathy, vascular occlusions, and uveitis.
To account for a seasonal effect on sleep quality, records were kept of the season in which the operation and the assessments took place. The year was divided into four seasons: spring (February to April), summer (May to July), autumn (August to October), and winter (November to January).
Questionnaire and Data Collection
Overall sleep quality was assessed with the PSQI questionnaire at four different time points over the course of 1 year: 1 month preoperatively, then 1, 6 (UVB-IOL group only), and 12 months postoperatively. The PSQI is a validated, self-rating sleep questionnaire that asks about sleep quality over the past 4 weeks and scores seven different sleep components (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medication, daytime dysfunction) on a scale of 0 to 3.18 The component scores are added together to calculate an overall score for subjective sleep quality (global score), with a maximum score of 21; the higher the score, the poorer the quality of sleep. Any global score between 0 and 5 is considered as “no sleep difficulties,” while a global score of 6 or higher is considered as “poor sleep quality.” A score for each component and a subjective global sleep score (overall sleep quality) were calculated for each individual at each preoperative and postoperative time point.
In order to assess anxiety levels pre- and postoperatively, a small subgroup (n = 54) of individuals completed a “Hospital Anxiety and Depression Scale” questionnaire preoperatively and 1 and 12 months postoperatively.
Data Analysis
Data were analyzed using statistical software (R-2.15.1; R Development Core Team 2012, University of Auckland, Auckland, New Zealand; available in the public domain at http://www.R-project.org). To account for the within-patient correlations with records nested within patients, data were fitted to a two-level multilevel binomial generalized linear model (using the lme4 library in R). All PSQI scores were expressed as a fraction of a maximum of 21. The most parsimonious cumulative linked mixed model (using the ordinal library in R) was used to analyze the individual component scores. We present P values with statistical significance assumed when P < 0.05. The χ2 test was used to investigate the data for seasonal differences and visual acuity differences between groups. Fisher's exact test was used to check for comparability between the groups in terms of overall good and poor sleep and suitable sex balance between the groups. For checking comparability of age distribution, a nonparametric Mann-Whitney-Wilcoxon test was used.
Data were included in the analysis when the participant completed the PSQI questionnaire preoperatively and at least once postoperatively. The BF-IOL group had no questionnaire data collected at 6 months postoperatively; therefore, this time point was excluded from the UVB-IOL data set when the two groups were combined for analysis.
The study was approved through the Oxfordshire Research Ethics Committee B, Oxford, UK (ref 08/H0605/02), and this study adhered to the tenets of the Declaration of Helsinki. Informed written consent was obtained from those patients willing and able to take part in the study.
Results
A total of 1482 participants were recruited and 961 participants completed the study. The demographics of those participants who did not complete the study do not differ from the demographics of those who completed the study. Of those who participated, 498 patients received a UVB-IOL and 463 patients received a BF-IOL. The timing of surgery regarding season showed that the distribution of the number of patients per season was comparable for the BF-IOL cohort (χ2 [3] = 0.7322; P = 0.867) but not for the UVB-IOL cohort (χ2 [3] = 82.47; P < 0.0001; Table 1). There was no significant correlation between anxiety and PSQI scores at 1 month (P = 0.670) or 12 months (P = 0.415) postoperatively. When considering anxiety alone, there was no significant difference 1 month following surgery (P = 0.745), but individuals were likely to have a lower anxiety score 12 months postoperatively (P = 0.05).
Table 1.
Descriptive Statistics of Data From Preoperative Cataract Patients
|
UV-IOL |
BF-IOL |
Whole Cohort |
|
| Participants, n | |||
| Recruited | 713 | 769 | 1482 |
| Completed study | 498 | 463 | 961 |
| Mean age, y (SD) | 74.19 (9.6) | 74.4 (9.7) | 76.94 (5.53) |
| Age range, y | 41–97 | 37–97 | 37–97 |
| Sex, n* | |||
| Men/women | 230/268 | 182/281 | 412/549 |
| Female, % | 53.82 | 60.69 | 57.12 |
| No other ocular pathology, %* | 73.2 | 67.17 | 70.29 |
| Other ocular pathologies, %* | 26.8 | 32.83 | 29.71 |
| PSQI poor sleep (≥6), % | 53 | 49.46 | 51.3 |
| Visual acuity* | |||
| ≤6/12, % | 89.11 | 80.35 | 84.88 |
| >6/12, % | 10.89 | 19.65 | 15.12 |
| Season at surgery | |||
| Spring, % | 36.35 | 23.76 | 30.3 |
| Summer, % | 28.71 | 24.4 | 26.64 |
| Autumn, % | 14.68 | 25.49 | 19.88 |
| Winter, % | 12.86 | 26.35 | 19.35 |
Divided according to lens type and also as a whole cohort.
Indicates that the data are not comparable (P > 0.05) between the two groups.
Sleep Quality of Individuals With Cataract in the Whole Cohort (UVB-IOL and BF-IOL)
Before cataract surgery, 51.3% of all patients reported poor sleep quality with women being 1.44 times as likely as men to report worse sleep quality (P < 0.0001; Table 1).
The distribution of poor and good sleepers between UVB-IOL and BF-IOL recipients were similar (Fisher's exact test, P = 0.273). However, neither sex nor the presence or absence of ocular pathology were equally proportioned between the two groups (P = 0.047 and P = 0.037, respectively). The distribution of the number of patients per visual acuity group was not equally proportioned between the two lens groups (χ2 [1] = 14.3425; P = 0.0002), with 8.76% more subjects <6/12 in the BF-IOL group (Table 1). A Mann-Whitney-Wilcoxon test showed that the distribution of age was the same between groups (W = 1002740, P = 0.237; Table 1).
Change in Sleep After Cataract in the Whole Cohort (UVB-IOL and BF-IOL)
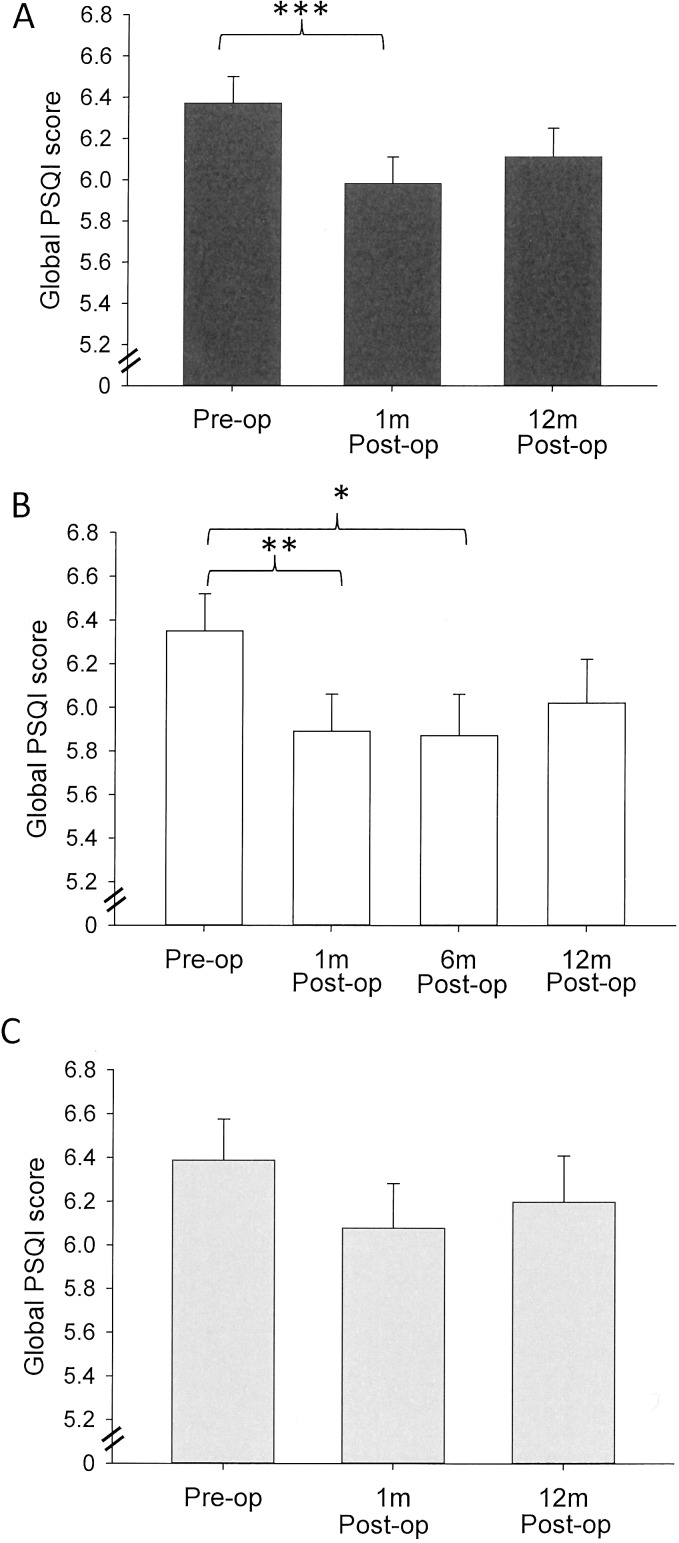
In the whole cohort, there was a significant improvement in the overall quality of sleep 1 month after surgery (P < 0.0001). Twelve months after surgery, there was a trend toward sleep improvement, but this was not statistically significant (P = 0.073; Fig. 1A). The type of lens implanted was irrelevant to the change in overall sleep quality (P = 0.674); and when analyzed separately, the minimal differences seen in the improvement in overall quality of sleep in the UVB-IOL but not the BF-IOL postoperatively is presumably due to factors other than lens type that cannot be resolved from this data. The improvement in the global sleep score was driven by four sleep parameters, specifically: sleep quality (an improvement at 1 month; P = 0.006); subjective sleep latency (shorter latency at 1 month, P < 0.0001; and at 12 months P = 0.0002); sleep duration (longer sleep at 1 month P = 0.004, and only weak evidence for longer sleep at 12 months, which would not be considered significant: P = 0.057); and sleep efficiency (an improvement at 1 month P = 0.001). By contrast, patients reported an increase in their daytime dysfunction at 1 month (P = 0.025) and 12 months (P = 0.029) post surgery. The type of lens implanted was not relevant to the change in the component scores following cataract surgery (P > 0.05 in all cases; Table 2).
Figure 1.
(A–C) Mean global PSQI scores with standard errors over time for the whole cohort (A) and separately for the UVB (B) and BF (C) lens types. Postop 1 month shows a significant improvement in global PSQI score. Although all postop global PSQI scores remained lower than preoperative levels, they were slightly increased at 12 months. Significant differences are marked with an asterisk. *P < 0.02. **P < 0.001. ***P < 0.0001.
Table 2.
Mean Component Scores of the PSQI
|
Time |
Before Surgery |
After Surgery |
||||||||||
|
1 Mo |
1 Mo |
6 Mo |
12 Mo |
|||||||||
| IOL Type | UVB | BF | Whole Cohort | UVB | BF | UVB and BF | UVB | BF | Whole Cohort | UVB | BF | Whole Cohort |
| Participants, n | 498 | 463 | 961 | 454 | 363 | 817 | 392 | NA | NA | 353 | 340 | 693 |
| Sleep quality | 0.84 | 0.91 | 0.88 | 0.75* | 0.85 | 0.80 | 0.72* | 0.79 | 0.87 | 0.83 | ||
| Sleep latency | 1.16 | 1.17 | 1.16 | 1.01* | 1* | 1.01 | 1.02 | 0.96* | 0.99* | 0.98* | ||
| Sleep duration | 0.80 | 0.83 | 0.82 | 0.69* | 0.72* | 0.71 | 0.66* | 0.70* | 0.79 | 0.74 | ||
| Habitual sleep efficacy | 1.40 | 1.3 | 1.35 | 1.23* | 1.28 | 1.25 | 1.20* | 1.27 | 1.29 | 1.28 | ||
| Sleep disturbance | 1.19 | 1.28 | 1.23 | 1.18 | 1.2 | 1.19 | 1.2 | 0.36 | 0.34 | 0.35 | ||
| Sleep medication | 0.36 | 0.33 | 0.34 | 0.33 | 0.37 | 0.35 | 0.38 | 0.36 | 0.34 | 0.35 | ||
| Daytime disfunction | 0.62 | 0.56 | 0.59 | 0.71* | 0.63 | 0.67 | 0.70* | 0.71* | 0.69* | 0.70* | ||
| Global score (SD) | 6.35 (3.82) | 6.39 (4.04) | 6.37 (3.92) | 5.894* (3.71) | 6.08 (3.88) | 5.98* (3.79) | 5.87* (3.81) | 6.02 (3.71) | 6.2 (3.90) | 6.11 (3.80) | ||
Measured over time and according to lens type: UVB-IOL or BF-IOL and the whole cohort (UVB-IOL and BF-IOL together).
Indicates a significant difference in the score compared with preoperative scores.
Change in Sleep After Cataract Separated by Lens Type
UVB-IOL Data.
A total of 53% patients rated their preoperative sleep to be of poor quality (Table 1). Women were 1.39 times as likely to report worse subjective sleep (P < 0.0001).
There was a significant improvement in the overall sleep quality in the whole group 1 month postoperatively (P = 0.0003) and 6 months postoperatively (P = 0.013), but not at 12 months postoperatively (P = 0.122; Fig. 1B).
The improvement was driven by four sleep parameters, specifically: subjective sleep quality (an improvement at 1 month [P = 0.01] and 6 months [P = 0.01], but not 12 months [P = 0.429]); sleep latency (shorter latency at 1 month [P < 0.0001], 6 months [P = 0.004], and 12 months [P = 0.0001]); sleep duration (longer sleep at 1 month [P < 0.0001], 6 months [P < 0.0001], and 12 months [P < 0.0001]); and sleep efficiency (an improvement at 1 month [P = 0.002] and 6 months [P = 0.006], but not at 12 months [P = 0.095]). By contrast, patients also reported an increase in their daytime dysfunction at 1 month (P = 0.01), 6 months (P = 0.016), and 12 months (P = 0.015) postoperatively (Table 2).
BF-IOL Data.
A total of 49.46% of patients rated their preoperative sleep to be of poor quality (Table 1). Women were 1.45 times as likely to report poor subjective sleep (P < 0.0001).
There was weak evidence for an improvement in overall sleep quality 1 month postoperatively, which would not be considered significant (P = 0.08), but not at 12 months postoperatively (P = 0.26667; Fig. 1C).
When separating the global PSQI into specific sleep parameters, the latency to sleep onset shortened significantly at 1 month (P = 0.0046) and at 12 months (P = 0.0007). Sleep duration also improved at 1 month (P = 0.0136). By contrast, there was only weak evidence of an increase in individuals' daytime dysfunction at 1 month, which would not be considered significant (P = 0.064) but patients reported a significant increase in their daytime dysfunction at 12 months (P = 0.018; Table 2).
A significant shortening in the sleep-specific parameters “latency to sleep onset” and an increase in the wake-related “daytime dysfunction” were consistently found and sustained over 12 months, irrespective of combining or separating the data sets (P < 0.05 in all cases). All other things being equal, an individual is twice as likely to have a shorter time to fall asleep after cataract surgery to their first eye.
Discussion
Approximately 50% of our patient cohort with cataract reported a global PSQI sleep score of 6 and above, which is indicative of problems with sleep. The distribution of sleep quality within this cataract population is equivalent to an age-matched population.19 Women always reported significantly more sleep disturbances than men, which is consistent with normative population data.19 Age, visual acuity, other ocular comorbidities, and the season during which the operation took place had no impact on the global sleep quality or the component scores postoperatively.
Overall sleep quality improved significantly following cataract surgery in the short term for the entire cohort, irrespective of the type of lens implanted. There were no significant differences in overall sleep scores between the two main categories of IOLs: UVB-IOL and BF-IOL. Although there might be a reduction in transmission of blue light into the eye expected with a BF-IOL compared with a UVB-IOL, the size of that reduction does not appear to be clinically significant and indeed, when compared with a crystalline lens, the BF-IOLs allow transmission of at least as much, if not more, short wavelength light in the visible spectrum.20
The extent to which a cataractous lens and different types of intraocular implants might contribute to a decrease in photic input to the clock has not been studied extensively. Both aging of the healthy lens and, to a greater extent, development of cataract, decrease transmission of short wavelength light in the visible spectrum to the retina.1,2 Light input to the circadian system is not only influenced by the health of the eye, but also by behavior such as physical activity. Without exposure to light of sufficient irradiance and spectral quality, circadian entrainment will be significantly impaired, particularly in the elderly due to senescence of the circadian system coupled with reduced exposure to light. Supplementary bright light in the home environment of the elderly has been shown to have a dramatic effect on their ability to consolidate their sleep–wake timing.21
In our study the only improvement that occurred consistently across the two cohorts and over time following cataract surgery, was the shortening in time to fall asleep (sleep latency). It seems likely that this is related to the increase in overall light transmission, and in particular the transmission of short wavelength light in the visible spectrum once the cataract has been removed.22 Although in vivo measurements of the amount of light reaching the retina before and after surgery have not been undertaken, the transmittance of the short-wavelengths (between 420 and 500 nm) was expected to improve on average by a factor of 4 according to a study by Giménez et al.23 They measured the change in retinal reflectance due to replacement of a crystalline, cataractous lens with artificial lens implants of different type to those in the present study.23 Furthermore, it has been shown that increased levels of environmental light can improve sleep latency.24
The significant improvement for the summarized sleep score 1 month postoperatively, which was driven by improving latency, duration, efficiency and subjective quality, was not sustained 12 months later. A number of factors could explain this attenuation. First, a temporary improvement in sleep can be a result of increased physical activity due to regained vision after cataract surgery as has been shown by Espindle et al.17 Second, there is evidence that the circadian system reacts to acute changes in light exposure, but habituates thereafter and restores the response to prechange levels, as assessed with the melatonin suppression test to light.25
A third explanation relates to findings that changes in light exposure in the elderly after cataract removal can shift the entire sleep–wake cycle and melatonin rhythm.26 The direction is influenced by the timing of light exposure (e.g., evening light causes sleep phase delay and morning light sleep phase advance), and the person's preference toward morningness or eveningness. A delay in the sleep–wake cycle due to evening light can thereby lead to a decrease in sleep quality. Finally, sleep problems are common in the elderly.27 Anxiety, depression, medication side effects, chronic disease, and caffeine or alcohol are known to negatively affect sleep quality.28 This is likely to be the reason for the worsening of daytime functioning over the same period when the improvement of subjective sleep quality was fading. Daytime functioning was addressed by asking the participant how often they have had trouble staying awake while driving, eating meals, or engaging in social activities and how much of a problem it had been to show enthusiasm to get things done. Rather than being sleep specific, this component is very generic and reflects overall well-being and mental health, and we assume that other unrecognized factors contributed to the patients' rating this individual components of the PSQI.
In summary, the key finding of this study is the sustained improvement in sleep latency after surgery irrespective of intraocular lens type implanted. This result highlights a significant benefit of cataract surgery in addition to improvement in visual acuity.
Acknowledgments
The authors thank Richard Packard, MD, FRCS, FRCOphth, for providing patients from Windsor and Daniel Lunn, MA, DPhil (Department of Statistics, Oxford), for statistical assistance.
Supported by Wellcome Trust Grant 090684/Z/09/Z and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust, Oxford University (REF A90305 and A92181). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Disclosure: I. Alexander, None; F.M. Cuthbertson, None; G. Ratnarajan, None; R. Safa, None; F. Mellington, None; R.G. Foster, None; S.M. Downes, None; K. Wulff, None
References
- 1. Mellerio J. Yellowing of the human lens: nuclear and cortical contributions. Vision Res. 1987; 27: 1581–1587 [DOI] [PubMed] [Google Scholar]
- 2. Weale RA. Age and the transmittance of the human crystalline lens. J Physiol. 1988; 395: 577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Priority eye diseases. Available at: http://www.who.int/blindness/causes/priority/en/index1.html. Accessed July 2014 [Google Scholar]
- 4. Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006; 90: 784–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner PL, Mainster MA. Circadian photoreception: ageing and the eye's important role in systemic health. Br J Ophthalmol. 2008; 92: 1439–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Foster RG, Hankins MW. Circadian vision. Curr Biol. 2007; 17: R746–R751 [DOI] [PubMed] [Google Scholar]
- 7. Hattar S, Lucas RJ, Mrosovsky N, Thompson S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003; 424: 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001; 4: 621–626 [DOI] [PubMed] [Google Scholar]
- 9. Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006; 497: 326–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim YH, Jung KI, Song CH. The effect of cataract on sleep time and quality in late adulthood. Aging Clin Exp Res. 2012; 24: 663–668 [DOI] [PubMed] [Google Scholar]
- 11. Asplund R. Ejdervik Lindblad B. The development of sleep in persons undergoing cataract surgery. Arch Gerontol Geriatr. 2002; 35: 179–187 [DOI] [PubMed] [Google Scholar]
- 12. Asplund R, Lindblad BE. Sleep and sleepiness 1 and 9 months after cataract surgery. Arch Gerontol Geriatr. 2004; 38: 69–75 [DOI] [PubMed] [Google Scholar]
- 13. Tanaka M, Hosoe K, Hamada T, Morita T. Change in sleep state of the elderly before and after cataract surgery. J Physiol Anthro. 2010; 29: 219–224 [DOI] [PubMed] [Google Scholar]
- 14. Ayaki M, Muramatsu M, Negishi K, Tsubota K. Improvements in sleep quality and gait speed after cataract surgery. Rejuvenation Res. 2013; 16: 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davison JA, Patel AS. Light normalizing intraocular lenses. Int Ophthalmol Clin. 2005; 45: 55–106 [PubMed] [Google Scholar]
- 16. Landers JA, Tamblyn D, Perriam D. Effect of a blue-light-blocking intraocular lens on the quality of sleep. J Cataract Refract Surg. 2009; 35: 83–88 [DOI] [PubMed] [Google Scholar]
- 17. Espindle D, Crawford B, Maxwell A, et al. Quality-of-life improvements in cataract patients with bilateral blue light-filtering intraocular lenses: clinical trial. J Cataract Refract Surg. 2005; 31: 1952–1959 [DOI] [PubMed] [Google Scholar]
- 18. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28: 193–213 [DOI] [PubMed] [Google Scholar]
- 19. Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. J Psycho Res. 2004; 56: 503–510 [DOI] [PubMed] [Google Scholar]
- 20. Kessel L, Lundeman JH, Herbst K, Andersen TV, Larsen M. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg. 2010; 36: 308–312 [DOI] [PubMed] [Google Scholar]
- 21. Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008; 299: 2642–2655 [DOI] [PubMed] [Google Scholar]
- 22. Cuthbertson FM, Peirson SN, Wulff K, Foster RG, Downes SM. Blue light-filtering intraocular lenses: review of potential benefits and side effects. J Cataract Refract Surg. 2009; 35: 1281–1297 [DOI] [PubMed] [Google Scholar]
- 23. Gimenez MC, Kanis MJ, Beersma DG, van der Pol BA, van Norren D, Gordijn MC. In vivo quantification of the retinal reflectance spectral composition in elderly subjects before and after cataract surgery: implications for the non-visual effects of light. J Biol Rhythm. 2010; 25: 123–131 [DOI] [PubMed] [Google Scholar]
- 24. Kirisoglu C, Guilleminault C. Twenty minutes versus forty-five minutes morning bright light treatment on sleep onset insomnia in elderly subjects. J Psychosom Res. 2004; 56: 537–542 [DOI] [PubMed] [Google Scholar]
- 25. Giménez MC, Beersma DG, Bollen P, van der Linden ML, Gordijn MC. Effects of reducing short wavelengths input on melatonin and sleep patterns in human: evidence for adaptation. In: Giménez MC. ed Light From Dawn to Dusk: Human Entrainment in a Changing Environment. Groningen, The Netherlands: University of Groningen; 2013: 83–96 [Google Scholar]
- 26. Giménez MC, Beersma DGM, Daan S, et al. Melatonin and sleep-wake rhythms before and after ocular lens replacement in elderly humans. In: Giménez MC. ed Light From Dawn to Dusk: Human Entrainment in a Changing Environment. Groningen, The Netherlands: University of Groningen; 2013: 69–82 [Google Scholar]
- 27. Kessel L, Siganos G, Jorgensen T, Larsen M. Sleep disturbances are related to decreased transmission of blue light to the retina caused by lens yellowing. Sleep. 2011; 34: 1215–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foster RG, Wulff K. The rhythm of rest and excess. Nat Rev Neurosci. 2005; 6: 407–414 [DOI] [PubMed] [Google Scholar]