Abstract
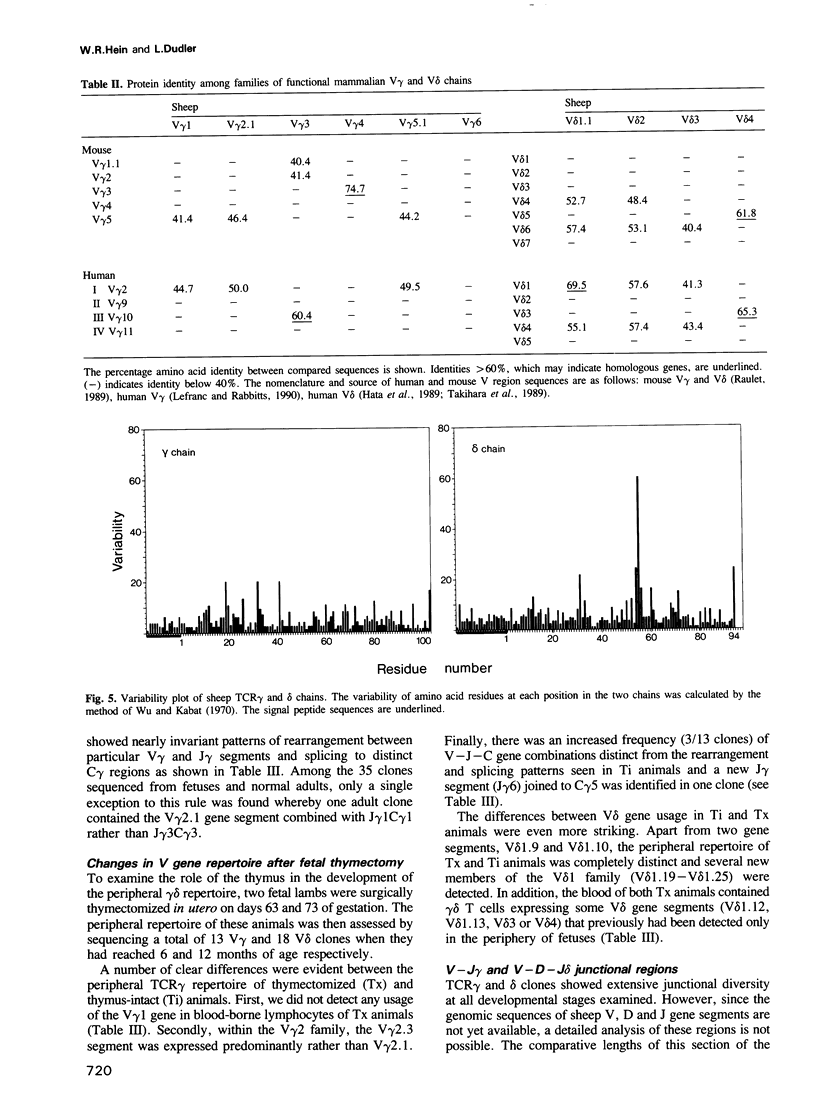
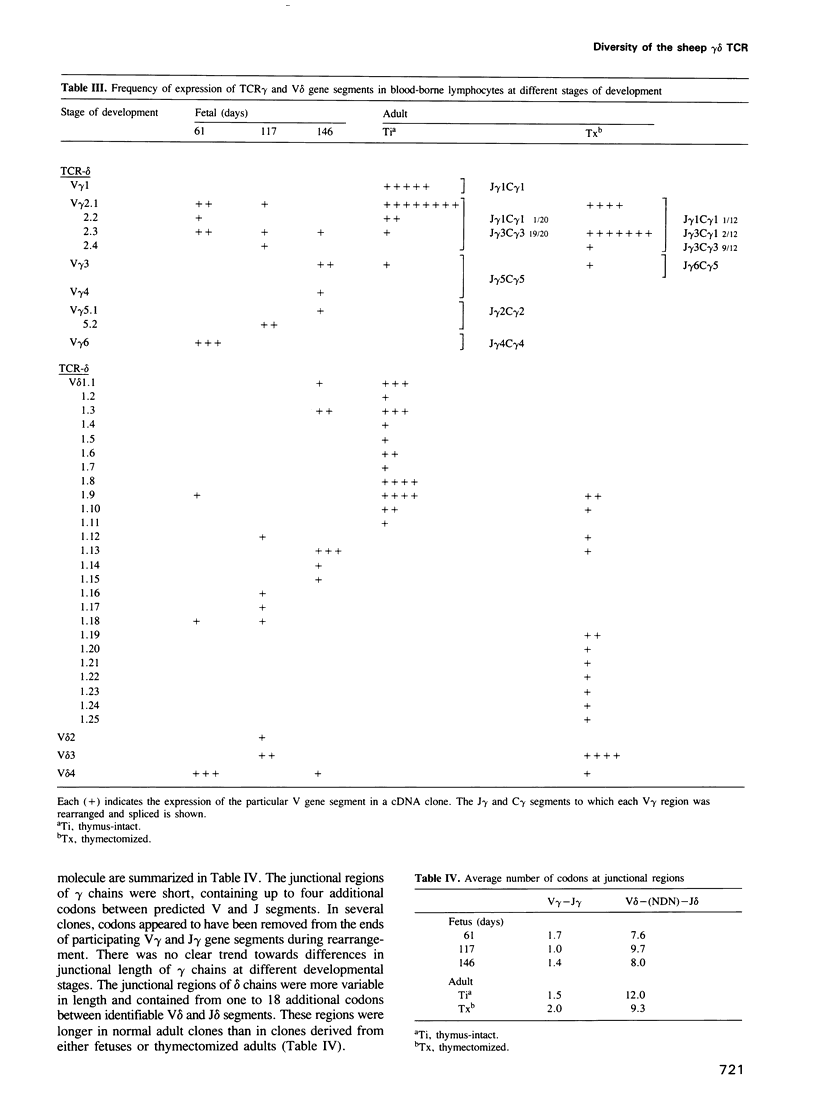
Sheep gamma delta T cells express an unprecedented repertoire of antigen receptors contributed by increased diversity in both variable and constant region gene segments. Variable region diversity results mainly from the utilization of a large family of duplicated V delta genes that have retained two distinct hypervariable segments comparable with the complementarity determining regions present in other antigen receptor V genes. This implies that sheep V delta chains have been intensely selected during evolution, probably at sites involved in ligand recognition. The sheep gamma delta heterodimer occurs in at least five isotypic variants formed by the association of a single C delta segment with one of five functional C gamma segments, each with distinctive hinge regions. Our analysis also shows that the establishment of a normal peripheral repertoire is both developmentally regulated and dependent on the continual presence of a functional thymus during ontogeny. The existence of an expanded V gene repertoire and multiple receptor isotypes together with the prominence of gamma delta T cells in the sheep immune system argues that this lineage of T cells has a more elaborate functional role in this evolutionary pathway.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. P., Havran W. L. The immunobiology of T cells with invariant gamma delta antigen receptors. Annu Rev Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- Asarnow D. M., Kuziel W. A., Bonyhadi M., Tigelaar R. E., Tucker P. W., Allison J. P. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988 Dec 2;55(5):837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Cron R. Q., Barrett T. A., Houlden B., Sperling A. I., Dent A., Hedrick S., Rellahan B., Matis L. A. Repertoire development and ligand specificity of murine TCR gamma delta cells. Immunol Rev. 1991 Apr;120:5–33. doi: 10.1111/j.1600-065x.1991.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Bucy R. P., Chen C. H., Cooper M. D. Analysis of gamma delta T cells in the chicken. Semin Immunol. 1991 Mar;3(2):109–117. [PubMed] [Google Scholar]
- Buresi C., Ghanem N., Huck S., Lefranc G., Lefranc M. P. Exon duplication and triplication in the human T-cell receptor gamma constant region genes and RFLP in French, Lebanese, Tunisian, and black African populations. Immunogenetics. 1989;29(3):161–172. doi: 10.1007/BF00373641. [DOI] [PubMed] [Google Scholar]
- Casorati G., De Libero G., Lanzavecchia A., Migone N. Molecular analysis of human gamma/delta+ clones from thymus and peripheral blood. J Exp Med. 1989 Nov 1;170(5):1521–1535. doi: 10.1084/jem.170.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Claverie J. M., Prochnicka-Chalufour A., Bougueleret L. Implications of a Fab-like structure for the T-cell receptor. Immunol Today. 1989 Jan;10(1):10–14. doi: 10.1016/0167-5699(89)90058-3. [DOI] [PubMed] [Google Scholar]
- Clevers H., MacHugh N. D., Bensaid A., Dunlap S., Baldwin C. L., Kaushal A., Iams K., Howard C. J., Morrison W. I. Identification of a bovine surface antigen uniquely expressed on CD4-CD8- T cell receptor gamma/delta+ T lymphocytes. Eur J Immunol. 1990 Apr;20(4):809–817. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Engel I., Hedrick S. M. Site-directed mutations in the VDJ junctional region of a T cell receptor beta chain cause changes in antigenic peptide recognition. Cell. 1988 Aug 12;54(4):473–484. doi: 10.1016/0092-8674(88)90068-2. [DOI] [PubMed] [Google Scholar]
- Hata S., Clabby M., Devlin P., Spits H., De Vries J. E., Krangel M. S. Diversity and organization of human T cell receptor delta variable gene segments. J Exp Med. 1989 Jan 1;169(1):41–57. doi: 10.1084/jem.169.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran W. L., Allison J. P. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988 Sep 29;335(6189):443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- Havran W. L., Allison J. P. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990 Mar 1;344(6261):68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- Havran W. L., Carbone A., Allison J. P. Murine T cells with invariant gamma delta antigen receptors: origin, repertoire, and specificity. Semin Immunol. 1991 Mar;3(2):89–97. [PubMed] [Google Scholar]
- Hein W. R., Dudler L., Beya M. F., Marcuz A., Grossberger D. T cell receptor gene expression in sheep: differential usage of TcR1 in the periphery and thymus. Eur J Immunol. 1989 Dec;19(12):2297–2301. doi: 10.1002/eji.1830191218. [DOI] [PubMed] [Google Scholar]
- Hein W. R., Dudler L., Marcuz A., Grossberger D. Molecular cloning of sheep T cell receptor gamma and delta chain constant regions: unusual primary structure of gamma chain hinge segments. Eur J Immunol. 1990 Aug;20(8):1795–1804. doi: 10.1002/eji.1830200826. [DOI] [PubMed] [Google Scholar]
- Hein W. R., Dudler L., Morris B. Differential peripheral expansion and in vivo antigen reactivity of alpha/beta and gamma/delta T cells emigrating from the early fetal lamb thymus. Eur J Immunol. 1990 Aug;20(8):1805–1813. doi: 10.1002/eji.1830200827. [DOI] [PubMed] [Google Scholar]
- Hein W. R., Mackay C. R. Prominence of gamma delta T cells in the ruminant immune system. Immunol Today. 1991 Jan;12(1):30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- Hein W. R., Marcuz A., Fichtel A., Dudler L., Grossberger D. Primary structure of the sheep T-cell receptor alpha chain. Immunogenetics. 1991;34(1):39–41. doi: 10.1007/BF00212310. [DOI] [PubMed] [Google Scholar]
- Hirt W., Saalmüller A., Reddehase M. J. Distinct gamma/delta T cell receptors define two subsets of circulating porcine CD2-CD4-CD8- T lymphocytes. Eur J Immunol. 1990 Feb;20(2):265–269. doi: 10.1002/eji.1830200206. [DOI] [PubMed] [Google Scholar]
- Hong S. C., Chelouche A., Lin R. H., Shaywitz D., Braunstein N. S., Glimcher L., Janeway C. A., Jr An MHC interaction site maps to the amino-terminal half of the T cell receptor alpha chain variable domain. Cell. 1992 Jun 12;69(6):999–1009. doi: 10.1016/0092-8674(92)90618-m. [DOI] [PubMed] [Google Scholar]
- Itohara S., Farr A. G., Lafaille J. J., Bonneville M., Takagaki Y., Haas W., Tonegawa S. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990 Feb 22;343(6260):754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Yssel H., Brocklehurst C., Spits H. A distinct wave of human T cell receptor gamma/delta lymphocytes in the early fetal thymus: evidence for controlled gene rearrangement and cytokine production. J Exp Med. 1990 Sep 1;172(3):847–859. doi: 10.1084/jem.172.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Rabbitts T. H. A nomenclature to fit the organization of the human T-cell receptor gamma and delta genes. Res Immunol. 1990 Sep;141(7):615–618. doi: 10.1016/0923-2494(90)90068-a. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Rabbitts T. H. Two tandemly organized human genes encoding the T-cell gamma constant-region sequences show multiple rearrangement in different T-cell types. Nature. 1985 Aug 1;316(6027):464–466. doi: 10.1038/316464a0. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Beya M. F., Matzinger P. Gamma/delta T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989 Aug;19(8):1477–1483. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Hein W. R. A large proportion of bovine T cells express the gamma delta T cell receptor and show a distinct tissue distribution and surface phenotype. Int Immunol. 1989;1(5):540–545. doi: 10.1093/intimm/1.5.540. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Bluestone J. A. Specificity of gamma delta receptor bearing T cells. Semin Immunol. 1991 Mar;3(2):75–80. [PubMed] [Google Scholar]
- McVay L. D., Carding S. R., Bottomly K., Hayday A. C. Regulated expression and structure of T cell receptor gamma/delta transcripts in human thymic ontogeny. EMBO J. 1991 Jan;10(1):83–91. doi: 10.1002/j.1460-2075.1991.tb07923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec C., Faure F., Ferradini L., Roman-Roman S., Jitsukawa S., Ferrini S., Moretta A., Triebel F., Hercend T. Further analysis of the T cell receptor gamma/delta+ peripheral lymphocyte subset. The V delta 1 gene segment is expressed with either C alpha or C delta. J Exp Med. 1990 Apr 1;171(4):1171–1188. doi: 10.1084/jem.171.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novacek M. J. Mammalian phylogeny: shaking the tree. Nature. 1992 Mar 12;356(6365):121–125. doi: 10.1038/356121a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Born W. Heat shock proteins as antigens for gamma delta T cells. Semin Immunol. 1991 Mar;3(2):81–87. [PubMed] [Google Scholar]
- Philpott K. L., Viney J. L., Kay G., Rastan S., Gardiner E. M., Chae S., Hayday A. C., Owen M. J. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992 Jun 5;256(5062):1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Brenner M. B., Band H. Biology of the human gamma delta T-cell receptor. Immunol Rev. 1991 Apr;120:137–183. doi: 10.1111/j.1600-065x.1991.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. The structure, function, and molecular genetics of the gamma/delta T cell receptor. Annu Rev Immunol. 1989;7:175–207. doi: 10.1146/annurev.iy.07.040189.001135. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Kabat E. A., Wu T. T. Subgroups of Tcr alpha chains and correlation with T-cell function. Immunogenetics. 1992;35(4):224–234. doi: 10.1007/BF00166827. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Wu T. T., Kabat E. A. Subgroups of variable region genes of beta chains of T-cell receptors for antigen. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4461–4463. doi: 10.1073/pnas.83.12.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L. Developmental biology of T cell receptors. Science. 1989 May 26;244(4907):943–950. doi: 10.1126/science.2658058. [DOI] [PubMed] [Google Scholar]
- Takeuchi N., Ishiguro N., Shinagawa M. Molecular cloning and sequence analysis of bovine T-cell receptor gamma and delta chain genes. Immunogenetics. 1992;35(2):89–96. doi: 10.1007/BF00189517. [DOI] [PubMed] [Google Scholar]
- Takihara Y., Reimann J., Michalopoulos E., Ciccone E., Moretta L., Mak T. W. Diversity and structure of human T cell receptor delta chain genes in peripheral blood gamma/delta-bearing T lymphocytes. J Exp Med. 1989 Feb 1;169(2):393–405. doi: 10.1084/jem.169.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Triebel F., Faure F., Graziani M., Jitsukawa S., Lefranc M. P., Hercend T. A unique V-J-C-rearranged gene encodes a gamma protein expressed on the majority of CD3+ T cell receptor-alpha/beta- circulating lymphocytes. J Exp Med. 1988 Feb 1;167(2):694–699. doi: 10.1084/jem.167.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F., Faure F., Mami-Chouaib F., Jitsukawa S., Griscelli A., Genevée C., Roman-Roman S., Hercend T. A novel human V delta gene expressed predominantly in the Ti gamma A fraction of gamma/delta+ peripheral lymphocytes. Eur J Immunol. 1988 Dec;18(12):2021–2027. doi: 10.1002/eji.1830181223. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]



