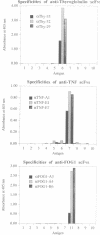
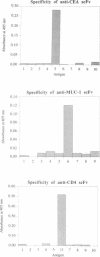
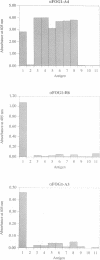
Abstract
Recently we demonstrated that human antibody fragments with binding activities against foreign antigens can be isolated from repertoires of rearranged V-genes derived from the mRNA of peripheral blood lymphocytes (PBLs) from unimmunized humans. The heavy and light chain V-genes were shuffled at random and cloned for display as single-chain Fv (scFv) fragments on the surface of filamentous phage, and the fragments selected by binding of the phage to antigen. Here we show that from the same phage library we can make scFv fragments encoded by both unmutated and mutated V-genes, with high specificities of binding to human self-antigens. Several of the affinity purified scFv fragments were shown to be a mixture of monomers and dimers in solution by FPLC gel filtration and the binding kinetics of the dimers were determined using surface plasmon resonance (k(on) = 10(5)-10(6) M-1s-1, k(off) = 10(-2)s-1 and Ka = 10(7) M-1). The kinetics of association are typical of known Ab-protein interactions, but the kinetics of dissociation are relatively fast. For therapeutic application, the binding affinities of such antibodies could be improved in vitro by mutation and selection for slower dissociation kinetics.
Full text
PDF
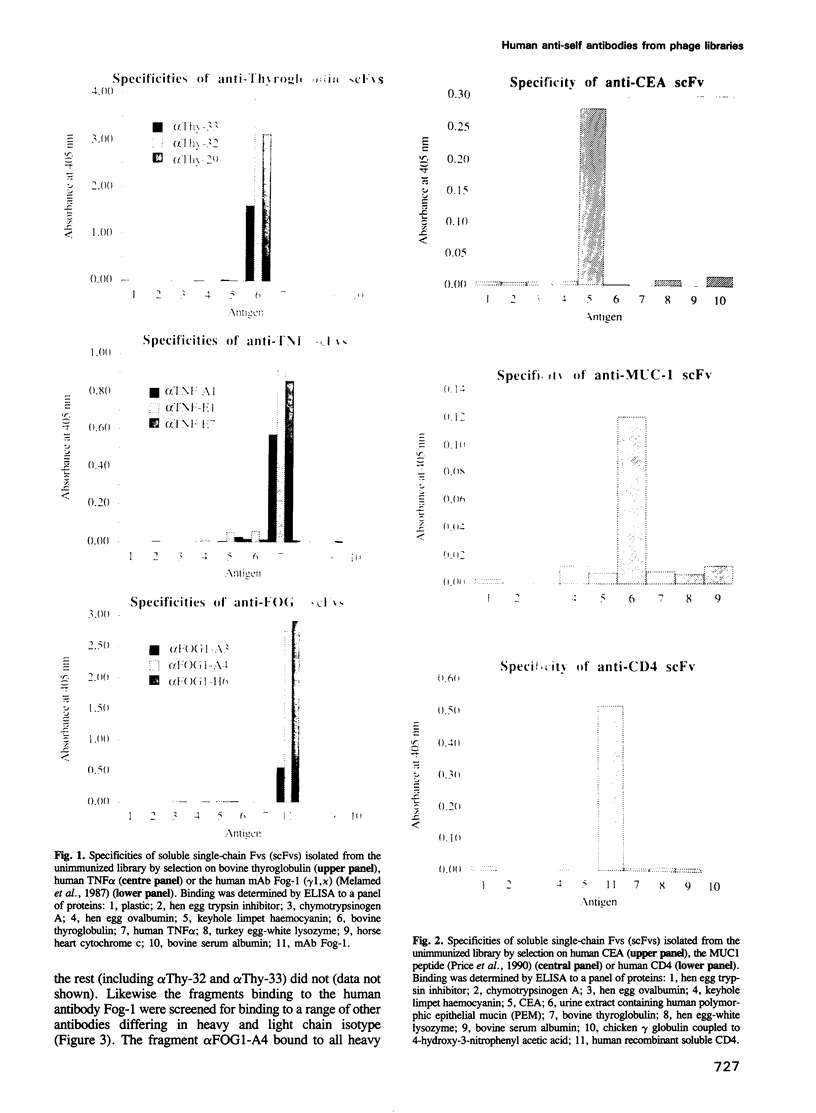

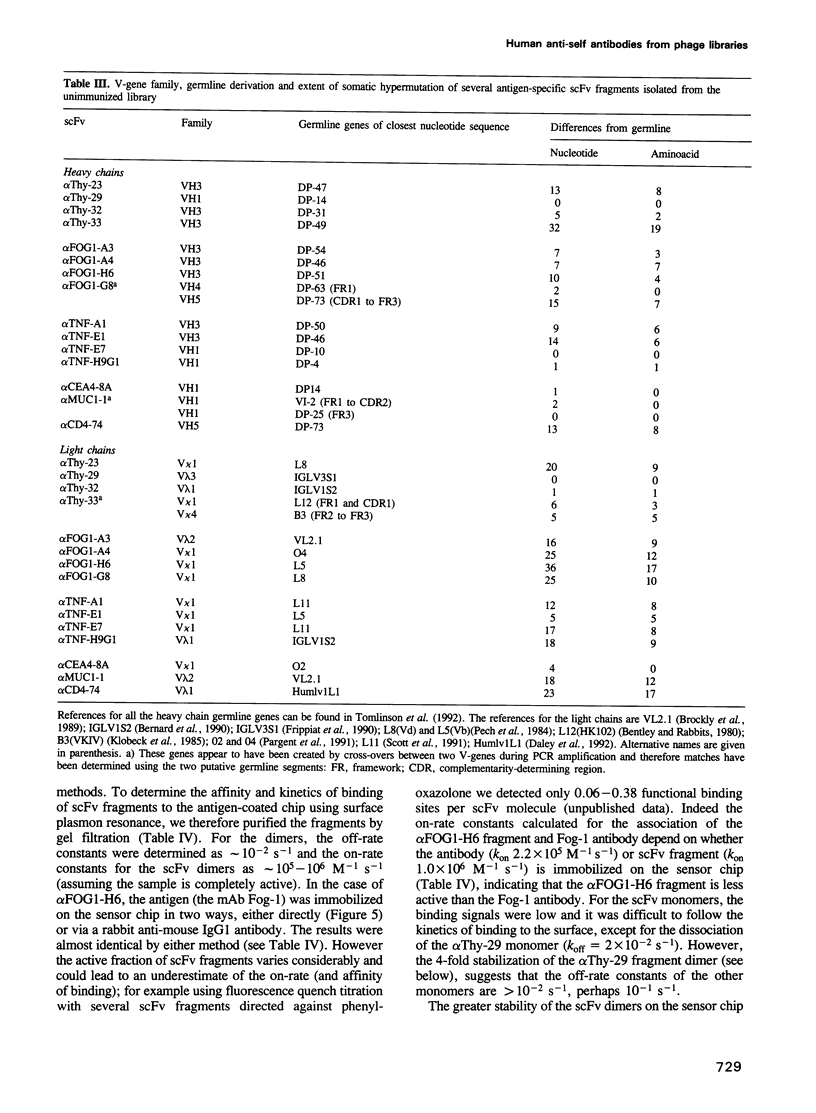
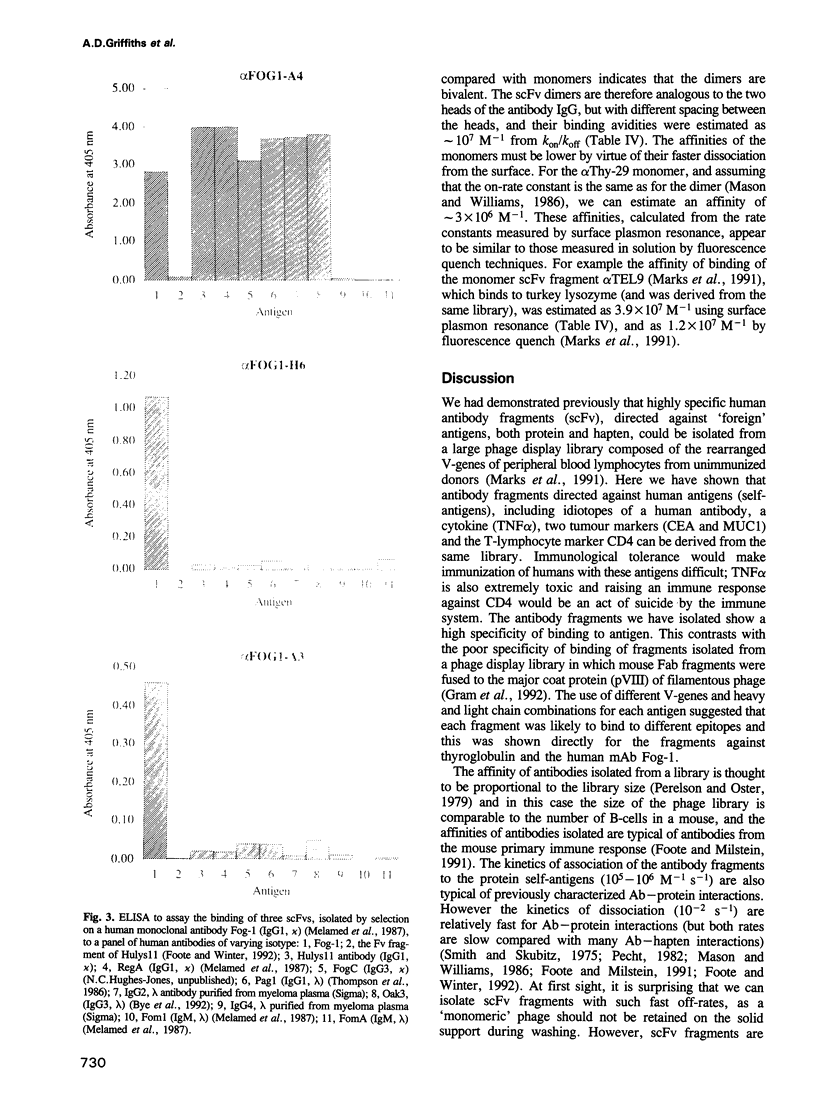
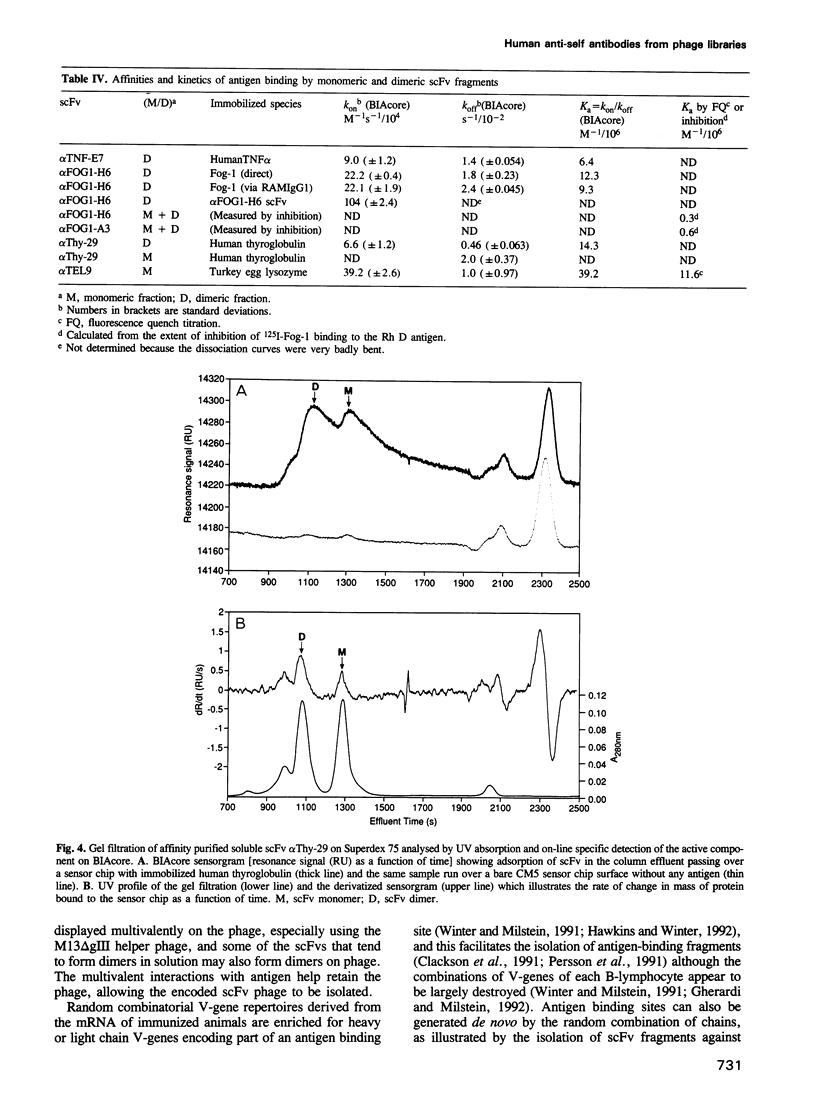


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein S., Pritchard-Briscoe H., Anderson T. A., Crosbie J., Gammon G., Loblay R. H., Basten A., Goodnow C. C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991 Mar 8;251(4998):1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Alexandre D., Chuchana P., Brockly F., Blancher A., Lefranc G., Lefranc M. P. First genomic sequence of a human Ig variable lambda gene belonging to subgroup I. Functional genes, pseudogenes and vestigial sequences are interspersed in the IGLV locus. Nucleic Acids Res. 1989 May 25;17(10):3975–3975. doi: 10.1093/nar/17.10.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. L., Szajnert M. F., Kaplan J. C., McColl L., Young B. D. The isolation of a human Ig V lambda gene from a recombinant library of chromosome 22 and estimation of its copy number. Nucleic Acids Res. 1984 Sep 11;12(17):6647–6661. doi: 10.1093/nar/12.17.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunol Today. 1991 May;12(5):154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Basten A., Brink R., Peake P., Adams E., Crosbie J., Hartley S., Goodnow C. C. Self tolerance in the B-cell repertoire. Immunol Rev. 1991 Aug;122:5–19. doi: 10.1111/j.1600-065x.1991.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Bendtzen K., Svenson M., Jønsson V., Hippe E. Autoantibodies to cytokines--friends or foes? Immunol Today. 1990 May;11(5):167–169. doi: 10.1016/0167-5699(90)90068-k. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Evolution of immunoglobulin V genes: evidence indicating that recently duplicated human V kappa sequences have diverged by gene conversion. Cell. 1983 Jan;32(1):181–189. doi: 10.1016/0092-8674(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Human immunoglobulin variable region genes--DNA sequences of two V kappa genes and a pseudogene. Nature. 1980 Dec 25;288(5792):730–733. doi: 10.1038/288730a0. [DOI] [PubMed] [Google Scholar]
- Berek C., Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987 Apr;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Bernard F., Chuchana P., Frippiat J. P., Buluwela L., Lefranc M. P. Genomic sequence of IGLV1S2, a human immunoglobulin variable lambda gene belonging to subgroup I. Nucleic Acids Res. 1990 Dec 11;18(23):7139–7139. doi: 10.1093/nar/18.23.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- Bona C. A. V genes encoding autoantibodies: molecular and phenotypic characteristics. Annu Rev Immunol. 1988;6:327–358. doi: 10.1146/annurev.iy.06.040188.001551. [DOI] [PubMed] [Google Scholar]
- Bouanani M., Bataille R., Piechaczyk M., Salhi S. L., Pau B., Bastide M. Autoimmunity to human thyroglobulin. Respective epitopic specificity patterns of anti-human thyroglobulin autoantibodies in patients with Sjögren's syndrome and patients with Hashimoto's thyroiditis. Arthritis Rheum. 1991 Dec;34(12):1585–1593. doi: 10.1002/art.1780341218. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Brockly F., Alexandre D., Chuchana P., Huck S., Lefranc G., Lefranc M. P. First nucleotide sequence of a human immunoglobulin variable lambda gene belonging to subgroup II. Nucleic Acids Res. 1989 May 25;17(10):3976–3976. doi: 10.1093/nar/17.10.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Albrandt K., Kipps T. J., Radoux V., Liu F. T., Carson D. A. Isolation and characterization of human VkIII germ-line genes. Implications for the molecular basis of human VkIII light chain diversity. J Immunol. 1987 Sep 1;139(5):1727–1733. [PubMed] [Google Scholar]
- Chen P. P., Albrandt K., Orida N. K., Radoux V., Chen E. Y., Schrantz R., Liu F. T., Carson D. A. Genetic basis for the cross-reactive idiotypes on the light chains of human IgM anti-IgG autoantibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8318–8322. doi: 10.1073/pnas.83.21.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. P., Robbins D. L., Jirik F. R., Kipps T. J., Carson D. A. Isolation and characterization of a light chain variable region gene for human rheumatoid factors. J Exp Med. 1987 Dec 1;166(6):1900–1905. doi: 10.1084/jem.166.6.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clackson T., Hoogenboom H. R., Griffiths A. D., Winter G. Making antibody fragments using phage display libraries. Nature. 1991 Aug 15;352(6336):624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- Combriato G., Klobeck H. G. V lambda and J lambda-C lambda gene segments of the human immunoglobulin lambda light chain locus are separated by 14 kb and rearrange by a deletion mechanism. Eur J Immunol. 1991 Jun;21(6):1513–1522. doi: 10.1002/eji.1830210627. [DOI] [PubMed] [Google Scholar]
- Daley M. D., Olee T., Peng H. Q., Soto-Gil R. W., Chen P. P., Siminovitch K. A. Molecular characterization of the human immunoglobulin V lambda I germline gene repertoire. Mol Immunol. 1992 Sep;29(9):1031–1042. doi: 10.1016/0161-5890(92)90034-u. [DOI] [PubMed] [Google Scholar]
- De Bellis D., Schwartz I. Regulated expression of foreign genes fused to lac: control by glucose levels in growth medium. Nucleic Acids Res. 1990 Mar 11;18(5):1311–1311. doi: 10.1093/nar/18.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Radic M. Z., Camper S. A., Hardy R. R., Carmack C., Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991 Jan 24;349(6307):331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- Foote J., Milstein C. Kinetic maturation of an immune response. Nature. 1991 Aug 8;352(6335):530–532. doi: 10.1038/352530a0. [DOI] [PubMed] [Google Scholar]
- Foote J., Winter G. Antibody framework residues affecting the conformation of the hypervariable loops. J Mol Biol. 1992 Mar 20;224(2):487–499. doi: 10.1016/0022-2836(92)91010-m. [DOI] [PubMed] [Google Scholar]
- Frippiat J. P., Chuchana P., Bernard F., Buluwela L., Lefranc G., Lefranc M. P. First genomic sequence of a human Ig variable lambda gene belonging to subgroup III. Nucleic Acids Res. 1990 Dec 11;18(23):7134–7134. doi: 10.1093/nar/18.23.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988 Sep 15;263(26):12820–12823. [PubMed] [Google Scholar]
- Gherardi E., Milstein C. Original and artificial antibodies. Nature. 1992 May 21;357(6375):201–202. doi: 10.1038/357201a0. [DOI] [PubMed] [Google Scholar]
- Glockshuber R., Malia M., Pfitzinger I., Plückthun A. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry. 1990 Feb 13;29(6):1362–1367. doi: 10.1021/bi00458a002. [DOI] [PubMed] [Google Scholar]
- Gorick B. D., Thompson K. M., Melamed M. D., Hughes-Jones N. C. Three epitopes on the human Rh antigen D recognized by 125I-labelled human monoclonal IgG antibodies. Vox Sang. 1988;55(3):165–170. doi: 10.1111/j.1423-0410.1988.tb05086.x. [DOI] [PubMed] [Google Scholar]
- Gram H., Marconi L. A., Barbas C. F., 3rd, Collet T. A., Lerner R. A., Kang A. S. In vitro selection and affinity maturation of antibodies from a naive combinatorial immunoglobulin library. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3576–3580. doi: 10.1073/pnas.89.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Güssow D., Clackson T. Direct clone characterization from plaques and colonies by the polymerase chain reaction. Nucleic Acids Res. 1989 May 25;17(10):4000–4000. doi: 10.1093/nar/17.10.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale G., Dyer M. J., Clark M. R., Phillips J. M., Marcus R., Riechmann L., Winter G., Waldmann H. Remission induction in non-Hodgkin lymphoma with reshaped human monoclonal antibody CAMPATH-1H. Lancet. 1988 Dec 17;2(8625):1394–1399. doi: 10.1016/s0140-6736(88)90588-0. [DOI] [PubMed] [Google Scholar]
- Hartley S. B., Crosbie J., Brink R., Kantor A. B., Basten A., Goodnow C. C. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991 Oct 24;353(6346):765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- Hawkins R. E., Winter G. Cell selection strategies for making antibodies from variable gene libraries: trapping the memory pool. Eur J Immunol. 1992 Mar;22(3):867–870. doi: 10.1002/eji.1830220336. [DOI] [PubMed] [Google Scholar]
- Hoogenboom H. R., Griffiths A. D., Johnson K. S., Chiswell D. J., Hudson P., Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991 Aug 11;19(15):4133–4137. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse W. D., Sastry L., Iverson S. A., Kang A. S., Alting-Mees M., Burton D. R., Benkovic S. J., Lerner R. A. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989 Dec 8;246(4935):1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenichen H. R., Pech M., Lindenmaier W., Wildgruber N., Zachau H. G. Composite human VK genes and a model of their evolution. Nucleic Acids Res. 1984 Jul 11;12(13):5249–5263. doi: 10.1093/nar/12.13.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K., Bell G. T. Human monoclonal antibody production. Current status and future prospects. J Immunol Methods. 1987 Jun 26;100(1-2):5–40. doi: 10.1016/0022-1759(87)90170-0. [DOI] [PubMed] [Google Scholar]
- Johnsson B., Löfås S., Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991 Nov 1;198(2):268–277. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- Jones D. H., McMillan A. J., Fersht A. R., Winter G. Reversible dissociation of dimeric tyrosyl-tRNA synthetase by mutagenesis at the subunit interface. Biochemistry. 1985 Oct 8;24(21):5852–5857. doi: 10.1021/bi00342a024. [DOI] [PubMed] [Google Scholar]
- Jönsson U., Fägerstam L., Ivarsson B., Johnsson B., Karlsson R., Lundh K., Löfås S., Persson B., Roos H., Rönnberg I. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. Biotechniques. 1991 Nov;11(5):620–627. [PubMed] [Google Scholar]
- Karlsson R., Michaelsson A., Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods. 1991 Dec 15;145(1-2):229–240. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- Klobeck H. G., Bornkamm G. W., Combriato G., Mocikat R., Pohlenz H. D., Zachau H. G. Subgroup IV of human immunoglobulin K light chains is encoded by a single germline gene. Nucleic Acids Res. 1985 Sep 25;13(18):6515–6529. doi: 10.1093/nar/13.18.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Meindl A., Combriato G., Solomon A., Zachau H. G. Human immunoglobulin kappa light chain genes of subgroups II and III. Nucleic Acids Res. 1985 Sep 25;13(18):6499–6513. doi: 10.1093/nar/13.18.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lautner-Rieske A., Huber C., Meindl A., Pargent W., Schäble K. F., Thiebe R., Zocher I., Zachau H. G. The human immunoglobulin kappa locus. Characterization of the duplicated A regions. Eur J Immunol. 1992 Apr;22(4):1023–1029. doi: 10.1002/eji.1830220422. [DOI] [PubMed] [Google Scholar]
- Lorenz W., Schäble K. F., Thiebe R., Stavnezer J., Zachau H. G. The J kappa proximal region of the human K locus contains three uncommon V kappa genes which are arranged in opposite transcriptional polarities. Mol Immunol. 1988 May;25(5):479–484. doi: 10.1016/0161-5890(88)90168-x. [DOI] [PubMed] [Google Scholar]
- Mach H., Middaugh C. R., Lewis R. V. Statistical determination of the average values of the extinction coefficients of tryptophan and tyrosine in native proteins. Anal Biochem. 1992 Jan;200(1):74–80. doi: 10.1016/0003-2697(92)90279-g. [DOI] [PubMed] [Google Scholar]
- Mach J. P., Forni M., Ritschard J., Buchegger F., Carrel S., Widgren S., Donath A., Alberto P. Use of limitations of radiolabeled anti-CEA antibodies and their fragments for photoscanning detection of human colorectal carcinomas. Oncodev Biol Med. 1980 Aug;1(1):49–69. [PubMed] [Google Scholar]
- Malthiéry Y., Lissitzky S. Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA. Eur J Biochem. 1987 Jun 15;165(3):491–498. doi: 10.1111/j.1432-1033.1987.tb11466.x. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Griffiths A. D., Malmqvist M., Clackson T. P., Bye J. M., Winter G. By-passing immunization: building high affinity human antibodies by chain shuffling. Biotechnology (N Y) 1992 Jul;10(7):779–783. doi: 10.1038/nbt0792-779. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Bonnert T. P., McCafferty J., Griffiths A. D., Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991 Dec 5;222(3):581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Griffiths A. D., Winter G. Molecular evolution of proteins on filamentous phage. Mimicking the strategy of the immune system. J Biol Chem. 1992 Aug 15;267(23):16007–16010. [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Melamed M. D., Thompson K. M., Gibson T., Hughes-Jones N. C. Requirements for the establishment of heterohybridomas secreting monoclonal human antibody to rhesus (D) blood group antigen. J Immunol Methods. 1987 Nov 23;104(1-2):245–251. doi: 10.1016/0022-1759(87)90511-4. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Burastero S. E., Ueki Y., Larrick J. W., Notkins A. L., Casali P. Probing the normal and autoimmune B cell repertoire with Epstein-Barr virus. Frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto's disease and systemic lupus erythematosus. J Immunol. 1988 Dec 15;141(12):4165–4172. [PubMed] [Google Scholar]
- Nemazee D., Russell D., Arnold B., Haemmerling G., Allison J., Miller J. F., Morahan G., Buerki K. Clonal deletion of autospecific B lymphocytes. Immunol Rev. 1991 Aug;122:117–132. doi: 10.1111/j.1600-065x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Bone marrow pre-B cells and the clonal anergy theory of immunologic tolerance. Int Rev Immunol. 1987 Mar;2(3):321–338. doi: 10.3109/08830188709044760. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Immunologic tolerance: collaboration between antigen and lymphokines. Science. 1989 Jul 14;245(4914):147–153. doi: 10.1126/science.2526369. [DOI] [PubMed] [Google Scholar]
- Pargent W., Meindl A., Thiebe R., Mitzel S., Zachau H. G. The human immunoglobulin kappa locus. Characterization of the duplicated O regions. Eur J Immunol. 1991 Aug;21(8):1821–1827. doi: 10.1002/eji.1830210807. [DOI] [PubMed] [Google Scholar]
- Pech M., Jaenichen H. R., Pohlenz H. D., Neumaier P. S., Klobeck H. G., Zachau H. G. Organization and evolution of a gene cluster for human immunoglobulin variable regions of the kappa type. J Mol Biol. 1984 Jun 25;176(2):189–204. doi: 10.1016/0022-2836(84)90420-0. [DOI] [PubMed] [Google Scholar]
- Pech M., Smola H., Pohlenz H. D., Straubinger B., Gerl R., Zachau H. G. A large section of the gene locus encoding human immunoglobulin variable regions of the kappa type is duplicated. J Mol Biol. 1985 Jun 5;183(3):291–299. doi: 10.1016/0022-2836(85)90001-4. [DOI] [PubMed] [Google Scholar]
- Pech M., Zachau H. G. Immunoglobulin genes of different subgroups are interdigitated within the VK locus. Nucleic Acids Res. 1984 Dec 21;12(24):9229–9236. doi: 10.1093/nar/12.24.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson A. S., Oster G. F. Theoretical studies of clonal selection: minimal antibody repertoire size and reliability of self-non-self discrimination. J Theor Biol. 1979 Dec 21;81(4):645–670. doi: 10.1016/0022-5193(79)90275-3. [DOI] [PubMed] [Google Scholar]
- Persson M. A., Caothien R. H., Burton D. R. Generation of diverse high-affinity human monoclonal antibodies by repertoire cloning. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2432–2436. doi: 10.1073/pnas.88.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. R., Hudecz F., O'Sullivan C., Baldwin R. W., Edwards P. M., Tendler S. J. Immunological and structural features of the protein core of human polymorphic epithelial mucin. Mol Immunol. 1990 Aug;27(8):795–802. doi: 10.1016/0161-5890(90)90089-i. [DOI] [PubMed] [Google Scholar]
- Rossi F., Guilbert B., Tonnelle C., Ternynck T., Fumoux F., Avrameas S., Kazatchkine M. D. Idiotypic interactions between normal human polyspecific IgG and natural IgM antibodies. Eur J Immunol. 1990 Sep;20(9):2089–2094. doi: 10.1002/eji.1830200930. [DOI] [PubMed] [Google Scholar]
- Ruf J., Carayon P., Lissitzky S. Various expressions of a unique anti-human thyroglobulin antibody repertoire in normal state and autoimmune disease. Eur J Immunol. 1985 Mar;15(3):268–272. doi: 10.1002/eji.1830150311. [DOI] [PubMed] [Google Scholar]
- Russell D. M., Dembić Z., Morahan G., Miller J. F., Bürki K., Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991 Nov 28;354(6351):308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. G., Crimmins D. L., McCourt D. W., Chung G., Schäble K. F., Thiebe R., Quenzel E. M., Zachau H. G., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. IV. The less frequently expressed VL are heterogeneous. J Immunol. 1991 Dec 1;147(11):4007–4013. [PubMed] [Google Scholar]
- Scott M. G., Crimmins D. L., McCourt D. W., Zocher I., Thiebe R., Zachau H. G., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. III. A single VKII gene and one of several JK genes are joined by an invariant arginine to form the most common L chain V region. J Immunol. 1989 Dec 15;143(12):4110–4116. [PubMed] [Google Scholar]
- Siminovitch K. A., Misener V., Kwong P. C., Song Q. L., Chen P. P. A natural autoantibody is encoded by germline heavy and lambda light chain variable region genes without somatic mutation. J Clin Invest. 1989 Nov;84(5):1675–1678. doi: 10.1172/JCI114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. P. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985 Jun 14;228(4705):1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- Smith H. R., Steinberg A. D. Autoimmunity--a perspective. Annu Rev Immunol. 1983;1:175–210. doi: 10.1146/annurev.iy.01.040183.001135. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Skubitz K. M. Kinetics in interactions between antibodies and haptens. Biochemistry. 1975 Apr 8;14(7):1496–1502. doi: 10.1021/bi00678a023. [DOI] [PubMed] [Google Scholar]
- Spooner C. E., Markowitz N. P., Saravolatz L. D. The role of tumor necrosis factor in sepsis. Clin Immunol Immunopathol. 1992 Jan;62(1 Pt 2):S11–S17. doi: 10.1016/0090-1229(92)90036-n. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Kekish O., Batter D., Grenier J., Balazs I., Henderson E., Zegers B. J. Aberrant recombination events in B cell lines derived from a kappa-deficient human. Nucleic Acids Res. 1985 May 24;13(10):3495–3514. doi: 10.1093/nar/13.10.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger B., Huber E., Lorenz W., Osterholzer E., Pargent W., Pech M., Pohlenz H. D., Zimmer F. J., Zachau H. G. The human VK locus. Characterization of a duplicated region encoding 28 different immunoglobulin genes. J Mol Biol. 1988 Jan 5;199(1):23–34. doi: 10.1016/0022-2836(88)90376-2. [DOI] [PubMed] [Google Scholar]
- Straubinger B., Thiebe R., Huber C., Osterholzer E., Zachau H. G. Two unusual human immunoglobulin V kappa genes. Biol Chem Hoppe Seyler. 1988 Jul;369(7):601–607. doi: 10.1515/bchm3.1988.369.2.601. [DOI] [PubMed] [Google Scholar]
- Thompson K. M., Hough D. W., Maddison P. J., Melamed M. D., Hughes-Jones N. The efficient production of stable, human monoclonal antibody-secreting hybridomas from EBV-transformed lymphocytes using the mouse myeloma X63-Ag8.653 as a fusion partner. J Immunol Methods. 1986 Nov 20;94(1-2):7–12. doi: 10.1016/0022-1759(86)90208-5. [DOI] [PubMed] [Google Scholar]
- Tomer Y., Shoenfeld Y. The significance of natural autoantibodies. Immunol Invest. 1988 Jul;17(5):389–424. doi: 10.3109/08820138809049846. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- Ward E. S., Güssow D., Griffiths A. D., Jones P. T., Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989 Oct 12;341(6242):544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]
- Welch M. J., Fong S., Vaughan J., Carson D. Increased frequency of rheumatoid factor precursor B lymphocytes after immunization of normal adults with tetanus toxoid. Clin Exp Immunol. 1983 Feb;51(2):299–304. [PMC free article] [PubMed] [Google Scholar]
- Winkler T. H., Fehr H., Kalden J. R. Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur J Immunol. 1992 Jul;22(7):1719–1728. doi: 10.1002/eji.1830220709. [DOI] [PubMed] [Google Scholar]
- Winter G., Milstein C. Man-made antibodies. Nature. 1991 Jan 24;349(6307):293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]