Abstract
Objectives:
Determine diagnostic accuracy of a clinical diagnosis of Parkinson disease (PD) using neuropathologic diagnosis as the gold standard.
Methods:
Data from the Arizona Study of Aging and Neurodegenerative Disorders were used to determine the predictive value of a clinical PD diagnosis, using 2 clinical diagnostic confidence levels, PossPD (never treated or not clearly responsive) and ProbPD (responsive to medications). Neuropathologic diagnosis was the gold standard.
Results:
Based on first visit, 9 of 34 (26%) PossPD cases had neuropathologically confirmed PD while 80 of 97 (82%) ProbPD cases had confirmed PD. PD was confirmed in 8 of 15 (53%) ProbPD cases with <5 years of disease duration and 72 of 82 (88%) with ≥5 years of disease duration. Using final diagnosis at time of death, 91 of 107 (85%) ProbPD cases had confirmed PD. Clinical variables that improved diagnostic accuracy were medication response, motor fluctuations, dyskinesias, and hyposmia.
Conclusions:
Using neuropathologic findings of PD as the gold standard, this study establishes the novel findings of only 26% accuracy for a clinical diagnosis of PD in untreated or not clearly responsive subjects, 53% accuracy in early PD responsive to medication (<5 years' duration), and >85% diagnostic accuracy of longer duration, medication-responsive PD. Caution is needed when interpreting clinical studies of PD, especially studies of early disease that do not have autopsy confirmation. The need for a tissue or other diagnostic biomarker is reinforced.
Classification of evidence:
This study provides Class II evidence that a clinical diagnosis of PD identifies patients who will have pathologically confirmed PD with a sensitivity of 88% and specificity of 68%.
Making an accurate diagnosis of Parkinson disease (PD) is critical for patient care as well as research related to epidemiology, genetics, imaging, biomarker discovery, and both symptomatic and disease-modifying treatments. Methods for diagnosing PD are limited by the lack of a tissue diagnostic test or other definitive biomarker test. Current diagnostic criteria for PD are based on the nonspecific clinical findings of rest tremor, cogwheel rigidity, and bradykinesia,1 but the gold standard remains to be neuropathologic confirmation.
A major goal to improve diagnostic accuracy in living patients has been to find a blood, CSF, or other tissue biomarker for PD. One inherent complicating factor for finding a biomarker in living patients with PD is the potential inaccuracy of a clinical rather than autopsy-confirmed diagnosis. Validation of an accurate diagnostic biomarker for PD may require neuropathologic confirmation of the PD diagnosis, as has been demanded for amyloid imaging in Alzheimer disease research.2
Numerous studies report that neuropathologic confirmation of a clinical diagnosis of PD may range from 65% to 93%, depending on the criteria used and the stage of disease.3–8 Recently, the use of dopaminergic neuroimaging has improved the diagnosis of PD in living patients, and appears to be sensitive but not specific, and has not yet been validated by postmortem examination.9 This study presents clinical and neuropathologic data on the diagnostic accuracy of longitudinally followed subjects with PD based on disease duration, medication responsiveness, and clinical signs.
METHODS
Subjects.
Subjects enrolled from 1997 to 2013 in an ongoing longitudinal clinical-neuropathologic study, the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND), with autopsies performed by the Banner Sun Health Research Institute Brain and Body Donation Program (www.brainandbodydonationprogram.org), were included.
Standard protocol approvals, registrations, and patient consents.
All subjects signed written informed consent approved by the Banner Sun Health Institutional Review Board.
Clinical assessments.
Subjects received annual standardized movement disorder examinations by a fellowship-trained movement disorders specialist (C.A., H.S., J.C., E.D.-D.) as previously described.10,11 Examinations included a full Unified Parkinson's Disease Rating Scale (UPDRS) (performed in the practically defined off state whenever possible),12 medication history, and neuropsychological test battery.11 Olfactory testing, using the University of Pennsylvania Smell Identification Test (UPSIT), began in 2005.13,14
At each assessment, subjects were evaluated for one or more of the cardinal signs of PD: rest tremor—a UPDRS motor score of ≥1 for lower lip or any limb; bradykinesia—a UPDRS motor score of ≥1 in 2 motor tests on the same side of the body (arm/leg) or a score of ≥2 in one motor test of a limb; and cogwheel rigidity—a UPDRS motor score of ≥1 of any limb. After each evaluation, subjects were given a movement disorders diagnosis: (1) probable PD (ProbPD): 2 of 3 cardinal signs, no symptomatic cause, improvement when treated with dopaminergic medications and continued response if still being treated, or if lack of current response, then an explanation for why treatment was no longer working (i.e., inadequate dose due to side effects); (2) possible PD (PossPD): 2 of 3 cardinal signs, no symptomatic cause, symptoms or signs present for ≤5 years, dopaminergic treatment had not been tried or an adequate trial had not clearly occurred (i.e., too low a dose, side effects that limited therapeutic dose, etc.); (3) progressive supranuclear palsy (PSP): meeting National Institute of Neurological Disorders and Stroke PSP clinical criteria15 for diagnosis; (4) parkinsonism not otherwise specified (ParkNOS): parkinsonism without response to an adequate dose of dopaminergic medication or disease duration of >5 years and had not been treated or been given an adequate trial of dopaminergic treatment, or appeared to have another etiology including an unclear neurodegenerative condition, dementia with parkinsonian features, or secondary parkinsonism; or (5) multiple system atrophy (MSA): autonomic dysfunction with or without parkinsonism poorly responsive to medication and/or cerebellar findings. At the time of death, all available medical records were reviewed and a final clinical diagnosis was given.
Neuropathologic assessments.
The postmortem diagnosis of PD was made based on previously reported neuropathologic criteria together with a clinical diagnosis of parkinsonism.16–19 This included subjects with a clinical diagnosis of ProbPD, PossPD, or parkinsonism with neuropathologic evidence of substantia nigra pigmented neuron loss and Lewy bodies. Gross and microscopic neuropathologic assessments were made by a single observer (T.B.) initially blinded to clinical history or clinical diagnosis, then able to review clinical information to make an appropriate clinical-neuropathologic diagnosis. Paraffin sections of the substantia nigra were stained immunohistochemically using a polyclonal antibody raised against an α-synuclein peptide fragment phosphorylated at serine 129, after epitope exposure with proteinase K, to identify Lewy bodies.17,20–23 Histologic evaluation of substantia nigra pigmented neuron loss was graded using hematoxylin & eosin–stained microscopic sections.17
Statistical analysis.
Diagnostic accuracy was assessed for the clinical diagnosis at the first visit and for the final clinical diagnosis. The sample included all subjects with ProbPD, PossPD, or other types of parkinsonism at the given time point. Positive predictive value (PPV) was the percentage of subjects with neuropathologically confirmed PD among those with the given clinical diagnosis. Negative predictive value (NPV) was the percentage of subjects without neuropathologically confirmed PD among those with other forms of parkinsonism. Sensitivity was the percentage of subjects with a clinical diagnosis of PD among those with neuropathologically confirmed PD, and specificity was the percentage of subjects without PD among those without neuropathologically confirmed PD. Mean UPSIT scores were compared between groups by using the 2-sample t test. UPSIT cutoff scores were chosen in order to maximize the Youden index (sensitivity + specificity − 1). Proportions were compared among groups by using the Pearson χ2 test. The Fisher exact test was used instead of the Pearson χ2 test if the minimum expected cell count was less than 5. The primary research question was to determine the diagnostic accuracy of a clinical diagnosis of PossPD or ProbPD using neuropathologic assessment as the reference standard, and the level of evidence was Class II.
RESULTS
Demographics.
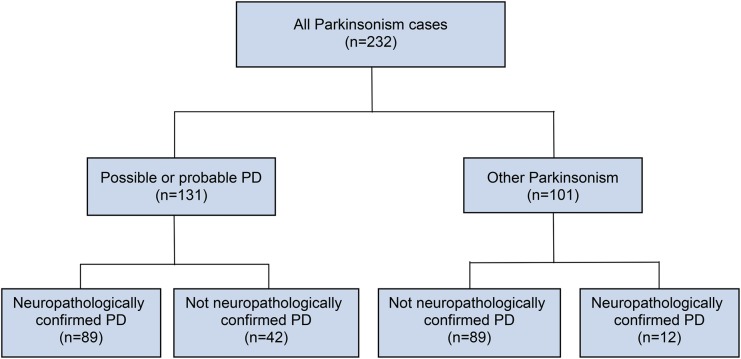
At the time of first visit, there were 232 cases of parkinsonism, 97 subjects had ProbPD, 34 PossPD, and 101 had other types of parkinsonism (ParkNOS, PSP, MSA) (figure). Age, sex, and disease duration at first visit and time of death are presented in table 1. When ProbPD was subdivided by disease duration, those with disease duration less than 5 years had an older age of disease onset (mean 76.0 years) than those with disease duration of at least 5 years (mean 64.0 years), although age at death was no different. The PossPD cases had shorter disease duration (mean 0.7 years) at first visit, but somewhat older age at onset (80.6 years) and age at death (87.5 years).
Figure. Flow diagram.
PD = Parkinson disease.
Table 1.
Demographics for subjects with parkinsonism followed to autopsy and percentage with a neuropathologically confirmed diagnosis of PD

Predictive value for a diagnosis of PossPD.
For the 34 subjects with PossPD (31 never treated and 3 with an inadequate treatment trial) at the first visit, only 9 had neuropathologically confirmed PD (PPV 26%, table 1). The sample size was too small to determine whether specific clinical signs improved diagnostic accuracy.
Predictive value for ProbPD.
For the 15 subjects with ProbPD who had disease duration of less than 5 years at first visit, only 8 had neuropathologically confirmed PD (PPV 53%, table 1). In subjects with ProbPD who had disease duration of at least 5 years, 72 of 82 (PPV 88%) had neuropathologically confirmed PD. Using a final clinical diagnosis of ProbPD at the time of death, the PPV was 84% (87/103) for disease duration of at least 5 years and 100% (4/4) for disease duration of less than 5 years.
Predictive value based on clinical signs.
Because response to dopaminergic medication is the key differentiating factor between ProbPD and PossPD, this was the key clinical finding that improved diagnostic accuracy (table 1). Rest tremor was not associated with autopsy confirmation of PD among subjects with either PossPD or ProbPD. Among subjects with PossPD, 6 of 28 subjects with rest tremor had PD vs 3 of 6 without rest tremor, while among subjects with ProbPD, 42 of 51 subjects with rest tremor had PD vs 35 of 43 without rest tremor. Because only 15 cases had ProbPD short duration, the comparison for rest tremor was not performed.
Requiring all 3 cardinal features (bradykinesia, rest tremor, and rigidity) at first visit, 5 of 12 (42%) PossPD cases had confirmed PD while 4 of 22 (18%) cases without all 3 signs had PD (p = 0.22). For ProbPD cases with 3 signs, 36 of 40 (90%) had PD while 41 of 54 (76%) without all 3 signs had PD (p = 0.08). Asymmetric onset also did not improve PPV (data not shown).
For the ProbPD group, the percentage with autopsy-confirmed PD differed (p = 0.006) if the subjects had motor fluctuations (47/51 [92%]) vs no motor fluctuations (31/44 [70%]) or dyskinesia (27/28 [96%] with and 50/66 [76%] without dyskinesia, p = 0.02). Because there were only 15 cases of ProbPD with disease duration less than 5 years, identifying key clinical factors that would increase diagnostic accuracy was not possible.
Olfactory testing and diagnostic accuracy.
Of the 16 PossPD cases tested at first visit, the mean UPSIT score for the 4 cases with autopsy-confirmed PD was 13.5, and the mean UPSIT score was 29.2 for the 12 who did not have PD (p < 0.001) (table 2). Using a cutoff score of 22, 3 of 4 (75%) PossPD cases with UPSIT <22 had PD and 1 of 12 (8%) of those with a score of ≥22 had PD (p = 0.03).
Table 2.
UPSIT scores for subjects who did and did not have neuropathologically confirmed PD

Of ProbPD cases with UPSIT testing at first visit, those with neuropathologically confirmed PD had significantly lower UPSIT scores (p < 0.001) (table 2). Using an UPSIT cutoff score of 20, 89% of the ProbPD cases with a score <20 had pathologically confirmed PD while 33% of cases with an UPSIT ≥20 had PD (p = 0.02). Data for ProbPD cases at the time of autopsy also revealed significant hyposmia in those with neuropathologically confirmed PD (table 2).
Subjects with ProbPD without neuropathologically confirmed PD.
Sixteen ProbPD cases at the time of death did not have neuropathologically confirmed PD (table 3). Mean age at symptom onset and death was higher than the neuropathologically confirmed PD group (tables 1 and 3). Seven had PSP with or without other neuropathologic findings, 6 had various neurodegenerative findings, and 3 had no clear neuropathologic findings to explain the parkinsonism, and they did not have drug-induced parkinsonism. One case of PSP had Lewy bodies but not in the substantia nigra.
Table 3.
Neuropathologic findings in subjects with ProbPD at death who did not have neuropathologic findings of PD at autopsy

Sensitivity and specificity: Clinical diagnosis in pathologically proven PD.
There were 106 subjects with a final clinical-neuropathologic diagnosis of PD (table 4). The sensitivity for the clinical diagnosis of ProbPD was 91 of 106 (86%). There were 5 cases clinically diagnosed with PSP who had a neuropathologic diagnosis of PD. Seven cases with a final clinical diagnosis of ParkNOS met neuropathologic criteria for PD. Some had ProbPD or PossPD at an earlier visit, but before their death, the clinical diagnosis was changed to ParkNOS by the examiner.
Table 4.
Demographics and clinical diagnoses for subjects with and without neuropathologically confirmed PD

The specificity of a final clinical diagnosis without ProbPD was 90% because only 16 of 157 subjects without neuropathologically confirmed PD had ProbPD (table 4).
NPV of a clinical diagnosis of other parkinsonism.
Of the 101 subjects with other types of parkinsonism at the first visit, 89 did not have neuropathologically confirmed PD (NPV 88%, table 1). At autopsy, 129 of 141 cases of other types of parkinsonism did not have neuropathologically confirmed PD (NPV 91%).
DISCUSSION
These data indicate that early in the course of a parkinsonian disorder, even if the subject is responsive to dopaminergic medication, the clinical diagnosis of PD may have relatively poor accuracy. For subjects who were never treated or possibly inadequately treated (PossPD), the PPV was very poor—only 26% at the time of first visit (mean symptom duration of 0.7 years). This is a critical finding given the number of studies attempting to find biomarkers or disease-modifying treatments in very early PD cases. These data improve on previously published clinical-neuropathologic correlation studies showing that appreciable numbers of subjects diagnosed with PD during life, especially those for whom signs and symptoms have been present for less than 5 years, do not have neuropathologically confirmed PD.3,4,24
Given the inaccuracy of the clinical diagnosis, these data are very sobering and have significant implications for studies that enroll subjects with early PD. The inaccuracy has the potential of severely compromising the likelihood of observing an adequate effect size in a trial. This inaccuracy was present despite that all cases were examined by a small group of movement disorder specialists as opposed to many neurologists and geriatricians who examined the cases in other studies.4 For subjects responsive to medication, ProbPD, longer disease duration improved diagnostic accuracy; disease duration ≥5 years had PPV of 88%. An unexpected finding was that the PPV was only 53% for ProbPD cases with <5 years' disease duration at first visit. A disease duration of >5 years was also found to be key to making the correct clinical diagnosis in an earlier study.3 In that study of 43 patients initially diagnosed with PD, only 28 (65%) had neuropathologically confirmed PD.3 After a mean follow-up period of 12 years, 41 still had a clinical diagnosis of PD at the final visit before death, but only 31 (76%) had PD pathologically.3
In a study of 100 cases,4 76 subjects with PD had neuropathologically confirmed PD. The study did not assess neuropathologic diagnosis in longitudinally followed subjects with early, untreated PD. Retrospective application of diagnostic criteria7 (presence of bradykinesia plus other factors including asymmetry, rest tremor, progression, response to levodopa, >5 years' response + dyskinesias, >10 years' disease course) improved the accuracy to 82% (73/89).25 The best predictors of pathologically proven PD were no atypical features of PD, an asymmetric onset, and no suggestion of a cause for another parkinsonian syndrome.25 Tremor-predominant disease had a 91% PPV, but it is critical to note that tremor was only present in 11 (14%) of their 76 cases, so that these investigators concluded that this may therefore have occurred by chance.25 The current data did not show improved PPV for ProbPD cases with rest tremor or with asymmetry at first visit. If all 3 cardinal signs were present, the PPV was 90% in the present study, and 88% to 92% in other studies.6,25 However, the current data did not show that having all 3 cardinal signs significantly differed from not having all of them.
The present data clearly showed that medication response improved PPV, as did the presence of motor fluctuations (92% PPV at first visit) or dyskinesia (96% PPV at first visit). That is not surprising because fluctuations and dyskinesia help to determine responsiveness to dopaminergic medication. However, in subjects responsive to dopaminergic medications but having disease duration <5 years, the PPV was only 53%. While sample size was small (n = 15), this finding supports the clinical and pathologic finding that subjects with other forms of parkinsonism may respond to dopaminergic medications early in the disease course.26
Hyposmia has been linked to PD,13,14 and while the study sample was small, this study demonstrates that low UPSIT scores significantly improve the PPV. The value of the UPSIT may be greatest in early disease duration cases, especially in cases with PossPD because 3 of 4 PossPD subjects with an UPSIT <22 had PD while only 1 of 12 with a score ≥22 had PD. As AZSAND, PARS,27 PPMI,28 and PRIPS29,30 continue, the issue of using hyposmia as an inclusion criterion for early PD studies will become clearer.
It was not surprising that many false-positive cases had a neuropathologic diagnosis of PSP or another neurodegenerative disorder. Most had a lack of response to dopaminergic medication or the loss of response to these medications while followed during life. In one study, 6 of 24 clinically diagnosed PD cases had PSP and 5 had MSA,4 and in a second study of 10 cases without pathologic PD, 6 had MSA and 2 had PSP.6 As for the false-negative rate of diagnostic criteria, previous studies found that approximately one-third of cases with pathologically confirmed PD were not clinically diagnosed as PD4,5,25,31 compared with the present study of only 10% for ProbPD.
One limitation of this study was the age of the PossPD cases. The mean age of disease onset for the ProbPD group was 67 years, and mean age at death was 80 years, suggesting the ProbPD cases are similar to those reported in other studies,32,33 including one discussed above25 (PD onset 64.5 years, age at death 76.5 years). However, the PossPD group had a mean age at onset of 80.6 years and a mean age at death of 87.5 years. Whether a diagnosis of PossPD in a younger cohort would have a similar poor PPV cannot be determined from AZSAND data. It is possible that some of these cases had bradykinesia related to other medical issues, such as arthritis (although the examining physician takes this into account), or that the research diagnosis of PossPD was too lenient, as suggested by the lower degree of certainty for having PD.
Thus, the diagnostic accuracy of a clinical diagnosis of PD (both PossPD and ProbPD) at first visit varies between 26% and 88%, with shorter duration of disease and subjects without a clear response to dopaminergic medication having markedly lower diagnostic accuracy. As clinical research studies attempt to find the earliest possible biomarkers for PD and early, disease-modifying treatments for PD, the low diagnostic accuracy at this stage needs to be addressed and will continue to be a critical impediment until autopsy- or biopsy-verified diagnostic biomarkers are developed.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to the subjects who have volunteered to participate in the Banner Sun Health Research Institute Brain and Body Donation Program. The authors thank Mr. Bruce Peterson for developing and maintaining the database, and other members of the Arizona Parkinson's Disease Consortium for helpful discussions, including Kathy Jo Davis, Dr. Padma Mahant, and Dr. Johan Samanta.
GLOSSARY
- AZSAND
Arizona Study of Aging and Neurodegenerative Disorders
- MSA
multiple system atrophy
- NPV
negative predictive value
- ParkNOS
parkinsonism not otherwise specified
- PD
Parkinson disease
- PossPD
possible PD
- PPV
positive predictive value
- ProbPD
probable PD
- PSP
progressive supranuclear palsy
- UPDRS
Unified Parkinson's Disease Rating Scale
- UPSIT
University of Pennsylvania Smell Identification Test
Footnotes
Editorial, page 386
AUTHOR CONTRIBUTIONS
C. Adler and J. Hentz wrote the first draft of the manuscript. All authors contributed to manuscript writing/revision. Authors involved in patient recruitment and clinical activities included C. Adler, H. Shill, J. Caviness, E. Driver-Dunckley, M. Sabbagh, S. Jacobson, and C. Belden, while authors involved in postmortem tissue analysis included L. Sue, B. Dugger, and T. Beach. Statistical analysis was performed by J. Hentz.
STUDY FUNDING
Supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson's Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer's Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer's Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901, and 1001 to the Arizona Parkinson's Disease Consortium), the Michael J. Fox Foundation for Parkinson's Research, and Mayo Clinic Foundation.
DISCLOSURE
C. Adler has received research funding from Phytopharm, Avid Radiopharmaceuticals, the Michael J. Fox Foundation, the NIH, US Department of Defense, and the Arizona Biomedical Research Foundation, and has received consulting fees from Impax, Ipsen, Merz, Novartis, Teva, and Xenoport. T. Beach has received research funding from the Avid Radiopharmaceuticals/Eli Lilly Corporation, Bayer Healthcare, GE Healthcare, Navidea Healthcare, the Michael J. Fox Foundation, the NIH, and the Arizona Biomedical Research Foundation. J. Hentz has received research funding from Eisai, CaridianBCT, the Michael J. Fox Foundation, the NIH, and the Arizona Biomedical Research Foundation. H. Shill has received research support from Schering-Plough/Merck, Avid Radiopharmaceuticals, UCB Biosciences, Adamas Pharmaceuticals, the International Essential Tremor Foundation, the Michael J. Fox Foundation, the NIH, US Department of Defense, and the Arizona Biomedical Research Foundation, and has received consulting fees from US World Meds. J. Caviness has received research funding from Amarin Pharmaceuticals, the Michael J. Fox Foundation, the NIH, and the Arizona Biomedical Research Foundation, and received consulting fees from Teva. E. Driver-Dunckley has received research funding from Ipsen, EMD Serono, Chelsea Therapeutics, Allon, Allergan, the Michael J. Fox Foundation, the NIH, and the Arizona Biomedical Research Foundation, and received consulting fees from Merz. M. Sabbagh has received research funding from Pfizer, Eisai, Neuronix, Lilly, Avid, Piramal, GE, Avanir, Elan, Functional Neuromodulation, Takeda, Merck, the Michael J. Fox Foundation, the NIH, and the Arizona Biomedical Research Foundation, and has received consulting fees from Biogen, Lilly, Piramal, Eisai, and royalties from Wiley and Ten Speed Press (Random House). L. Sue has received research funding from the Michael J. Fox Foundation, the NIH, and the Arizona Biomedical Research Foundation. S. Jacobson has received research funding from the Michael J. Fox Foundation, the NIH, and the Arizona Biomedical Research Foundation, and has received royalties from American Psychiatric Publishing, Inc. C. Belden has received research funding from Quintiles, Genentech, Pfizer-Wyeth, Avid, Elan, Eli Lilly, Avanir, DART Neurosciences, Neuronix, and Takeda/Zinfandel, International Essential Tremor Foundation, the Michael J. Fox Foundation, the NIH, and the Arizona Biomedical Research Foundation. B. Dugger has received research funding from the Michael J. Fox Foundation, the NIH, the Arizona Biomedical Research Foundation, and Avid Radiopharmaceuticals. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Adler CH. Differential diagnosis of Parkinson's disease. Med Clin North Am 1999;83:349–367 [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Rieves D, Ganley C. Brain amyloid imaging: FDA approval of florbetapir F18 injection. N Engl J Med 2012;367:885–887 [DOI] [PubMed] [Google Scholar]
- 3.Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism: a prospective study. Can J Neurol Sci 1991;18:275–278 [DOI] [PubMed] [Google Scholar]
- 4.Hughes AJ, Daniel SE, Kilford L, Lees AJ. The accuracy of the clinical diagnosis of Parkinson's disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol 1993;50:140–148 [DOI] [PubMed] [Google Scholar]
- 6.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology 2001;57:1497–1499 [DOI] [PubMed] [Google Scholar]
- 7.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988;51:745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jellinger KA. Morphological substrates of parkinsonism with and without dementia: a retrospective clinico-pathological study. J Neural Transm Suppl 2007:91–104 [DOI] [PubMed] [Google Scholar]
- 9.Brooks DJ. Parkinson's disease: diagnosis. Parkinsonism Relat Disord 2012;18(suppl 1):S31–S33 [DOI] [PubMed] [Google Scholar]
- 10.Adler CH, Hentz JG, Shill HA, et al. Probable RBD is increased in Parkinson's disease but not in essential tremor or restless legs syndrome. Parkinsonism Relat Disord 2011;17:456–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler CH, Hentz JG, Joyce JN, Beach T, Caviness JN. Motor impairment in normal aging, clinically possible Parkinson's disease, and clinically probable Parkinson's disease: longitudinal evaluation of a cohort of prospective brain donors. Parkinsonism Relat Disord 2002;9:103–110 [DOI] [PubMed] [Google Scholar]
- 12.Fahn S, Elton RL; Members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne CD, editors. Recent Developments in Parkinson's Disease, Volume II. Florham Park, NJ: Macmillan; 1987:153–163 [Google Scholar]
- 13.Stern MB, Doty RL, Dotti M, et al. Olfactory function in Parkinson's disease subtypes. Neurology 1994;44:266–268 [DOI] [PubMed] [Google Scholar]
- 14.McKinnon J, Evidente V, Driver-Dunckley E, et al. Olfaction in the elderly: a cross-sectional analysis comparing Parkinson's disease with controls and other disorders. Int J Neurosci 2010;120:36–39 [DOI] [PubMed] [Google Scholar]
- 15.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology 1996;47:1–9 [DOI] [PubMed] [Google Scholar]
- 16.Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank 2008;9:229–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 2009;117:613–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol 2009;8:1150–1157 [DOI] [PubMed] [Google Scholar]
- 19.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39 [DOI] [PubMed] [Google Scholar]
- 20.Beach TG, Adler CH, Dugger BN, et al. Submandibular gland biopsy for the diagnosis of Parkinson's disease. J Neuropathol Exp Neurol 2013;72:130–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 2010;119:689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beach TG, White CL, Hamilton RL, et al. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol 2008;116:277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker DG, Lue LF, Adler CH, et al. Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol 2013;240:190–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uitti RJ, Calne DB, Dickson DW, Wszolek ZK. Is the neuropathological “gold standard” diagnosis dead? Implications of clinicopathological findings in an autosomal dominant neurodegenerative disorder. Parkinsonism Relat Disord 2004;10:461–463 [DOI] [PubMed] [Google Scholar]
- 25.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology 1992;42:1142–1146 [DOI] [PubMed] [Google Scholar]
- 26.Rajput AH, Rozdilsky B, Rajput A, Ang L. Levodopa efficacy and pathological basis of Parkinson syndrome. Clin Neuropharmacol 1990;13:553–558 [DOI] [PubMed] [Google Scholar]
- 27.Siderowf A, Jennings D, Eberly S, et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov Disord 2012;27:406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marek K, Jennings D, Lasch S, et al. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011;95:629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg D, Godau J, Seppi K, et al. The PRIPS Study: screening battery for subjects at risk for Parkinson's disease. Eur J Neurol 2013;20:102–108 [DOI] [PubMed] [Google Scholar]
- 30.Berg D, Marek K, Ross GW, Poewe W. Defining at-risk populations for Parkinson's disease: lessons from ongoing studies. Mov Disord 2012;27:656–665 [DOI] [PubMed] [Google Scholar]
- 31.Hughes AJ, Daniel SE, Lees AJ. The clinical features of Parkinson's disease in 100 histologically proven cases. Adv Neurol 1993;60:595–599 [PubMed] [Google Scholar]
- 32.Abbott RD, Ross GW, White LR, et al. Environmental, life-style, and physical precursors of clinical Parkinson's disease: recent findings from the Honolulu-Asia Aging Study. J Neurol 2003;250(suppl 3):III30– III–39. [DOI] [PubMed] [Google Scholar]
- 33.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest Study. Neurology 2009;72:1121–1126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.