Abstract
Objective:
To determine whether asymptomatic persons with Alzheimer disease (AD) neuropathologic change differ in the trajectory of their cognitive performance compared to asymptomatic persons without AD neuropathologic change.
Methods:
Longitudinal performance on standard neuropsychological tests was examined in participants who died within 2 years of their last cognitive assessment and who were never diagnosed with mild cognitive impairment or dementia (Clinical Dementia Rating global score of 0 at all assessments). Using cognitive and neuropathologic data collected between 2005 and 2013 from the 34 National Institute on Aging–sponsored Alzheimer's Disease Centers, cognitive trajectories were compared for persons with and without evidence of AD neuropathologic change. We evaluated rates of decline in 4 domains (episodic memory, language, attention/working memory, executive function). The significance of the differences (β) in rates of decline was tested using linear regression, adjusting for age, education, sex, and other neuropathologic lesions.
Results:
Participants who had low to high levels of AD neuropathologic change (n = 131) showed a greater rate of decline on the attention/working memory domain score (β = −0.11; 95% confidence interval = −0.19, −0.02; p = 0.02) when compared to 80 participants who died without evidence of AD neuropathologic change.
Conclusions:
Clinically normal individuals who come to autopsy with AD neuropathologic change exhibit subtle evidence of declining cognitive trajectories for attention/working memory.
Neuropathologic and biomarker changes of Alzheimer disease (AD) begin decades before the onset of symptoms.1–3 Less is known about subtle cognitive changes that may occur before a person develops noticeable symptoms. Better understanding such changes would help to define the cognitive consequences of biological changes occurring in AD. One or more neuropsychological features that frequently deteriorate prior to onset of noticeable symptoms might be promising for eventual use in selecting subjects for clinical trials.
Recent changes in the neuropathologic definition of AD offer the opportunity to look more closely at persons with AD neuropathologic change who died and came to autopsy while still asymptomatic. Older classification schemes such as the National Institute on Aging (NIA)-Reagan Institute criteria required the presence of clinical symptoms in addition to AD neuropathologic change to assign a diagnosis of AD.4 Hence, neuropathologic changes of AD in persons with preclinical stages of AD may routinely have been labeled as “age-related” or as having histologic findings that “suggest” diagnosis of AD.5 The most recent standard for neuropathologic assessment of AD is the NIA-Alzheimer's Association (AA) guidelines,6,7 which address only underlying AD neuropathologic change, regardless of the person's symptoms.
We sought to use this opportunity to determine whether asymptomatic persons with AD neuropathologic change differ in their cognitive status compared to asymptomatic persons without AD neuropathologic change. We also sought to determine the trajectory of these potential changes over time.
METHODS
Study sample.
Data for this analysis were obtained from the National Alzheimer's Coordinating Center Uniform Data Set (NACC UDS)8 and the Neuropathology Data Set (NPDS). The analytic sample included data entered between September 2005 and June 2013 from 34 current and past Alzheimer's Disease Centers (ADCs). The UDS records information on demographics and clinical characteristics for patients with and without dementia.9 Standard collected information includes health history, functional and cognitive impairment history, neuropsychological testing, and neurologic examination. Assessments are made approximately annually. A subset of UDS subjects also consent to autopsy, in which case their neuropathologic features are recorded in the NPDS. For these subjects, data in UDS and NPDS are linked.
The analytic sample comprised subjects with normal cognition at their last ADC visit who died and went to autopsy. Subjects who died more than 2 years after their last UDS clinical assessment were excluded. Normal cognition was defined using the Clinical Dementia Rating (CDR) global score, an instrument that grades subjects' cognitive and functional abilities. Subjects with a CDR global score of 0 at their last clinical assessment were considered to have normal cognition and thus to be “asymptomatic.”10 Subjects with CDR scores of 0.5 or higher were considered to exhibit clinical characteristics consistent with mild cognitive impairment (MCI) or dementia and were excluded.
Defining neuropathologic AD.
Subjects meeting study inclusion criteria were then characterized as having neuropathologic AD (AD-NP) or non-AD-NP. The definition of AD-NP was based on a modification of the NIA-AA criteria for neuropathologic AD “ABC score.”6,7 Braak stage (B score) for neurofibrillary tangles11 and CERAD (Consortium to Establish a Registry for Alzheimer's Disease) neuritic plaque frequency5 (C score) were recorded in the NPDS; however, at the time of data analysis, a Thal phase for β-amyloid (Aβ) plaques12 (A score) was not recorded in the NPDS. In order to capture the most frequent plaque type, we included “diffuse plaque,” which is most likely an early form of Aβ plaque formation and is defined as plaques with no apparent dystrophic neurites, as detected by silver impregnation methods, ubiquitin, or tau immunohistochemistry (IHC). All types of Aβ plaques, including diffuse plaques, are also readily identified using Aβ IHC.
Subjects with sparse, moderate, or frequent diffuse plaques were considered to have a Thal Aβ plaque phase of 1 or higher and met AD-NP inclusion criteria for this study. Likewise, subjects with sparse, moderate, or frequent neuritic plaques had a neuritic plaque C score of 1 or higher and also met study inclusion criteria. Limiting the sample to subjects with either diffuse or neuritic plaques is similar to including all subjects meeting NIA-AA criteria for low to high AD neuropathologic change. The resulting study sample included only those subjects with amyloid plaques, excluding those without, regardless of Braak stage and clinical diagnosis.13
The remaining subjects not meeting criteria for AD-NP constituted the comparison group of non-AD-NP. No additional exclusion or inclusion criteria were applied to this group.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from all participants. Research using the NACC database was approved by the University of Washington Institutional Review Board.
Measures of decline.
Cognitive decline was measured using the UDS neuropsychological battery.14 The battery includes the Wechsler Memory Scale-Revised Logical Memory IA Story Units Recalled and Logical Memory IIA-Delayed Story Units Recalled, which are administered to test subjects' story retention ability.15 The Boston Naming Test presents subjects with drawings of objects and asks subjects to name the objects.16 Animal and vegetable naming tests require subjects to generate lists of words in a given category.17 The Wechsler Adult Intelligence Scale-Revised Digit Symbol requires subjects to use a symbol-to-number legend,18 while Digit Span Forward and Backward tests ask subjects to repeat a series of numbers.15 Finally, Trail Making Tests A and B assess visuomotor and perceptual scanning skills by recording the time it takes a subject to connect the lines between a set of numbers or letters and numbers from smallest to largest.19
The groupings of neuropsychological tests by cognitive domain were based on findings published by Hayden et al., who performed a factor analysis of the UDS battery resulting in 4 distinct cognitive domains. The episodic memory domain is measured by the 2 Logical Memory tests, the language domain by the Boston Naming Test and the object naming tests, attention/working memory by the Digit Span tests, and executive function by the Trail Making tests and Digit Symbol test.20
At every available visit, each test within a domain was converted to a z score by subtracting the mean and dividing by the SD of all UDS initial-visit scores among cognitively normal subjects defined as having a CDR global score of 0. The z scores for the tests within each domain were then averaged to obtain a standardized domain-specific composite score. Finally, the domains were averaged to create a global composite score. Domains missing data on at least one test were considered missing. Subjects missing the entire neuropsychological battery at all visits were excluded.
Statistical analysis.
Rate of cognitive decline was estimated using linear regression with generalized estimating equations, which allowed us to account for the nonindependence of visits for the same subject, as well as clustering of subjects within an ADC.21 Time was measured as years since initial visit. Each of the 4 cognitive domains, as well as the global composite, was an outcome measure (dependent variable) in a separate regression model, resulting in 5 separate models.
Average rate of decline for the AD-NP subjects was compared to average rate of decline in the non-AD-NP subjects using a Wald test. Two adjusted models were run for each outcome measure. The first model was adjusted for age at initial visit, education, and sex. We did not adjust for race in the model because nonwhite race was so uncommon for both groups. The second model was adjusted for the same characteristics as well as the presence of ischemic, hemorrhagic, or vascular pathology and presence of Lewy body pathology. Because some tests, specifically Logical Memory, often see score improvement due to practice effects, we attempted to adjust for this by including an indicator variable in the model for initial visit vs follow-up visit; however, this variable was not statistically significant in any of the models and was dropped. All regression models were run with an independent correlation structure and robust standard errors in R 2.14.2 using the “geeglm” package.
RESULTS
At the time of data abstraction, there were 2,509 UDS subjects with neuropathologic data. Limiting the sample to those who died within 2 years of their last UDS visit reduced the sample to 2,033 subjects. Applying the inclusion criteria of a CDR global score of 0 at the most recent visit resulted in 225 subjects, 211 of whom had at least some neuropsychological data. Of these, 131 (62%) met AD-NP criteria and 80 (38%) did not.
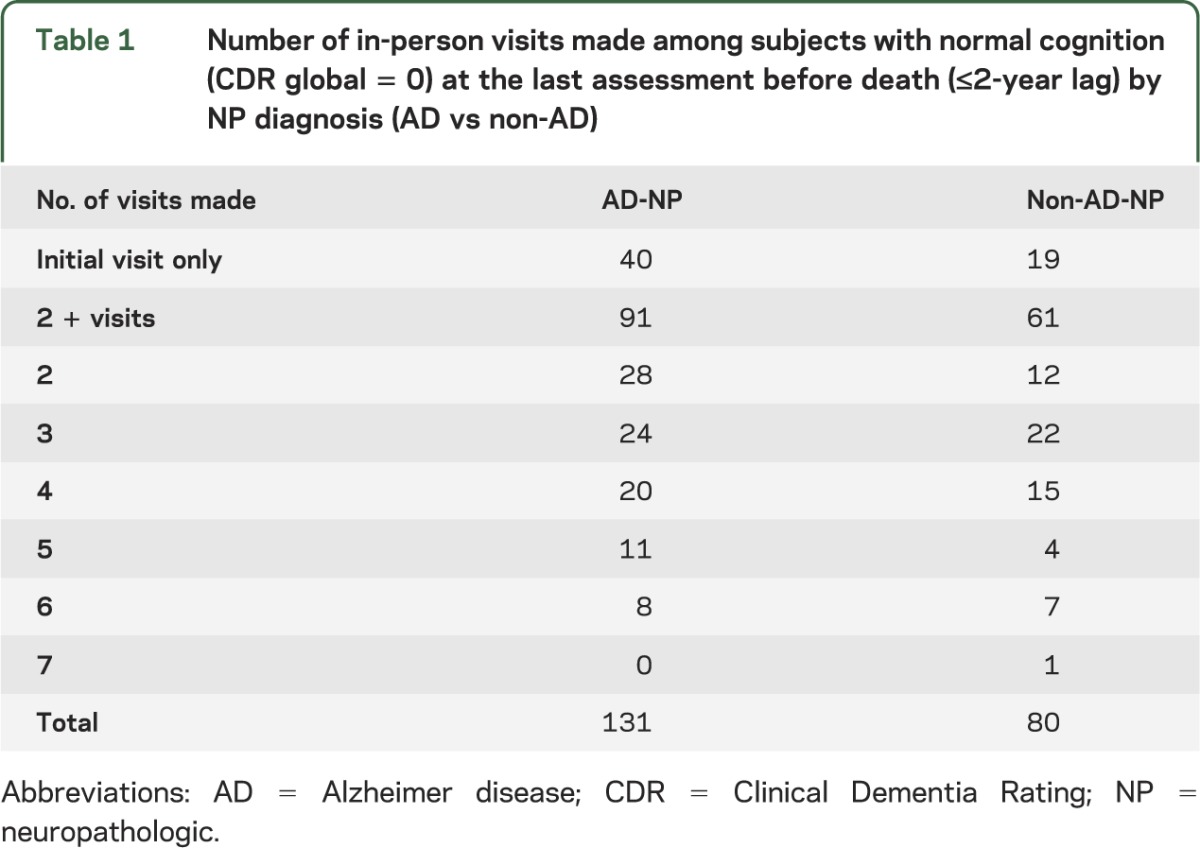
As shown in table 1, most subjects contributed at least 2 visits (72%). Subjects making only one visit before death were still included in the model because their data contributed to estimating mean cognition at baseline.
Table 1.
Number of in-person visits made among subjects with normal cognition (CDR global = 0) at the last assessment before death (≤2-year lag) by NP diagnosis (AD vs non-AD)
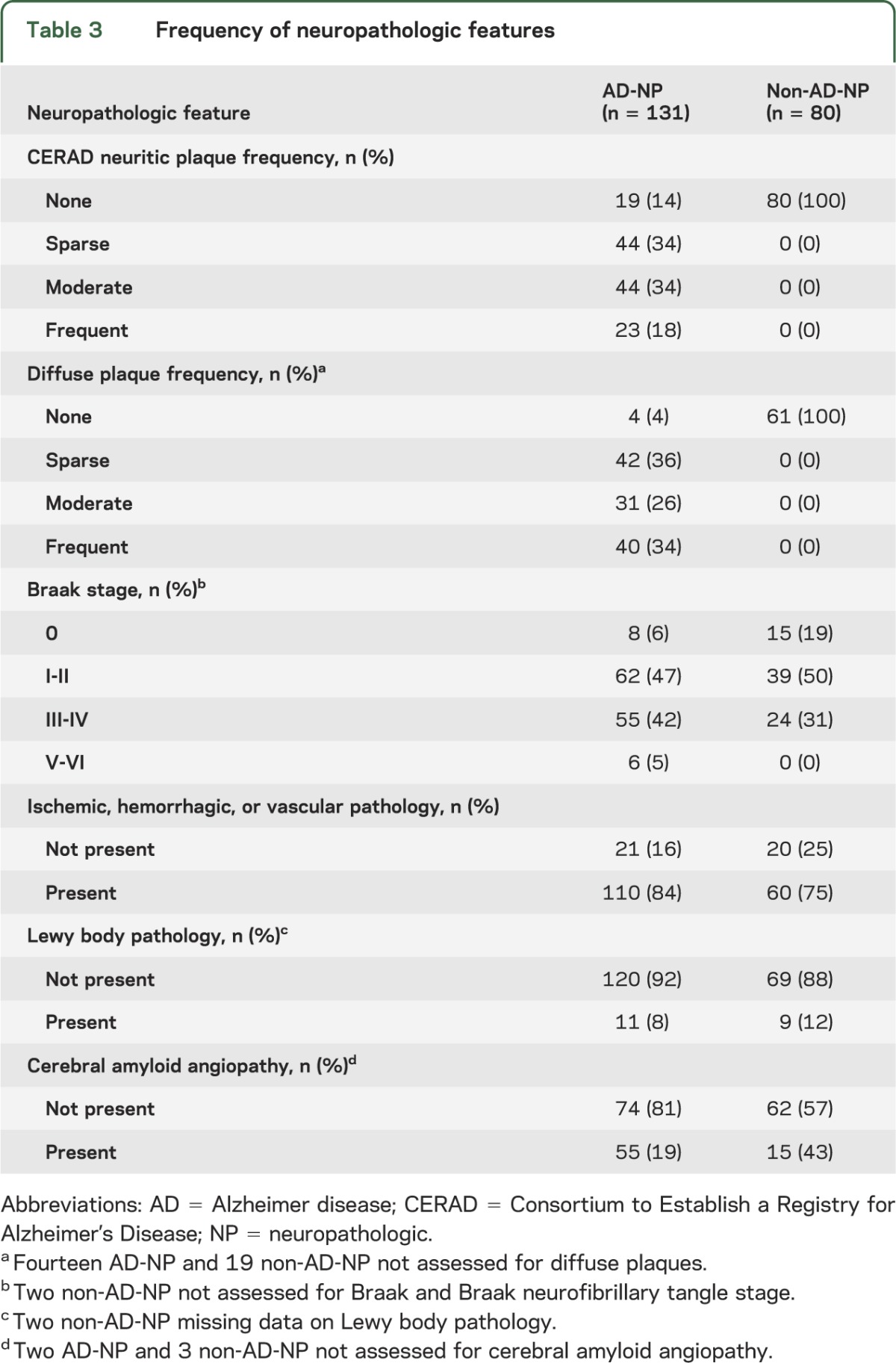
Demographic and neuropathologic characteristics of the 2 groups are described in tables 2 and 3. NP-AD and non-NP-AD subjects were similar in terms of age, education, and sex. However, AD-NP subjects had slightly more ischemic, hemorrhagic, or vascular pathology, whereas non-AD-NP subjects had slightly more Lewy body pathology.
Table 2.
Frequency of subject characteristics

Table 3.
Frequency of neuropathologic features
Average rates of cognitive decline in the various domains for each group were estimated from the regression models (table 4). Rates of decline are interpreted as the average annual change in score, where the score is measured in units of SDs from the baseline average. For example, subjects with AD-NP had a global cognitive decline of 0.02 SDs per year, on average.
Table 4.
Annual mean change in subjects with normal cognition (CDR = 0) at last visit prior to death by AD-NP status using 4 cognitive domains and a global composite score adjusted for underlying non-AD pathologic features
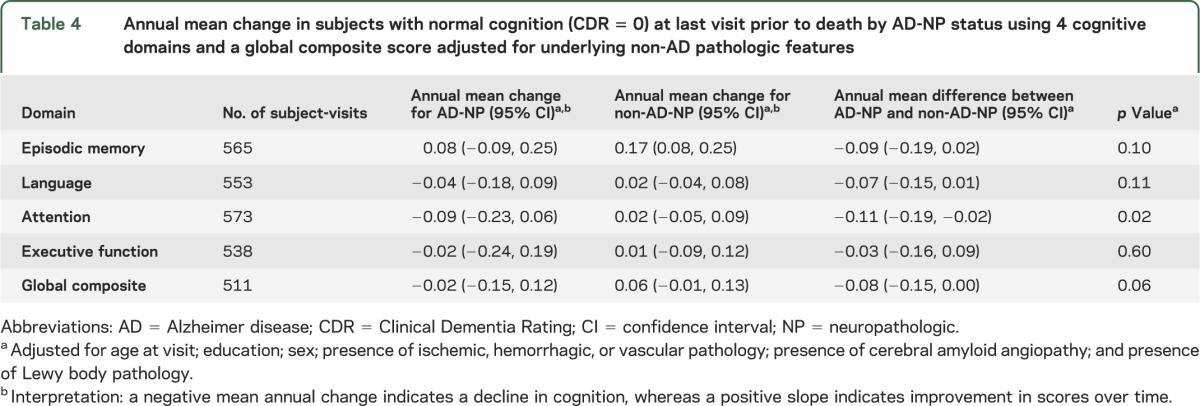
When looked at by themselves, changes over time in each group were not statistically significant from zero (except for an increase in score for episodic memory for the non-AD-NP group). This increase may reflect a practice effect, which can be pronounced on episodic memory measures.22 Comparisons between the 2 groups were more telling. Compared with the group without AD-NP changes, the AD-NP group showed a statistically significant decrease in attention/working memory scores over time.
Models not adjusted for neuropathology resulted in nearly identical estimates (table e-1 on the Neurology® Web site at Neurology.org). The mean scores for each domain are also presented for the first and last visits (tables e-2 and e-3). On the first assessment, there were no significant differences between the AD-NP and non-AD-NP groups. By the time of the last visit, the 2 groups differed significantly in attention/working memory and in the global composite score. These data are presented as ancillary, as they utilize data from first and last visits but not data from middle visits for persons having more than 2 visits. The slope data presented in table 4 are thus more robust, as they utilize data from all visits. Nonetheless, the static data corroborate the slope data, emphasizing attention/working memory as a key difference between the AD-NP and non-AD-NP groups. Estimates of slopes of individual tests are shown in table e-4. We also evaluated the effect of using a more narrow AD neuropathologic definition: at least moderate neuritic plaques and Braak stage ≥III. Sample size for the AD-NP group decreased to 31. Effect sizes were similar to those in table 4, but no comparisons were significant (table e-5).
DISCUSSION
We sought to determine whether persons with AD neuropathologic change but without symptoms of MCI or dementia have subtle cognitive changes detectable only by neuropsychological test performance, especially longitudinal performance. We found that, compared with persons without AD neuropathologic change, persons with AD neuropathologic change showed decline in the attention/working memory domain score. It must be emphasized that this decline was subtle and modest.
Declines in episodic memory have traditionally been viewed as the hallmark of cognitive change in early AD; however, recent studies have documented declines in attention/working memory before onset of AD symptoms.23–26 Subtle cognitive changes in the preclinical stage of AD may be more readily determined by changes over time compared to the person's baseline rather than differences compared to population norms.27,28
In terms of methodology used by studies investigating neuropsychological changes in preclinical AD, one study reviewed 73 articles. The vast majority of these studies used neuroimaging to determine AD status or were case-control studies comparing people who eventually developed AD symptoms with those who did not.26 In this review, only 3 studies used autopsy findings, each of which had fewer than 10 AD cases.29–31
Several newer studies have used autopsy-verified AD to evaluate neuropsychological changes in preclinical AD, with mixed findings. One study compared 21 asymptomatic persons with AD-NP and 27 without, with no differences in cognitive trajectories.32 A second study evaluated 97 asymptomatic persons at 7 ADCs; 40% met some level of criteria for AD-NP. Neuropsychological test scores were lower with more advanced pathology.33 A third studied 32 asymptomatic persons with AD-NP and 89 without. They found no cross-sectional differences between the groups at baseline or last evaluation; however, there were differences in slope of decline for several tests (word list delayed recall, verbal fluency, constructional praxis).28 A fourth studied 296 asymptomatic persons. Seventy-six percent had Aβ deposits. Increased measures of AD-NP change were associated with lower episodic and working memory scores.34
The current study adds to the literature by being one of the few studies on neuropsychological changes to use autopsy findings determined independently of the subject's cognitive status and by being the largest of only 3 studies reporting longitudinal cognitive data on persons with autopsy-proven AD-NP.28,32 The cases studied likely include more cases of very early preclinical AD than other studies, especially studies that utilized biomarker or imaging data. We cannot be sure how many in the AD-NP group would have been positive for in vivo biomarkers or neuroimaging. This group of people who had AD-NP changes but who are clinically normal may have a different cognitive profile from those who are biomarker positive and clinically normal.
A more narrow definition of AD neuropathology resulted in loss of significance of the difference between the 2 groups (table e-5), perhaps because of smaller sample size. Another potential contributing factor may be elimination of diffuse plaques from the analysis. Diffuse plaques have been shown to have a deleterious cognitive effect.33
Understanding preclinical AD is especially important for future clinical trials. Disease-modifying therapies for AD will likely be more useful in the preclinical AD stage than later.3,27,35,36 Biomarkers are currently the main criteria that would be used to identify preclinical AD; however, changes in neuropsychological tests have been posited as a means to identify people in the later stage of preclinical AD (i.e., people with both amyloid deposition and evidence of neurodegeneration on biomarkers, as well as subtle cognitive changes).37 It should be noted that such neuropsychological changes occur after neuronal degeneration, rather than after amyloid deposits alone. Increasing the enrollment of these subjects in clinical trials would likely enrich the study population with subjects who would be more likely to progress to symptomatic AD, which would decrease the sample size and/or length of follow-up needed in trials.36,38
The current study has suggested measures of attention/working memory as prime candidates for future development for this role. The neuropsychological battery used by UDS was primarily designed for evaluation of people with symptoms, and it is likely to be less useful for discrimination of people at the high end of function (ceiling effect). Thus, it must be stressed that the current study has not verified these specific tests (Digit Span Forward and Backward) for roles in clinical trials, but rather has identified this domain as potentially fruitful for identifying groups of people likely to be in later preclinical AD.
The study's limitations must be addressed. First, retrospectively fitting UDS neuropathology data to the NIA-AA criteria has shortcomings and we were not able to derive a Thal phase. Persons earlier in the study period might have undergone less sensitive autopsy techniques that would have missed the presence of Aβ deposits. This might have led to underassessment of cases that would have met NIA-AA criteria if they had been fully assessed. This would tend to bias detection of differences between the 2 groups towards the null, as persons falsely classified as non-AD-NP would be more likely to have subtle neuropsychological changes, unlike correctly classified members of that group.
Second, there are possible biases in the nature of UDS subjects. More highly educated subjects are more likely to volunteer as normal controls and consent to autopsy. There were few nonwhite subjects in either the AD-NP or non-AD-NP groups. These recruitment issues limit the generalizability of the study. Also, although ADCs contribute data using the same standardized protocols, there is some variation by center as to how data are gathered and recorded.
Third, there were some missing neuropsychological test data; however, the frequency of missing data was ≤8% across all visits for all domain-specific scores, and the frequency of missing data for the global composite was 10%. Missing data percentages were similar among AD-NP and non-AD-NP groups. All subjects failed to meet criteria for cognitive impairment, supporting the assumption that missing test data were not missing due to cognitive impairment and thus did not bias our findings.
Fourth, the cognitive tests in the UDS battery are older measures. More sensitive tests now available, such as the Free and Cued Selective Reminding Test, may have yielded significant effects for episodic memory. Thus, firm conclusions regarding the absence of changes in episodic memory are not possible.
Fifth, the constructs of attention and working memory are broad and can be subdivided into multiple components that often overlap with the construct of executive function. Digit Span Forward and Backward have shown validity as measures of attention/working memory20,39,40 but cannot be assumed to represent all aspects of attention/working memory such as cognitive control, switching, or response inhibition. Future studies incorporating more comprehensive assessment of attention are needed.
Sixth, 79% of subjects were aged 80 years or older. Findings regarding the nature of preclinical AD may not be generalizable to younger patients with AD.
Despite these limitations, this study has a major strength in that it allows us to evaluate neuropsychological changes in a group of persons who had the same standardized tests administered over multiple visits and who then underwent autopsy confirming the presence or absence of AD neuropathologic changes. Thus, these data allow us to draw reasonable inferences regarding subtle neuropsychological changes that people with underlying AD-NP manifest while they are still otherwise asymptomatic.
This study has shown that attention/working memory is a potential priority for development of more in-depth neuropsychological tests to identify people in the later stages of the preclinical phase of AD.
Supplementary Material
GLOSSARY
- AA
Alzheimer's Association
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADC
Alzheimer's Disease Center
- CDR
Clinical Dementia Rating
- IHC
immunohistochemistry
- MCI
mild cognitive impairment
- NACC
National Alzheimer's Coordinating Center
- NIA
National Institute on Aging
- NP
neuropathologic
- NPDS
Neuropathology Data Set
- UDS
Uniform Data Set
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
S.E. Monsell contributed to study concept and design, statistical analysis, interpretation of data, and drafting the manuscript. C. Mock contributed to the study concept and design, interpretation of data, and drafting the manuscript. J. Hassenstab contributed to the study concept and design, interpretation of data, and editing of the manuscript for content. C.M. Roe contributed to the study concept and design, interpretation of data, and editing of the manuscript for content. N.J. Cairns contributed to the study concept and design and editing of the manuscript for content. J.C. Morris contributed to the study concept and design and editing of the manuscript for content. W. Kukull contributed to the study concept and design and editing of the manuscript for content.
STUDY FUNDING
The NACC database is supported by NIA grant UO1 AG016976.
DISCLOSURE
S. Monsell, C. Mock, J. Hassanstab, C. Roe, and N. Cairns report no disclosures relevant to the manuscript. Neither Dr. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. Dr. Morris has participated or is currently participating in clinical trials of antidementia drugs sponsored by the following companies: Janssen Immunotherapy and Pfizer. Dr. Morris has served as a consultant for Lilly USA. He receives research support from Eli Lilly/Avid Radiopharmaceuticals and is funded by NIH grants #P50AG005681; P01AG003991; P01AG026276; and U19AG032438. W. Kukull reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med 2012;367:795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Wiste HJ, Weigand SD, et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 2013;18:1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIA and Reagan Institute Working Group. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging 1997;18:S1–S2 [PubMed] [Google Scholar]
- 5.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991;41:479–486 [DOI] [PubMed] [Google Scholar]
- 6.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–216 [DOI] [PubMed] [Google Scholar]
- 9.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord 2007;21:249–258 [DOI] [PubMed] [Google Scholar]
- 10.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–1800 [DOI] [PubMed] [Google Scholar]
- 13.Monsell SE, Mock C, Roe CM, et al. Comparison of symptomatic and asymptomatic persons with Alzheimer disease neuropathology. Neurology 2013;80:2121–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): The Neuropsychological Test Battery. Alzheimer Dis Assoc Disord 2009;23:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wechsler D. WMS-R: Wechsler Memory Scale–Revised Manual. Cleveland, OH: Psychological Corp., Harcourt Brace Jovanovich; 1987 [Google Scholar]
- 16.Kaplan E. Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983 [Google Scholar]
- 17.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989;39:1159–1165 [DOI] [PubMed] [Google Scholar]
- 18.Wechsler D. WAIS-R: Manual: Wechsler Adult Intelligence Scale–Revised. Cleveland, OH: Harcourt Brace Jovanovich [for] Psychological Corp; 1981 [Google Scholar]
- 19.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 2nd ed. Tucson, AZ: Neuropsychology Press; 1993 [Google Scholar]
- 20.Hayden KM, Jones RN, Zimmer C, et al. Factor structure of the National Alzheimer's Coordinating Centers uniform dataset neuropsychological battery: an evaluation of invariance between and within groups over time. Alzheimer Dis Assoc Disord 2011;25:128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22 [Google Scholar]
- 22.Dodge HH, Wang C-N, Chang C-CH, Ganguli M. Terminal decline and practice effects in older adults without dementia: the MoVIES project. Neurology 2011;77:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balota DA, Tse C-S, Hutchison KA, Spieler DH, Duchek JM, Morris JC. Predicting conversion to dementia of the Alzheimer's type in a healthy control sample: the power of errors in Stroop color naming. Psychol Aging 2010;25:208–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tse C-S, Balota DA, Moynan SC, Duchek JM, Jacoby LL. The utility of placing recollection in opposition to familiarity in early discrimination of healthy aging and very mild dementia of the Alzheimer's type. Neuropsychology 2010;24:49–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol 2009;66:1476–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twamley EW, Ropacki SAL, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. J Int Neuropsychol Soc JINS 2006;12:707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knopman DS, Caselli RJ. Appraisal of cognition in preclinical Alzheimer's disease: a conceptual review. Neurodegener Dis Manag 2012;2:183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley KP, Jicha GA, Davis D, et al. Prediction of preclinical Alzheimer's disease: longitudinal rates of change in cognition. J Alzheimers Dis JAD 2011;25:707–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman WP, Price JL, Storandt M, et al. Absence of cognitive impairment or decline in preclinical Alzheimer's disease. Neurology 2001;56:361–367 [DOI] [PubMed] [Google Scholar]
- 30.Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol 1998;57:1168–1174 [DOI] [PubMed] [Google Scholar]
- 31.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology 2000;55:370–376 [DOI] [PubMed] [Google Scholar]
- 32.Driscoll I, Resnick SM, Troncoso JC, An Y, O'Brien R, Zonderman AB. Impact of Alzheimer's pathology on cognitive trajectories in nondemented elderly. Ann Neurol 2006;60:688–695 [DOI] [PubMed] [Google Scholar]
- 33.Price JL, McKeel DW, Jr, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 2009;30:1026–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol 2012;72:599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 2012;78:1576–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vellas B, Aisen PS, Sampaio C, et al. Prevention trials in Alzheimer's disease: an EU-US task force report. Prog Neurobiol 2011;95:594–600 [DOI] [PubMed] [Google Scholar]
- 37.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol 2013;12:957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grill JD, Monsell SE. Choosing Alzheimer's disease prevention clinical trial populations. Neurobiol Aging 2014;35:466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessels RPC, van den Berg E, Ruis C, Brands AMA. The backward span of the Corsi Block-Tapping Task and its association with the WAIS-III Digit Span. Assessment 2008;15:426–434 [DOI] [PubMed] [Google Scholar]
- 40.Tulsky DS, Price LR. The joint WAIS-III and WMS-III factor structure: development and cross-validation of a six-factor model of cognitive functioning. Psychol Assess 2003;15:149–162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


